Abstract
Numerous studies have shown that the active form of vitamin D, 1,25(OH)2D3, can exert growth inhibitory effects on human breast cancer cells and mammary tumor growth. However, the molecular mechanisms remain to be fully delineated. This study demonstrates for the first time that CCAAT enhancer-binding protein α (C/EBPα), a member of the C/EBP family of transcription factors, is induced by 1,25(OH)2D3 and is a potent enhancer of VDR transcription in MCF-7 breast cancer cells. 1,25(OH)2D3 was found to induce C/EBPα as well as VDR expression in MCF-7 cells. C/EBPα was not detected in MDA-MB-231 cells that are poorly responsive to 1,25(OH)2D3. Antiproliferative effects of 1,25(OH)2D3 and induction of VDR were observed in MDA-MB-231 cells transfected with C/EBPα, and knockdown of C/EBPα suppressed VDR and antiproliferative effects of 1,25(OH)2D3 in MCF-7 cells. Transfection of C/EBPα in MCF-7 cells resulted in a dose-dependent enhancement of hVDR transcription. Our studies show that C/EBPα can bind to Brahma (Brm), an ATPase that is a component of the SWI/SNF complex, and cooperate with Brm in the regulation of hVDR transcription in MCF-7 cells. Because the levels of VDR in MCF-7 breast cancer cells correlate with the antiproliferative effects of 1,25(OH)2D3 and because C/EBPα has been suggested as a potential tumor suppressor in breast cancer, these findings provide important mechanisms whereby 1,25(OH)2D3 may act to inhibit growth of breast cancer cells. These findings also identify C/EBPα as a 1,25(OH)2D3 target in breast cancer cells and provide evidence for C/EBPα as a candidate for breast cancer treatment.
Vitamin D, to exert its effects, must be metabolized to its most active form, 1,25-dihydroxyvitaminD3 (1,25(OH)2D3)2 (1). The actions of 1,25(OH)2D3 include not only maintenance of calcium homeostasis but also effects on numerous other systems, including effects on the immune system and the growth and differentiation of a number of malignant cells including breast cancer cells (1). 1,25(OH)2D3 acts by binding to a high affinity intracellular receptor protein (the vitamin D receptor or VDR). 1,25(OH)2D3 bound to the VDR heterodimerizes with the retinoid X receptor and along with coactivators and additional accessory nuclear proteins interacts with vitamin D response elements in the promoter of target genes and modulates their transcription (1).
In vivo studies have suggested a role for vitamin D and 1,25(OH)2D3 in breast cancer prevention as well as in treatment of breast cancer. It has been demonstrated that rats fed diets low in vitamin D and calcium develop significantly more mammary tumors when treated with 7,12-dimethylbenz(a)anthracene than rats fed control diets with adequate vitamin D and calcium (2). In other in vivo studies using N-methyl-N-nitrosourea-treated rats 1,25(OH)2D3 or analogs of 1,25(OH)2D3 were found to inhibit the progression of mammary tumor growth (3, 4). When rats were treated with 1,25(OH)2D3 or analogs of 1,25(OH)2D3 prior to treatment with N-methyl-N-nitrosourea, tumor incidence was reduced or prevented (5, 6). In addition, in patient studies it has been noted that the presence of vitamin D receptors in breast carcinomas is correlated with improved prognosis in breast patients (7). Also, low serum levels of 1,25(OH)2D3 are associated with increased breast cancer risk or disease progression (8, 9). 1,25(OH)2D3 or its analogs also exerts potent growth inhibitory effects on human breast cancer cells (10, 11). We have earlier reported that a combination of 1,25(OH)2D3 and retinoic acid lowers the threshold for killing of breast cancer cells by taxol and adriamycin thus suggesting a novel option for the treatment of breast cancer (12, 13). Although these studies provide compelling evidence for the use of 1,25(OH)2D3 or less calcemic analogs of 1,25(OH)2D3 that have similar growth-inhibitory effects, little is known about the mechanisms involved in mediating the cellular responsiveness to 1,25(OH)2D3 and the downstream targets involved.
