Abstract
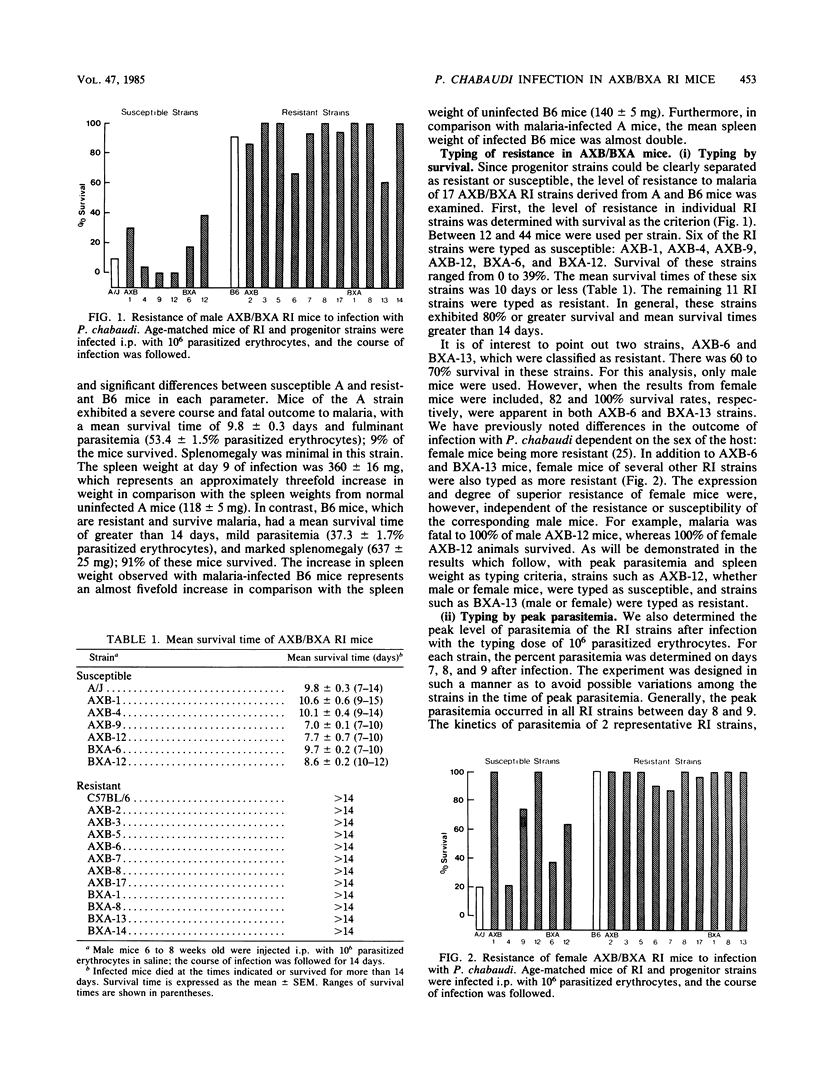
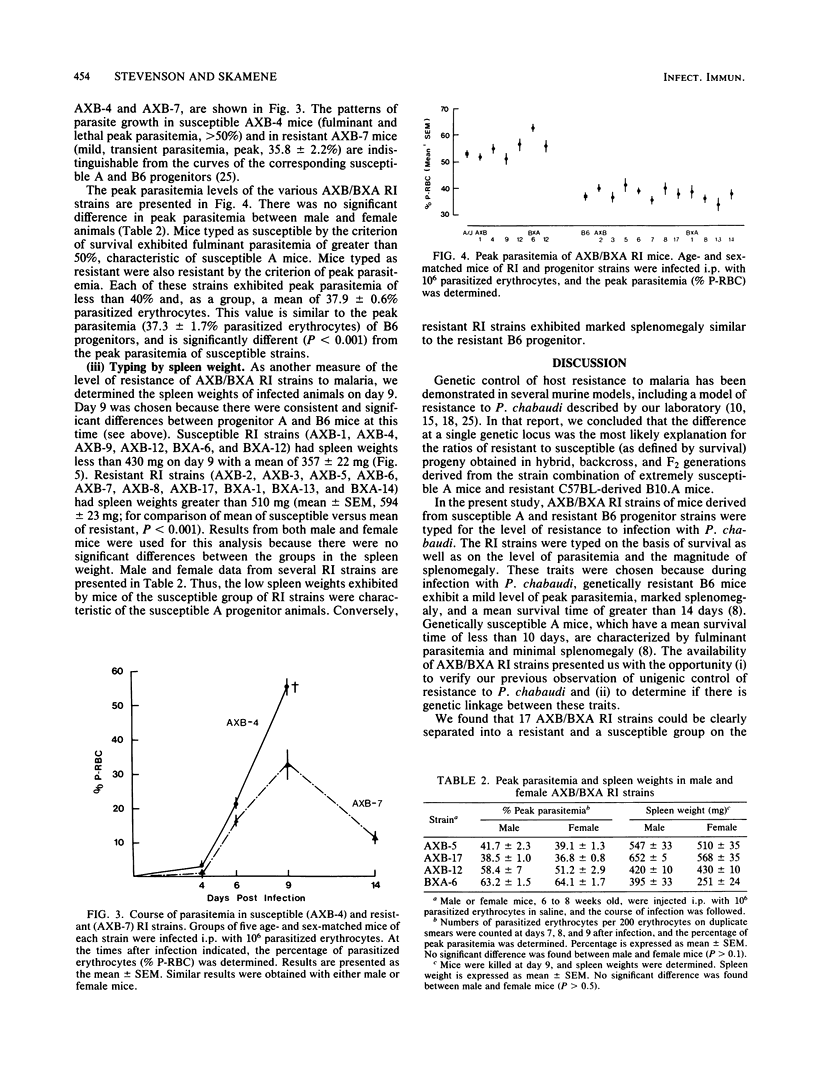
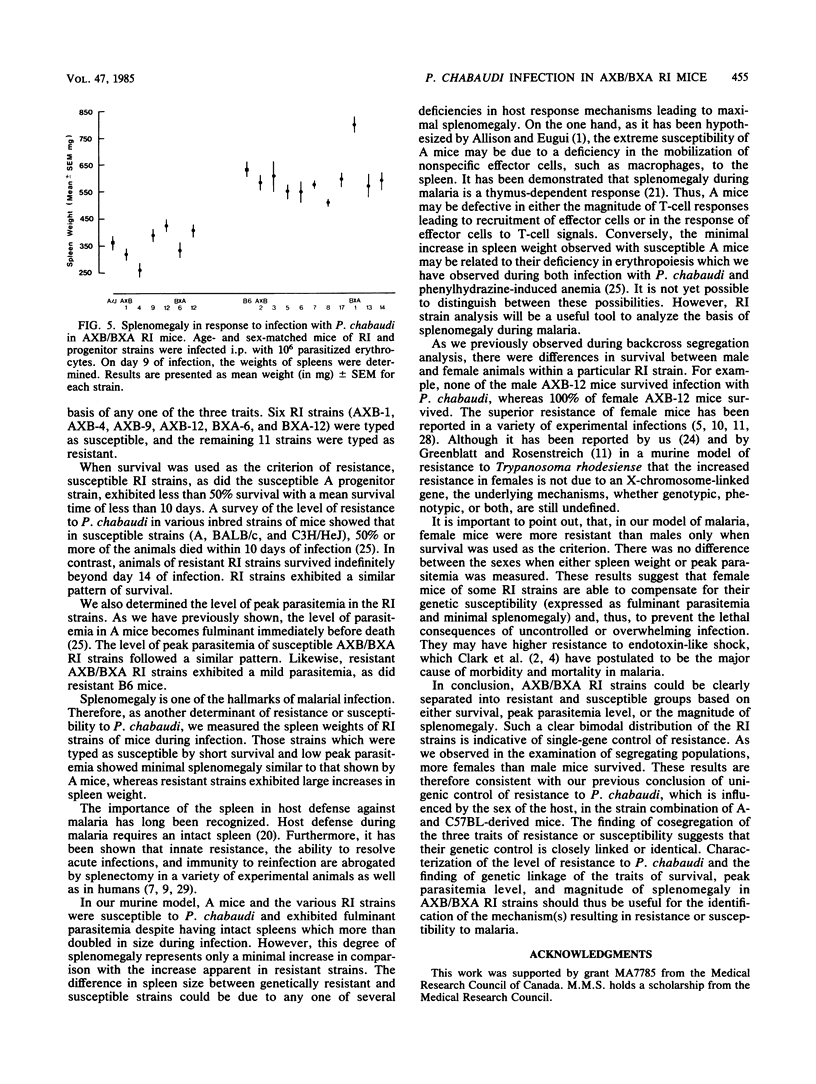
The level of resistance to infection with Plasmodium chabaudi is genetically controlled. We have previously reported that a single dominant gene is responsible for the variation in host resistance to malaria between susceptible A/J- and resistant C57BL-derived mice. In the present study, recombinant inbred strain analysis was performed with AXB/BXA recombinant inbred strains derived from A/J and C57BL/6 progenitors. Typing of 17 AXB/BXA recombinant inbred strains confirmed the unigenic control of inheritance in this particular strain combination and allowed us to demonstrate genetic linkage between the traits of resistance (defined as a prolonged survival and a low peak parasitemia) and the magnitude of splenomegaly. The influence of sex on the course of infection, which we previously reported in the examination of segregating populations (Stevenson et al., Infect. Immun. 38:80-88, 1982), was again demonstrated in the survey of RI strains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet. 1978 Jul 8;2(8080):75–77. doi: 10.1016/s0140-6736(78)91386-7. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson C., Owen M. E. Effect of host sex on passive immunity in mice infected with Nematospiroides dubius. Int J Parasitol. 1978 Oct;8(5):359–364. doi: 10.1016/0020-7519(78)90033-4. [DOI] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of blood-stage murine malaria parasites by hydrogen peroxide. Infect Immun. 1983 Jan;39(1):456–459. doi: 10.1128/iai.39.1.456-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eling W. M. Chronic, patent Plasmodium berghei malaria in splenectomized mice. Infect Immun. 1982 Mar;35(3):880–886. doi: 10.1128/iai.35.3.880-886.