Abstract
Human choices are remarkably susceptible to the manner in which options are presented. This so-called “framing effect” represents a striking violation of standard economic accounts of human rationality, although its underlying neurobiology is not understood. We found that the framing effect was specifically associated with amygdala activity, suggesting a key role for an emotional system in mediating decision biases. Moreover, across individuals, orbital and medial prefrontal cortex activity predicted a reduced susceptibility to the framing effect. This finding highlights the importance of incorporating emotional processes within models of human choice and suggests how the brain may modulate the effect of these biasing influences to approximate rationality.
A central tenet of rational decision-making is logical consistency across decisions, regardless of the manner in which available choices are presented. This assumption, known as “extensionality” (1) or “invariance” (2), is a fundamental axiom of game theory (3). However, the proposition that human decisions are “description-invariant” is challenged by a wealth of empirical data (4, 5). Kahneman and Tversky originally described this deviation from rational decision-making, which they termed the “framing effect,” as a key aspect of prospect theory (6, 7).
Theories of decision-making have tended to emphasize the operation of analytic processes in guiding choice behavior. However, more intuitive or emotional responses can play a key role in human decision-making (8-10). Thus, when taking decisions under conditions when available information is incomplete or overly complex, subjects rely on a number of simplifying heuristics, or efficient rules of thumb, rather than extensive algorithmic processing (11). One suggestion is that the framing effect results from systematic biases in choice behavior arising from an affect heuristic underwritten by an emotional system (12, 13). However, despite the substantial role of the framing effect in influencing human decision-making, the underlying neurobiological basis is not understood.
We investigated the neurobiological basis of the framing effect by means of functional magnetic resonance imaging (fMRI) and a novel financial decision-making task. Participants (20 university students or graduates) received a message indicating the amount of money that they would initially receive in that trial (e.g., “You receive £50”). Subjects then had to choose between a “sure” option and a “gamble” option presented in the context of two different frames. The “sure” option was formulated as either the amount of money retained from the initial starting amount (e.g., keep £20 of the £50; “Gain” frame) or as the amount of money lost from the initial amount (e.g., lose £30 of the £50; “Loss” frame). The “gamble” option was identical in both frames and was represented as a pie chart depicting the probability of winning or losing (Fig. 1) (14).
Fig. 1.
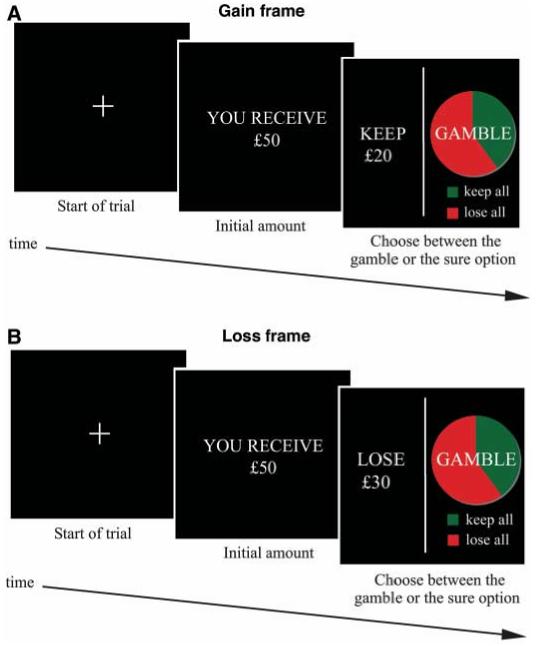
The financial decision-making task. At the beginning of each trial, participants were shown a message indicating the starting amount of money that they would receive (e.g., ‘You receive £50’) (duration 2 s). Subjects were instructed that they would not be able to retain the whole of this initial amount, but would next have to choose between a sure option and a gamble option (4 s). The sure option was presented in the Gain frame trials (A) as an amount of money retained from the starting amount (e.g., keep £20 of the £50) and in the Loss frame trials (B) as an amount of money lost from the starting amount (e.g., lose £30 of the £50). The gamble option was represented as a pie chart depicting the probability of winning (green) or losing (red) all of the starting money. The expected outcomes of the gamble and sure options were equivalent. Gain frame trials were intermixed pseudo-randomly with Loss frame trials. No feedback concerning trial outcomes was given during the experiment.
