Abstract
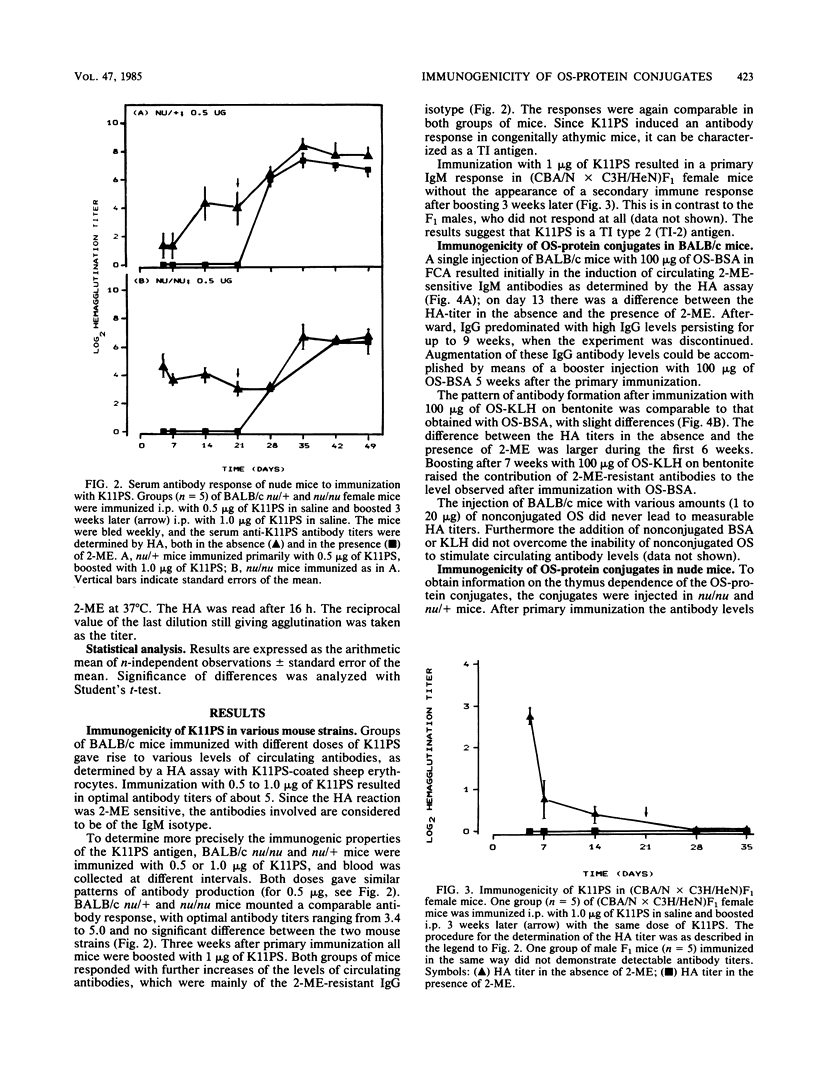
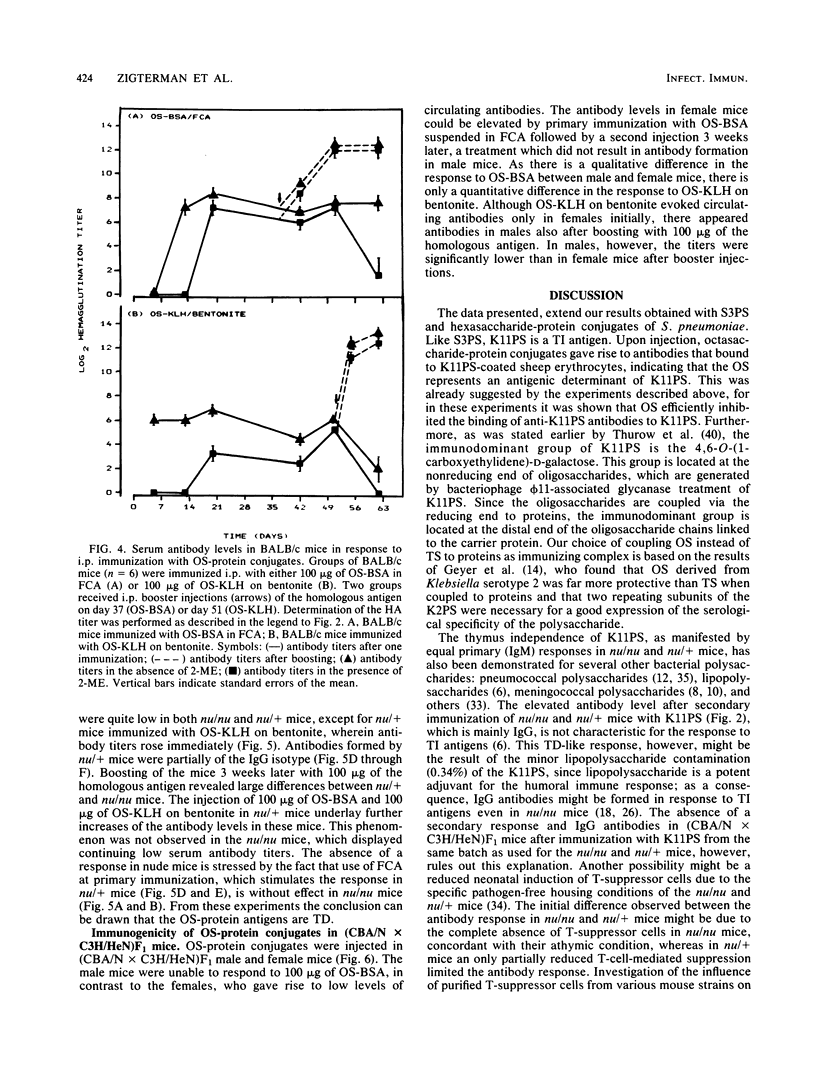
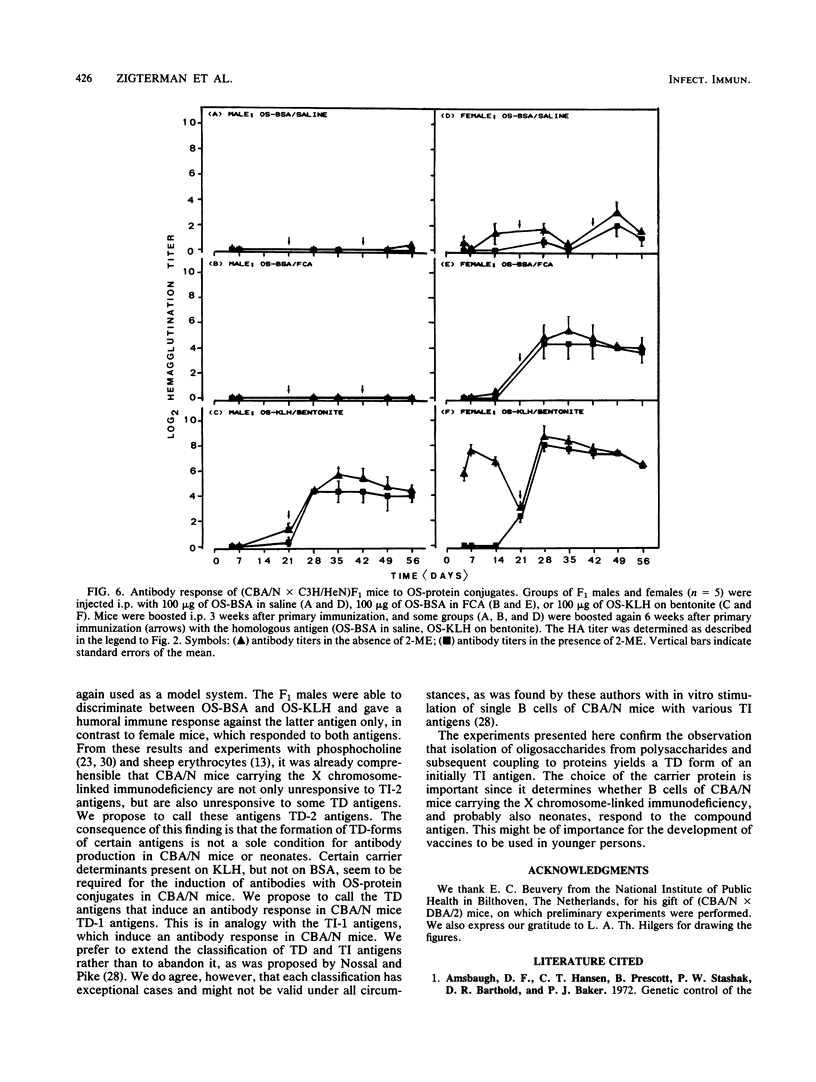
The tetrasaccharide repeating unit of the capsular polysaccharide of Klebsiella serotype 11, K11PS, comprises the following sequence: [----3)-beta-D-GlcpA-(1----3)-alpha-D-Galp-(1----3)-beta-D-Glcp-(1 ----] with a 4,6-O-(1-carboxyethylidene)-alpha-D-galactopyranosyl residue linked to O-4 of the glucuronic acid residue. Octasaccharide (OS) derived from K11PS by bacteriophage phi 11-associated glycanase, was coupled to bovine serum albumin and to keyhole limpet hemocyanin. The immunogenicity of various antigens after intraperitoneal immunization was studied by measuring the levels of circulating antibodies. Injection of BALB/c mice with K11PS resulted in induction of 2-mercaptoethanol-sensitive immunoglobulin M antibodies. The responses observed in BALB/c nu/nu mice and in male (CBA/N X C3H/HeN)F1 mice indicate that K11PS is a thymus-independent type 2 antigen. Immunization of BALB/c mice with either OS-bovine serum albumin or OS-keyhole limpet hemocyanin resulted in the induction of circulating 2-mercaptoethanol-resistant immunoglobulin G antibodies. Results in BALB/c nu/nu mice indicate that the OS-protein conjugates are thymus-dependent antigens. Since the OS-keyhole limpet hemocyanin conjugate induced antibodies in both (CBA/N X C3H/HeN)F1 females and males, we propose to refer to this kind of antigen as a thymus-dependent type 1 antigen, whereas OS-bovine serum albumin, which evoked immunoglobulins in (CBA/N X C3H/HeN)F1 females only, can be referred to as a thymus-dependent type 2 antigen.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972 Oct 1;136(4):931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Insel R. A. A polysaccharide-protein complex from Haemophilus influenzae type b. I. Activity in weanling rabbits and human T lymphocytes. J Infect Dis. 1981 Dec;144(6):509–520. doi: 10.1093/infdis/144.6.509. [DOI] [PubMed] [Google Scholar]