Recently, we reported that CCAAT/enhancer-binding protein β (C/EBPβ) is a 1,25(OH)2D3 target gene in kidney and in osteoblastic cells (14). The C/EBP family of transcription factors has been reported to be involved in the regulation of growth, differentiation, and inflammation and the expression of cell type-specific genes (15, 16). C/EBPβ has been shown to be essential for the development of the murine mammary gland (17, 18). C/EBPα has been reported to play a critical role in both proliferation and differentiation in numerous cell types (16, 19–21). It was suggested that C/EBPα could be considered a potential tumor suppression gene in breast cancer (22). In our previous studies we found that C/EBPβ cooperates with VDR in 1,25(OH)2D3-induced transcription (14).
In the present study we extend our initial observations in kidney and osteoblastic cells to cooperative effects between 1,25(OH)2D3 and the C/EBP family of transcription factors in breast cancer cells. We report for the first time that C/EBPα is induced by 1,25(OH)2D3 in MCF-7 breast cancer cells and that C/EBPα cooperates with Brm, an ATPase that is a component of the SWI/SNF chromatin-remodeling complex, and is a potent enhancer of VDR transcription. The induction of C/EBPα is accompanied by induction of p21WAF1/Cip1 and p27Kip1. Because the levels of VDR correlate with the antiproliferative effects of 1,25(OH)2D3 and because 1,25(OH)2D3 and C/EBPα up-regulate p21, these findings provide important mechanisms whereby 1,25(OH)2D3 may act to inhibit growth of breast cancer cells. These findings also identify C/EBPα as a 1,25(OH)2D3 target in breast cancer cells and provide evidence for C/EBPα as a candidate for breast cancer treatment.
EXPERIMENTAL PROCEDURES
Materials—[γ-32P]ATP (3000 Ci (111 TBq)/mmol) was purchased from PerkinElmer Life Sciences. Polyvinylidene difluoride membranes and pre-stained molecular weight markers were obtained from Bio-Rad Laboratories, Inc. (Hercules, CA). T4 poly nucleotide kinase for labeling double stranded oligonucleotides for electrophoretic mobility shift assay was purchased from Invitrogen. VDR, β-actin, Brm, Brg-1, and C/EBPα antiserum and secondary antibodies against mouse and rabbit antisera were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). 1,25(OH)2D3 was a generous gift from Milan Uskokovic (Hoffmann-LaRoche, Nutley, NJ).
Cell Culture—Fetal bovine serum (FBS) and charcoal-stripped FBS were from Gemini Biological Products (Calabasas, CA). Cell culture media, 0.25% trypsin-EDTA and penicillin, streptomycin, and neomycin mixture were purchased from Invitrogen. MCF-7 and MBA-MD-231 breast cancer cells were obtained from the American Type Culture Collection (Manassas, VA.) and were cultured in DMEM supplemented with 10% heat-inactivated FBS and 1% penicillin, streptomycin, and neomycin antibiotic mixture. Cells were grown in a humidified incubator with an atmosphere of 95% air-5% CO2 at 37 °C. For treatments, cells were grown to desired confluency, and their medium was changed to DMEM supplemented with 2% charcoal-dextran-treated FBS. Treatments with vehicle or 1,25(OH)2D3 were done for the durations and with concentrations mentioned in the figure legends.
Plasmids, Transfections, and Assay of Luciferase Activity—The luciferase reporter construct of human VDR promoter (-1500 to +60) was generated as previously described (23). The deletion construct was prepared using KpnI and NotI and re-ligating the remaining promoter construct containing (-646/+60). The C/EBP site at -919/-911 was mutated by site-directed mutagenesis using a site-directed mutagenesis kit from Stratagene. The oligonucleotides used to generate the C/EBPβ mutated site (shown in lowercase underlined letters; C/EBP site shown in bold) were as follows: 5′-CCA GAA GAT Tat gtt acg atT ACT ATT TAT TTA TAC-3′ and 5′-GTA TAA ATA AAT AGT Aat cgt aac aTA ATC TTC TGG. All these promoter constructs were used for transcription assays in MCF-7 cells. The C/EBPα promoter construct was kindly gifted by (A. Peres-Castillo, Madrid Spain) (24). The C/EBPα,-β, and -δ expression vectors were a gift of Simon Williams, Texas Tech University (Lubbock, TX). The dominant negative (DN) C/EBP construct was a gift from Dr. C. Vinson, NCI, National Institutes of Health. pCMV-Brm and pCMV-mutant Brm (with the ATPase site mutated, which acts as a dominant negative inhibitor) were obtained from M. Yaniv (25). pCMV-AML-1/ETO expression vector was from S. W. Hiebert (Vanderbilt University, Nashville, TN) Empty vectors were transfected to keep the total DNA concentration equal. Cells were transfected using Lipofectamine 2000 (Invitrogen) treated as described under “Results” in the appropriate medium supplemented with 2% charcoal-dextran-treated FBS. After treatment with vehicle or the compounds noted at the concentrations and times indicated under “Results,” cells were harvested and dual luciferase assay was performed according to the Dual Luciferase assay kit manufacturer's protocol (Promega, Madison, WI). For experiments shown in Fig. 5, MDA-MB-231 cells were co-transfected with C/EBPα expression plasmid and green fluorescent protein expression plasmid with G418 resistance using Lipofectamine 2000 and incubated at 37 °C for 20 h. Control cells were transfected with vector alone. Normal growth medium (DMEM as indicated in “cell culture” above) was added the next day. Three days after transfection, selection began with increasing amounts of G418 to a final concentration of 600 μg/ml. After 2 weeks of G418 selection, cells were used for Western blot analysis, VDR transcription assays, and assessment of cell proliferation in the presence or absence of 1,25(OH)2D3.