The behavioral results indicated that subjects’ decisions were significantly affected by our framing manipulation, with a marked difference in choices between the two frames (Fig. 2A). Specifically, and in accordance with predictions arising from prospect theory, subjects were risk-averse in the Gain frame, tending to choose the sure option over the gamble option [gambling on 42.9% of trials; significantly different from 50% (P < 0.05, t19 = 1.96)], and were risk-seeking in the Loss frame, preferring the gamble option [gambling on 61.6% of trials; significantly different from 50% (P < 0.005, t19 = 3.31)]. This effect of frame was consistently expressed across different probabilities and starting amounts (fig. S1).
Fig. 2.
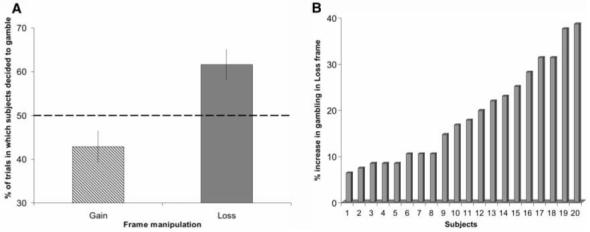
Behavioral results. (A) Percentages of trials in which subjects chose the gamble option in the Gain frame and the Loss frame. Subjects showed a significant increase in the percentage of trials in which the gamble option was chosen in the Loss frame with respect to the Gain frame [61.6%> 42.9% (P < 0.001, t19 = 8.06)]. The dashed line represents riskneutral behavior (choosing the gamble option in 50% of trials). Error bars denote SEM. (B) Each bar represents, for each individual subject, the percentage difference between how often subjects chose the gamble option in the Loss frame as compared to the Gain frame. A hypothetical value of zero represents a complete indifference to the framing manipulation (i.e., fully ‘rational’ behavior). All participants, to varying degrees, showed an effect of the framing manipulation.
Reaction times for decisions were not affected by frame [Gain frame, 1895 ms; Loss frame, 1884 ms (P> 0.1)]; this result provides evidence that difficulty was well matched between the two frames. Moreover, subjects performed highly accurately on “catch” trials (14) (fig. S2) where the expected outcomes of the sure and gamble options were unbalanced, indicating their continued engagement with the task throughout the experiment. Despite the marked though variable impact of the frame on subjects’ choice behavior (Fig. 2B), the majority (16/20) of subjects seemed unaware of any biasing effect when specifically questioned in a debriefing session that followed the experiment.
Subjects performed the behavioral task inside an fMRI scanner, allowing us to obtain continuous measures of regional brain activity. The subjects’ individual decisions during the entire fMRI experiment were recorded and used to construct four regressors of interest: sure decisions in the Gain frame (Gsure), gamble decisions in the Gain frame (G gamble), sure decisions in the Loss frame (Lsure), and gamble decisions in the Loss frame (Lgamble).
Given that the frame effect relates to subjects’ asymmetrical pattern of decisions across frames, the key experimental contrast of interest is the interaction between the decision to gamble (or not) and the valence of the frame: [(G sure þ Lgamble) − (Ggamble þ Lsure)]. It is noteworthy that this interaction contrast is balanced with respect to both decision type and frame valence. Consequently, we could identify brain areas that were more active when subjects chose in accordance with the frame effect (i.e., G sure þ Lgamble), as opposed to when their decisions ran counter to their general behavioral tendency Ggamble þ Lsure. This contrast revealed significant activation in the bilateral amygdala (Fig. 3, A and B). To ensure that this activation in the amygdala was not being driven by a significant effect in one frame alone (e.g., Loss frame), we conducted an independent analysis for each frame. This confirmed that robust activation in the amygdala was equally observed for simple effects of decision type (sure or gamble) in each frame separately. Thus, amygdala activation was significantly greater when subjects decided to choose the sure option in the Gain frame [G sure − Ggamble] [Montreal Neurological Institute (MNI) space coordinates (x, y, z) 18, −4, −24; Z score = 4.0], and the gamble option in the Loss frame [Lgamble − Lsure] [MNI space coordinates −16, 0, −26; Z score = 3.80; 12,2, −22; Z score = 4.67], in keeping with a central role in mediating the frame effect.
Fig. 3.