- Avery O. T., Goebel W. F. CHEMO-IMMUNOLOGICAL STUDIES ON CONJUGATED CARBOHYDRATE-PROTEINS : V. THE IMMUNOLOGICAL SPECIFITY OF AN ANTIGEN PREPARED BY COMBINING THE CAPSULAR POLYSACCHARIDE OF TYPE III PNEUMOCOCCUS WITH FOREIGN PROTEIN. J Exp Med. 1931 Jul 31;54(3):437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C., Miedema F., van Delft R. W., Haverkamp J., Leussink A. B., te Pas B. J., Teppema K. S., Tiesjema R. H. Preparation and physicochemical and immunological characterization of polysaccharide-outer membrane protein complexes of Neisseria meningitidis. Infect Immun. 1983 Apr;40(1):369–380. doi: 10.1128/iai.40.1.369-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C., van Delft R. W., Miedema F., Kanhai V., Nagel J. Immunological evaluation of meningococcal group C polysaccharide-tetanus toxoid conjugate in mice. Infect Immun. 1983 Aug;41(2):609–617. doi: 10.1128/iai.41.2.609-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C., van Rossum F., Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982 Jul;37(1):15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild R. L., Braley-Mullen H. Characterization of the murine immune response to type 6 pneumococcal polysaccharide. Infect Immun. 1983 Feb;39(2):615–622. doi: 10.1128/iai.39.2.615-622.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Deficient production of a thymus-dependent high affinity antibody subset in mice (CBA/N) with an X-linked B lymphocyte defect. J Immunol. 1976 Aug;117(2):701–702. [PubMed] [Google Scholar]
- Geyer H., Schlecht S., Himmelspach K. Immunochemical properties of oligosaccharide-protein conjugates with Klebsiella-K2-specificity. II. Protective capacity of the conjugates and anti-conjugate antibodies against infection with Klebsiella pneumoniae 01:K2 in mice. Med Microbiol Immunol. 1982;171(3):135–143. doi: 10.1007/BF02123621. [DOI] [PubMed] [Google Scholar]
- Goebel W. F. STUDIES ON ANTIBACTERIAL IMMUNITY INDUCED BY ARTIFICIAL ANTIGENS : I. IMMUNITY TO EXPERIMENTAL PNEUMOCOCCAL INFECTION WITH AN ANTIGEN CONTAINING CELLOBIURONIC ACID. J Exp Med. 1939 Feb 28;69(3):353–364. doi: 10.1084/jem.69.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger M., Roy N., Glaudemans C. P. Inhibition of aldobiouronates in the precipitation of pneumococcal type II and III systems. Biochemistry. 1969 Dec;8(12):4822–4824. doi: 10.1021/bi00840a025. [DOI] [PubMed] [Google Scholar]
