Abstract
Cyclic sulfonyl imines derived from ketones were identified as stable and readily prepared compounds that serve as superior electrophiles for N-heterocyclic carbene (NHC)-catalyzed annulations with α,β-unsaturated aldehydes to afford highly substituted γ-lactams. Their superior reactivity and properties are highlighted by the first example of NHC-catalyzed reactions of enals with low catalyst loadings (0.5 mol %) and broad substrate scope encompassing alkyl, aryl, and heteroaromatic substituents. These findings and supporting studies suggest an alternative, ene-like mechanism rather than the catalytic generation of a catalyst-bound homoenolate equivalent.
Nucleophilic additions to aldehydes and ketones provide reliable methods for the preparation of secondary and tertiary alcohols. Similar tactics can be employed for the preparation of certain amines via additions to aldimines; however, suitable ketimine derivatives are often difficult to prepare and handle due to their inherent lability.1 We have found this to be a particular problem in the case of nucleophile-catalyzed reactions, such as those promoted by N-heterocyclic carbenes (NHCs),2 in which the nucleophilic catalyst can promote the decomposition of imines. Thus, despite considerable effort from our group, and that of Scheidt, NHC-catalyzed additions of enals to imine electrophiles have been limited to aromatic aldehyde derived N-sulfonyl imines,3 azomethine imines,4 and N-phenyl nitrones.5
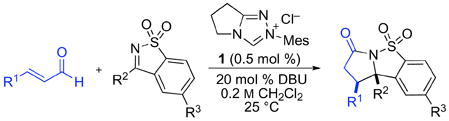
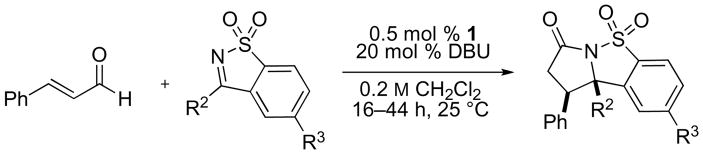
In this report, we document a simple and convenient solution to the synthesis of stereochemically-defined tertiary amine-derivatives via formal homoenolate additions to chemically stable, yet highly reactive, ketimines derived from saccharin.6 In addition to providing a powerful, diastereoselective approach to γ-lactams, this is the first report of NHC-catalyzed homoenolate additions to any class of electrophiles with low catalyst loadings (0.5 mol %), for a broad range of enal and imine substrates. These findings highlight the unique and underappreciated synthetic utility of cyclic sulfonyl imines; they are readily prepared and stable to common transformations, including nucleophilic catalysts (eq 1).
 |
(1) |
Most prior reports of NHC-catalyzed additions of enals to electrophiles including imines, aldehydes, or ketones employed imidazolium salts as precatalysts for homoenolate generation.7 Our studies with saccharin derivatives therefore began with the use of IMes·HCl and DBU in protic solvents, which provided the desired product in moderate yield. In contrast to our previous experience with homoenolate additions,8 the use of triazolium precatalyst 19 proved most effective for lactam formation, and completely suppressed the formation of aldehyde dimers. To our further surprise, a screen of conditions (see Supporting Information) revealed that 0.5 mol % of precatalyst 1 was sufficient for quantitative conversion at room temperature.
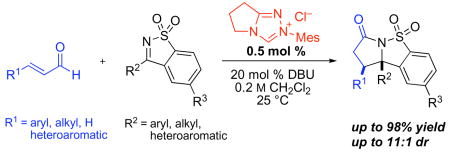
We applied our optimized conditions to the reaction of cinnamaldehyde with a number of different ketimines (table 1). Electronically varied aromatic (entries 1–3) and heteroaromatic substituents (entries 4, 6, 7) were well tolerated. In most cases the cis-product predominated, although an electron-poor aromatic substituent (entry 5) resulted in a small preference for the trans-product – a trend that was amplified by installing an ortho-substituent (entry 6). When alkyl-substituted ketimines were employed the diastereoselectivities improved, albeit at the expense of product yield. These substrates could be coaxed towards higher yields by using more precatalyst (entries 10, 11).