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugui E. M., Allison A. C. Differences in susceptibility of various mouse strains to haemoprotozoan infections: possible correlation with natural killer activity. Parasite Immunol. 1980 Winter;2(4):277–292. doi: 10.1111/j.1365-3024.1980.tb00059.x. [DOI] [PubMed] [Google Scholar]
- GREENBERG J., NADEL E. M., COATNEY G. R. [The influence of strain, sex and age of mice on infection with Plasmodium berghei]. J Infect Dis. 1953 Jul-Aug;93(1):96–100. doi: 10.1093/infdis/93.1.96. [DOI] [PubMed] [Google Scholar]
- Garnham P. C. The role of the spleen in protozoal infections with special reference to splenectomy. Acta Trop. 1970;27(1):1–14. [PubMed] [Google Scholar]
- Greenblatt H. C., Rosenstreich D. L. Trypanosoma rhodesiense infection in mice: sex dependence of resistance. Infect Immun. 1984 Jan;43(1):337–340. doi: 10.1128/iai.43.1.337-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Rosenstreich D. L., Taylor B. A., Osterman J. V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980 Sep;125(3):1395–1399. [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Haidaris C. G., Haynes J. D., Meltzer M. S., Allison A. C. Serum containing tumor necrosis factor is cytotoxic for the human malaria parasite Plasmodium falciparum. Infect Immun. 1983 Oct;42(1):385–393. doi: 10.1128/iai.42.1.385-393.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. J., Weidanz W. P., Long C. A. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect Immun. 1984 Mar;43(3):981–985. doi: 10.1128/iai.43.3.981-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Allan J. S., Carlin J. M., Vande Waa J. A., Divo A. A., Akood M. A. Association between human serum-induced crisis forms in cultured Plasmodium falciparum and clinical immunity to malaria in Sudan. Infect Immun. 