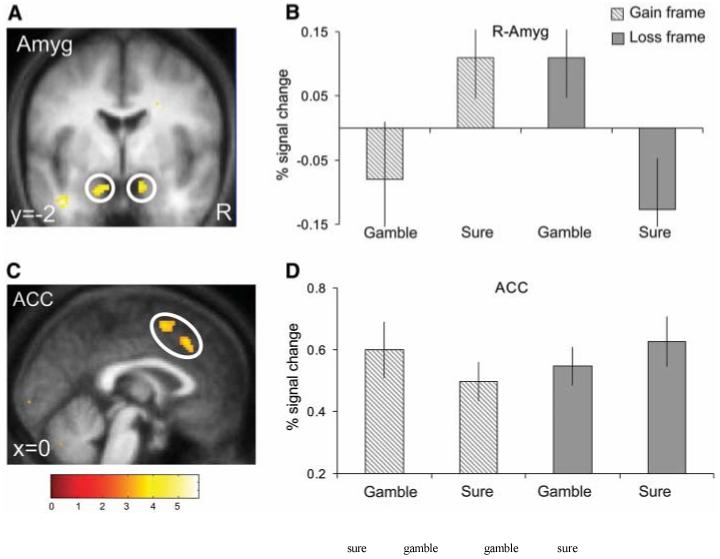
fMRI results (A) Interaction contrast [(G sure + Lgamble) - (Ggamble + Lsure)] : brain activations reflecting subjects’ behavioral tendency to choose the sure option in the Gain frame and the gamble option in the Loss frame (i.e., in accordance with the frame effect). Bilateral amygdala (Amyg) activation [MNI space coordinates (x, y, z)]: left hemisphere, −14, 2, −24 (peak Z score = 3.97); right hemisphere, 12, 2, −20 (Z score = 3.82). (C) Reverse interaction contrast [(Ggamble + Lsure) − (Gsure + L gamble)]:brain activations reflecting the decision to choose counter to subjects’ general behavioral tendency. Anterior cingulate cortex (ACC) activation: 2, 24, 44 (Z score = 3.65); −2, 8, 56 (Z score = 3.78). Effects in (A) and (C) were significant at P < 0.001; for display purposes they are shown at P < 0.005. (B and D) Plots of percentage signal change for peaks in right amygdala (12, 2, −20) (B) and ACC (2, 24, 44) (D). Error bars denote SEM.
A different pattern of brain activation was identified when subjects made decisions that ran counter to their general behavioral tendency. In this reverse interaction contrast [(Ggamble + Lsure) − (G sure + Lgamble)], we observed enhanced activity in the anterior cingulate cortex (ACC) (Fig. 3, C and D) (and to a lesser extent in the bilateral dorsolateral prefrontal cortex at an uncorrected threshold of P < 0.005; fig. S3) when subjects chose the gamble option in the Gain frame and the sure option in the Loss frame.
In light of the substantial intersubject variability in behavioral susceptibility to the frame, we next identified subject-specific differences in neural activity associated with their decision bias (that is, the decision x frame interaction) (Fig. 2A). Using the overall susceptibility of each subject to the frame manipulation as a between-subjects statistical regressor, operationalized as a “rationality index” (14), we found a significant correlation between decreased susceptibility to the framing effect and enhanced activity in the orbital and medial prefrontal cortex (OMPFC), specifically in the right orbitofrontal cortex (R-OFC; r = 0.8, P < 0.001) and the ventromedial prefrontal cortex (VMPFC; r = 0.75, P < 0.001) (Fig. 4). In summary, those subjects who acted more rationally exhibited greater activation in OMPFC associated with the frame effect.
Fig. 4.