- Ivars F., Nyberg G., Holmberg D., Coutinho A. Immune response to bacterial dextrans. II. T cell control of antibody isotypes. J Exp Med. 1983 Nov 1;158(5):1498–1510. doi: 10.1084/jem.158.5.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. Subclass-restricted IgG polyclonal antibody production in mice injected with lipid A-rich lipopolysaccharides. J Exp Med. 1981 Feb 1;153(2):324–338. doi: 10.1084/jem.153.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K., Jann B., Orskov F., Orskov I., Westphal O. Immunchemische Untersuchungen an K-Antigenen von Escherichia Coli. II. Das K-Antigen von E. coli 08:K42(A):H-. Biochem Z. 1965 Jun 3;342(1):1–22. [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Ashton F. E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984 Jan;43(1):407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981 Sep;127(3):1011–1018. [PubMed] [Google Scholar]
- Kamerling J. P., Gerwig G. J., Vliegenthart J. F., Clamp J. R. Characterization by gas-liquid chromatography-mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem J. 1975 Dec;151(3):491–495. doi: 10.1042/bj1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Shigemoto S., Watanabe T., Yamamura Y. Demonstration of phosphorylcholine-specific IgE B cells in CBA/N mice. J Immunol. 1979 Sep;123(3):1039–1043. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Metcalf E. S., Scher I., Klinman N. R. Susceptibility to in vitro tolerance induction of adult B cells from mice with an X-linked B-cell defect. J Exp Med. 1980 Feb 1;151(2):486–491. doi: 10.1084/jem.151.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Prescott B., Cross S. S., Stashak P. W., Baker P. J. Regulation of the antibody response to type III pneumococcal polysaccharide. V. Ontogeny of factors influencing the magnitude of the plaque-forming cell response. J Immunol. 1976 Feb;116(2):279–287. [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. A reappraisal of "T-independent" antigens. II. Studies on single, hapten-specific B cells from neonatal CBA/H or CBA/N mice fail to support classification into TI-1 and TI-2 categories. J Immunol. 1984 Apr;132(4):1696–1701. [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- Quintáns J., Kaplan R. B. Failure of CBA/N mice to respond to thymus-dependent and thymus-independent phosphorylcholine antigens. Cell Immunol. 1978 Jul;38(2):294–301. doi: 10.1016/0008-8749(78)90060-6. [DOI] [PubMed] [Google Scholar]