Table 1.
NHC-catalyzed annulation of cinnamaldehyde with various sulfonyl ketimines.a
 | ||||
|---|---|---|---|---|
| Entry | R2 = | R3 = | % yieldb | cis/transc |
| 1 | Ph | H | 89 | 3d:1 |
| 2 | p-MeO-C6H4 | H | 85 | 4:1 |
| 3 | m-MeO-C6H4 | H | 96 | 3:1 |
| 4 | thiophen-2-yl | H | 90 | 4:1 |
| 5 | p-CN-C6H4 | H | 83 | 1:1 |
| 6e | 3-Br-pyridin-4-yl | H | 77 | 1:2 |
| 7e | 5-pyrimidinyl | H | 68 | >20:1 |
| 8 | Ph | Me | 96 | 4:1 |
| 9 | Ph | OMe | 98 | 4:1 |
| 10 | Me | H | 60 (72)e | 9d:1 |
| 11 | nBu | H | 13 (78)e | 11:1 |
See Supporting Information for reaction details.
Combined, isolated yields of diastereomeric lactams after chromatography.
Diastereomeric ratio determined by 1H NMR analysis of unpurified reaction mixtures.
Structure determined by X-ray analysis.
5 mol % 1 used.
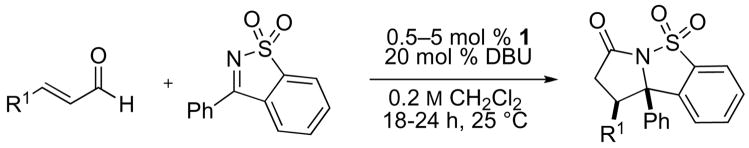
The annulation reactions with cyclic sulfonyl ketimines were not restricted to the use of cinnamaldehyde derivatives (table 2, entries 1–4) as was the case for aldehyde or ketone electrophiles. Alkyl and alkenyl substituted enals were suitable (entries 5–7) and even acrolein was viable (entry 8). Notably, an unprotected aldehyde in the substrate survives the annulation (entry 4).
Table 2.
NHC-catalyzed annulation of various enals with phenyl sulfonyl imine.a
 | ||||
|---|---|---|---|---|
| entry | R1 = | % cat. 1 | % yieldb | cis/transc |
| 1 | p-MeO-C6H4 | 0.5 | 95 | 4:1 |
| 2 | 2-furyl | 0.5 | 98 | 4:1 |
| 3 | p-CF3-C6H4 | 0.5 | 89 | 2:1 |
| 4 | p-CHO-C6H4 | 0.5 | 55 | 1:1 |
| 5 | 1-propenyl | 5.0 | 75 | 6:1 |
| 6d | 1-propyl | 5.0 | 96 | 5:1 |
| 7d | Me | 5.0 | 78 | 3:1 |
| 8e | H | 5.0 | 80 | n/a |
See Supporting Information for reaction details.
Combined, isolated yields of diastereomeric lactams after chromatography.
Diastereomeric ratio determined by 1H NMR analysis of unpurified reaction mixtures.
2.0 equiv of aldehyde used.
3.0 equiv of aldehyde added in 2 portions.