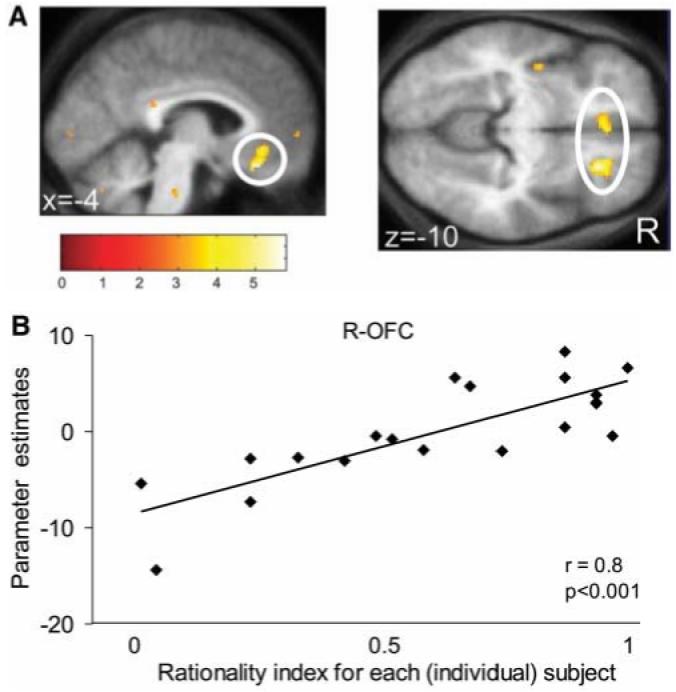
Rationality across subjects: fMRI correlational analysis. Regions showing a significant correlation between rationality index [between-subjects measure of susceptibility to the framing manipulation; see (14)] and the interaction contrast image [(G sure+ Lgamble) - (Ggamble + Lsure)] are highlighted. (A) Orbital and medial prefrontal cortex (OMPFC) [MNI space coordinates (x, y, z)]: VMPFC (left panel), −4, 34, −8 (Z score = 4.56); OMPFC and R-OFC circled in right panel [R-OFC: 24, 30, −10 (Z score = 5.77)]. Effects were significant at P < 0.001; for display purposes they are shown at P < 0.005. (B) Plot of the correlation of parameter estimates for R-OFC with the rationality index for each subject (r = 0.8, P < 0.001).
Our data provide a neurobiological account of the framing effect, both within and across individuals. Increased activation in the amygdala was associated with subjects’ tendency to be risk-averse in the Gain frame and risk-seeking in the Loss frame, supporting the hypothesis that the framing effect is driven by an affect heuristic underwritten by an emotional system. The amygdala plays a key role in value-related prediction and learning, both for negative (aversive) and positive (appetitive) outcomes (15-17). Furthermore, in simple instrumental decision-making tasks in animals, the amygdala appears to mediate decision biases that come from value-related predictions (18). In humans, the amygdala is also implicated in the detection of emotionally relevant information present in contextual and social emotional cues (19). It was previously shown that activation in the amygdala during the passive viewing of surprised faces is significantly modulated by the valence of preceding verbal contextual information (20). Our data extend the role of the amygdala to include processing the type of contextual positive or negative emotional information communicated by the frame in the context of a decision-making task.
In our study, activation of the amygdala was driven by the combination of a subject’s decision and the frame in which it took place, rather than by the valence of the frame per se. Consequently, our findings indicate that frame-related valence information is incorporated into the relative assessment of options to exert control over the apparent risk sensitivity of individual decisions. The observation that the frame has such a pervasive impact on complex decision-making supports an emerging role for the amygdala in decision-making (21, 22).
When subjects’ choices ran counter to their general behavioral tendency, there was enhanced activity in the ACC. This suggests an opponency between two neural systems, with ACC activation consistent with the detection of conflict between predominantly “analytic” response tendencies and a more “emotional” amygdala-based system (23, 24).
Previous descriptions of the frame effect have been predominantly confined to between-subjects investigations. Our experimental design allowed us to distinguish the anatomical bases of the frame effect, both within and between subjects. Interestingly, amygdala activity did not predict the substantial intersubject difference in terms of susceptibility to the frame effect. Instead, subjects’ tendency to be susceptible to the frame showed a robust correlation with neural activity in the OMPFC. It is noteworthy that there are strong reciprocal connections between the amygdala and the OMPFC (25), although each may contribute to distinct functional roles in decision-making (26). Lesions of the OMPFC cause impairments in decision-making; these are often characterized as an inability to adapt behavioral strategies according to the consequences of decisions, leading to impulsivity (27,28). It is thought that the OMPFC, incorporating inputs from the amygdala, represents the motivational value of stimuli (or choices), which allows it to integrate and evaluate the incentive value of predicted outcomes in order to guide future behavior (29, 30). Our data raise an intriguing possibility that more “rational” individuals have a better and more refined representation of their own emotional biases that enables them to modify their behavior in appropriate circumstances, as for example when such biases might lead to suboptimal decisions. As such, our findings support a model in which the OMPFC evaluates and integrates emotional and cognitive information, thus underpinning more “rational” (i.e., description-invariant) behavior.