- Rieger-Hug D., Stirm S. Comparative study of host capsule depolymerases associated with Klebsiella bacteriophages. Virology. 1981 Aug;113(1):363–378. doi: 10.1016/0042-6822(81)90162-8. [DOI] [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Lehle G., Weiler E., Kölsch E. Immune response against the T-independent antigen alpha (1 leads to 3) dextran. I. Demonstration of an unexpected IgG response of athymic and germ-free-raised euthymic BALB/c mice. Eur J Immunol. 1982 Feb;12(2):120–125. doi: 10.1002/eji.1830120205. [DOI] [PubMed] [Google Scholar]
- Snippe H., van Houte A. J., van Dam J. E., De Reuver M. J., Jansze M., Willers J. M. Immunogenic properties in mice of hexasaccharide from the capsular polysaccharide of Streptococcus pneumoniae type 3. Infect Immun. 1983 Jun;40(3):856–861. doi: 10.1128/iai.40.3.856-861.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. E., Zopf D. A., Johnson B. M., Miller C. B., Paul W. E. The immune response to an isomaltohexosyl-protein conjugate, a thymus-dependent analogue of alpha(1 replaced by 6) dextran. J Immunol. 1982 Mar;128(3):1350–1354. [PubMed] [Google Scholar]
- Stirm S., Freund-Mölbert E. Escherichia coli capsule bacteriophages. II. Morphology. J Virol. 1971 Sep;8(3):330–342. doi: 10.1128/jvi.8.3.330-342.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Artificial Salmonella vaccines. Prog Allergy. 1983;33:120–143. doi: 10.1159/000407424. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Coupling of acid labile Salmonella specific oligosaccharides to macromolecular carriers. J Immunol Methods. 1979;25(4):323–335. doi: 10.1016/0022-1759(79)90025-5. [DOI] [PubMed] [Google Scholar]
- Thurow H., Niemann H., Stirm S. Bacteriophage-borne enzymes in carbohydrate chemistry. Part I. On the glycanase activity associated with particles of Klebsiella bacteriophage No. 11. Carbohydr Res. 1975 May;41:257–271. doi: 10.1016/s0008-6215(00)87024-x. [DOI] [PubMed] [Google Scholar]
- Tsai C. S. Determination of degree of polymerization of N-acetyl chitooligoses by chromatographic methods. Anal Biochem. 1970 Jul;36(1):114–122. doi: 10.1016/0003-2697(70)90338-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