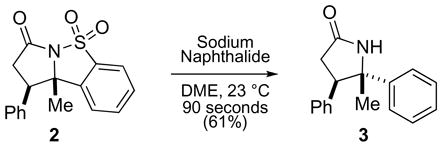
Although structurally interesting in their own right, the obtained γ-lactams could be rapidly converted to the corresponding desulfonated γ-lactams 3 (eq 2).10
 |
(2) |
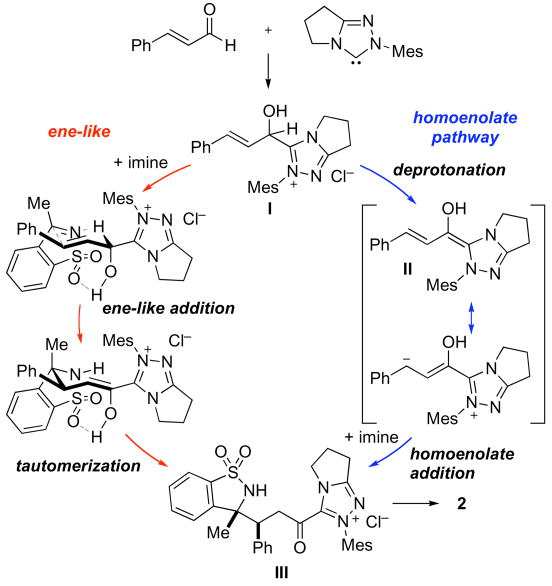
In contrast to the corresponding N-sulfonyl aldimines, which required an imidazolium precatalyst in a protic solvent, forcing conditions (60 °C and 15 mol % catalyst), and were limited to cinnamaldehydes or related substrates,3 the cyclic sulfonylimines could be coupled with enals by the use of only 0.5 mol % of a triazolium precatalyst at room temperature in an aprotic solvent.11 The great difference in reactivity, reaction conditions, and substrate scope prompted further consideration of the reaction mechanism. In one point, the acyclic and cyclic imines substrates were similar: both undergo rapid, reversible reactions with the nucleophilic N-heterocyclic carbene catalyst. Studies with molar equivalents of saccharin derivatives and triazolium precatalyst 1 revealed rapid (<1 min) and quantitative addition reactions. We therefore considered mechanisms whereby the resulting imine-catalyst adduct serves as the key reactive species. Our studies demonstrated instead that quantitative addition of the catalyst to the imine inhibits, rather than promotes, the annulation.
An alternative explanation for these discrepancies would be a different reaction pathway. Our prior studies invoked enal-catalyst adduct I, which is deprotonated to form Breslow intermediate II that serves as homoenolate equivalent that attacks the electrophilic imine. To better rationalize the observed reactivity and stereochemical outcome, we have considered an ene-like transition state (red in scheme 1). The presumed six membered cyclic transition state could be stabilized by a hydrogen bond to the sulfonyl oxygen and allows for the concomitant transfer of the acyl proton to the imine nitrogen atom and formation of the new carbon-carbon bond. Our hypothesis is supported by the reactivity of the intermediate catalyst-enal adduct I. When I is generated in the absence of an imine, and the imine is subsequently added, formation of the product is not observed, suggesting that formation of Breslow intermediate II may be detrimental to the reaction. This pathway would rationalize both the cis-selectivity of the annulation reaction and the superiority of aprotic solvents. Furthermore, it could explain the high activity of the catalyst, as deprotonation of I is often the rate-determining step in azolium catalyzed reactions.12
Scheme 1.
Possible reaction pathways for NHC-catalyzed annulations of enals and cyclic sulfonyl ketimines.
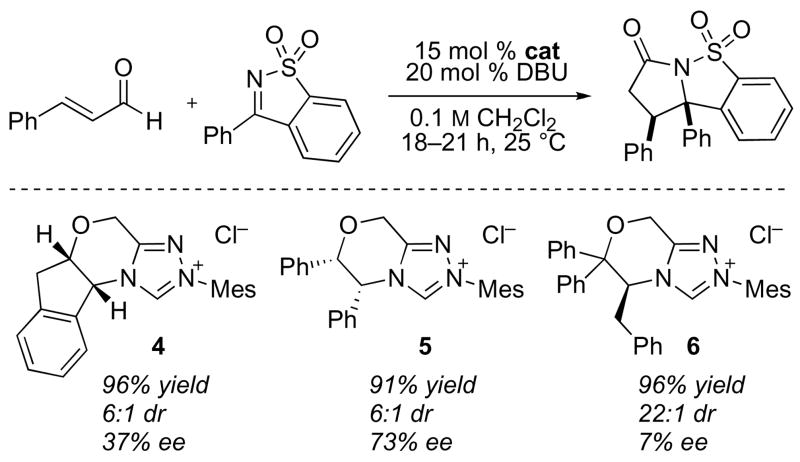
Preliminary efforts on the development of a catalytic, enantioselective variant are encouraging but highlight the established challenges of asymmetric additions to ketimines (Scheme 2). A number of previously reported chiral triazolium precatalysts give the desired product in excellent yield and up to 73% ee.5,13 Importantly, the use of precatalyst 65 dramatically improves the diastereoselectivity.