Our findings suggest a model in which the framing bias reflects an affect heuristic by which individuals incorporate a potentially broad range of additional emotional information into the decision process. In evolutionary terms, this mechanism may confer a strong advantage, because such contextual cues may carry useful, if not critical, information. Neglecting such information may ignore the subtle social cues that communicate elements of (possibly unconscious) knowledge that allow optimal decisions to be made in a variety of environments. However, in modern society, which contains many symbolic artifacts and where optimal decision-making often requires skills of abstraction and decontextualization, such mechanisms may render human choices irrational (31).
Supplementary Material
Acknowledgments
Supported by a Wellcome Trust Programme Grant (R.J.D.) and a Wellcome Trust studentship (B.D.M.). We thank H. Spiers, P. Sterzer, and J. Hughes for helpful discussions during the analysis of the study and P. Bossaerts for useful comments on the manuscript.
References and Notes
- 1.Arrow KJ. Econ. Inq. 1982;20:1. [Google Scholar]
- 2.Tversky A, Kahneman D. J. Bus. 1986;59:S251. [Google Scholar]
- 3.von Neumann J, Morgenstern O. Theory of Games and Economic Behavior. Princeton Univ. Press; Princeton, NJ: 1944. [Google Scholar]
- 4.Kahneman D, Tversky A. Choices, Values, and Frames. Cambridge Univ. Press; New York: 2000. [Google Scholar]
- 5.McNeil BJ, Pauker SG, Sox HC, Jr., Tversky A. N. Engl. J. Med. 1982;306:1259. doi: 10.1056/NEJM198205273062103. [DOI] [PubMed] [Google Scholar]
- 6.Kahneman D, Tversky A. Econometrica. 1979;47:263. [Google Scholar]
- 7.Tversky A, Kahneman D. Science. 1981;211:453. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- 8.Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. Science. 1994;264:1102. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- 9.Greene J, Haidt J. Trends Cogn. Sci. 2002;6:517. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- 10.Loewenstein GF, Weber EU, Hsee CK, Welch N. Psychol. Bull. 2001;127:267. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- 11.Gilovich T, Griffin DW, Kahneman D, editors. Heuristics and Biases: The Psychology of Intuitive Judgment. Cambridge Univ. Press; New York: 2002. [Google Scholar]
- 12.Slovic P, Finucane M, Perers E, MacGregor D. In: Heuristics and Biases: The Psychology of Intuitive Judgment. Gilovich T, Griffin DW, Kahneman D, editors. Cambridge Univ. Press; New York: 2002. pp. 397–421. [Google Scholar]
- 13.Gabaix X, Laibson D. In: The Psychology of Economic Decisions, Vol. 1: Rationality and Well-Being. Brocas I, Carrillo JD, editors. Oxford Univ. Press; Oxford: 2003. pp. 169–183. [Google Scholar]
- 14.See supporting material on Science Online.
- 15.Baxter MG, Murray EA. Nat. Rev. Neurosci. 2002;3:563. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 16.LeDoux JE. The Emotional Brain. Simon & Schuster; New York: 1996. [Google Scholar]
- 17.Paton JJ, Belova MA, Morrison SE, Salzman CD. Nature. 2006;439:865. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbit LH, Balleine BW. J. Neurosci. 2005;25:962. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adolphs R. Brain Res. 2006;1079:25. doi: 10.1016/j.brainres.2005.12.127. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, et al. J. Cogn. Neurosci. 2004;16:1730. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- 21.Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Science. 2005;310:1680. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 22.Balleine BW, Killcross S. Trends Neurosci. 2006;29:272. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Psychol. Rev. 2001;108:624. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 24.Miller EK, Cohen JD. Annu. Rev. Neurosci. 2001;24:167. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 25.Amaral DG, Price JL, Pitkanen A, Carmichael ST. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton JP, editor. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- 26.Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. J. Neurosci. 2004;24:4718. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bechara A, Damasio AR, Damasio H, Anderson SW. Cognition. 1994;50:7. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 28.Rolls ET, Hornak J, Wade D, McGrath J. J. Neurol. Neurosurg. Psychiatry. 1994;57:1518. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Neuron. 2003;39:855. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 30.Schoenbaum G, Roesch MR, Stalnaker TA. Trends Neurosci. 2006;29:116. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanovich K, West F. In: Heuristics and Biases: The Psychology of Intuitive Judgment. Gilovich T, Griffin DW, Kahneman D, editors. Cambridge Univ. Press; New York: 2002. pp. 421–440. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