1983 Sep;41(3):1302–1311. doi: 10.1128/iai.41.3.1302-1311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel E M, Greenberg J, Jay G E, Coatney G R. Backcross Studies on the Genetics of Resistance to Malaria in Mice. Genetics. 1955 Sep;40(5):620–626. doi: 10.1093/genetics/40.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Shear H. L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J Immunol. 1984 Jan;132(1):424–431. [PubMed] [Google Scholar]
- Oster C. N., Koontz L. C., Wyler D. J. Malaria in asplenic mice: effects of splenectomy, congenital asplenia, and splenic reconstitution on the course of infection. Am J Trop Med Hyg. 1980 Nov;29(6):1138–1142. doi: 10.4269/ajtmh.1980.29.1138. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. Splenomegaly, enhanced phagocytosis, and anemia are thymus-dependent responses to malaria. Infect Immun. 1978 Jun;20(3):728–731. doi: 10.1128/iai.20.3.728-731.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepczyk C. M., Clark I. A. Demonstration of a lipopolysaccharide-induced cytostatic effect on malarial parasites. Infect Immun. 1981 Aug;33(2):343–347. doi: 10.1128/iai.33.2.343-347.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Lyanga J. J., Skamene E. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun. 1982 Oct;38(1):80–88. doi: 10.1128/iai.38.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Dockrell H. M., Playfair J. H. Endotoxin-induced serum factor kills malarial parasites in vitro. Infect Immun. 1981 Jul;33(1):83–89. doi: 10.1128/iai.33.1.83-89.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trischmann T. M., Bloom B. R. Genetics of murine resistance to Trypanosoma cruzi. Infect Immun. 1982 Feb;35(2):546–551. doi: 10.1128/iai.35.2.546-551.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