Scheme 2.
Enantioselective annulation of cyclic ketimines.
In summary, we have expanded the utility of NHC-catalyzed homoenolate additions by 1) identifying saccharin-derived ketimines as stable and useful electrophiles; 2) demonstrating broad scope of both the enal and imine reaction partners; and 3) documenting, for the first time, that NHC-catalyzed reactions of enals can proceed with low (0.5 mol %) catalyst loadings.
Supplementary Material
Experimental procedures and characterization data for all new compounds. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgments
We are grateful to the NIH (GM-079339) and generous gifts from Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, and Roche for the support of this research program. M.R. received a postdoctoral fellowship from the German Academic Exchange Service (DAAD). We thank Justin Struble for the preparation of the precatalysts and Pat Sheppard for X-ray analyses.
References
- 1.Weinreb SM. Top Curr Chem. 1997;190:131–184. [Google Scholar]
- 2.(a) Enders D, Niemeier O, Henseler A. Chem Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]; (b) Marion N, Díez-González S, Nolan SP. Angew Chem Int Ed. 2007;46:2988–3000. doi: 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]
- 3.He M, Bode JW. Org Lett. 2005;7:3131–3134. doi: 10.1021/ol051234w. [DOI] [PubMed] [Google Scholar]
- 4.Chan A, Scheidt KA. J Am Chem Soc. 2007;129:5334–5335. doi: 10.1021/ja0709167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips EM, Reynolds TE, Scheidt KA. J Am Chem Soc. 2008;130:2416–2417. doi: 10.1021/ja710521m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For the synthesis of saccharin derived ketimines see: Davis FA, Towson JC, Vashi DB, ThimmaReddy R, McCauley JP, Jr, Harakal ME, Gosciniak DJ. J Org Chem. 1990;55:1254–1261.
- 7.For examples, see references 3, 4 and: Sohn SS, Rosen EL, Bode JW. J Am Chem Soc. 2004;126:14370–14371. doi: 10.1021/ja044714b.Burstein C, Glorius F. Angew Chem Int Ed. 2004;43:6205–6208. doi: 10.1002/anie.200461572.Nair V, Vellalath S, Poonoth M, Mohan R, Suresh E. Org Lett. 2006;8:507–509. doi: 10.1021/ol052926n.
- 8.Struble JR, Kaeobamrung J, Bode JW. Org Lett. 2008;10:957–960. doi: 10.1021/ol800006m. [DOI] [PubMed] [Google Scholar]
- 9.Sohn SS, Bode JW. Org Lett. 2005;7:3873–3876. doi: 10.1021/ol051269w. [DOI] [PubMed] [Google Scholar]
- 10.Ji S, Gortler LB, Waring A, Battisti AJ, Bank S, Closson WD, Wriede PA. J Am Chem Soc. 1967;89:5311–5312. [Google Scholar]
- 11.Notably, the acyclic aldimines from ref. 3 were unreactive under these reaction conditions, even at higher catalyst loading (15 mol %).
- 12.White MJ, Leeper FJ. J Org Chem. 2001;66:5124–5131. doi: 10.1021/jo010244h. [DOI] [PubMed] [Google Scholar]; (b) Rovis, T. personal communication.
- 13.He M, Struble JR, Bode JW. J Am Chem Soc. 2006;128:8418–8420. doi: 10.1021/ja062707c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and characterization data for all new compounds. This material is available free of charge via the internet at http://pubs.acs.org.