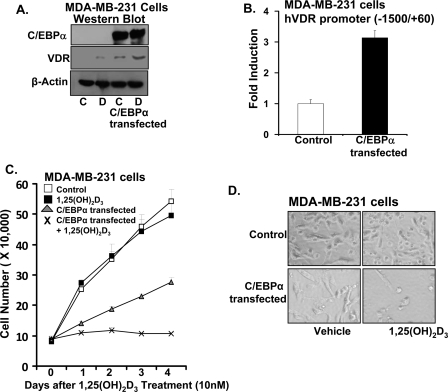
FIGURE 5.
Effect of C/EBPα expression in MDA-MB-231 cells. A, Western blot analysis. MDA-MB-231 cells were transfected with green fluorescent protein expression vector (with G418 resistance) and C/EBPα. Cells were selected on increasing concentration of G418 (200–600 μg/ml) for 2 weeks. Western blot was performed using nuclear extracts from control or C/EBPα-transfected cells treated with vehicle or 1,25(OH)2d3 (10 nm) for 24 h. Detection was by immunoblotting using C/EBPα, VDR, and β-actin antibodies. Two additional experiments yielded similar results. B, control or C/EBPα-transfected MDA-MB-231 cells were transfected with hVDR promoter (0.5 μg). C/EBPα-transfected cells show a significant increase in hVDR promoter activity compared with control, vector-transfected cells (p < 0.05 compared with control). C, proliferation of MDA-MB-231 cells in response to 1,25(OH)2d3 in the presence of absence of C/EBPα. Cells were transfected with vector or C/EBPα as described above and treated with vehicle or 1,25(OH)2d3 (10 nm). Differences in proliferation between MDA-MB-231 cells treated with vehicle or 1,25(OH)2d3 were not observed. C/EBPα expression resulted in a significant decrease in MDA-MB-231 cell proliferation (1–4 days; p < 0.05 compared with control vector-transfected cells). 1,25(OH)2d3 significantly enhanced the inhibition of growth in C/EBPα-transfected MDA-MB-231 cells (1–4 days; p < 0.05 compared with C/EBPα-transfected cells). D, light microscopic image of MDA-MB-231 cells in the presence or absence of C/EBPα treated for 4 days with vehicle or 1,25(OH)2d3 (10 nm) (magnification, 200×).
Electrophoretic Mobility Shift Assay—Complementary oligonucleotides were synthesized based on the region of the hVDR promoter containing the wild-type (-919/-911) or the mutated C/EBP site (prepared by the University of Medicine and Dentistry Molecular Resource Facility, Newark, NJ). The complementary oligonucleotides were annealed, end-radiolabeled, and purified as described before (14) and were used for the electrophoretic mobility shift assay. The sequences of the oligonucleotides used were 5′-CCA GAA GAT TAT GTT GTA ATT ACT ATT TAT TTA TAC-3′ and 5′-TAA ATA AAT AGT AAT TAC AAC ATA ATC TTC TGG-3′ for the wild-type and 5′-CCA GAA GAT TAT GTT ACG ATT ACT ATT TAT TTA TAC-3′ and 5′-GTA TAA ATA AAT AGT AAT CGT AAC ATA ATC TTC TGG-3′ for the mutant construct. Briefly, 5 μg of the nuclear preparations from C/EBPα-transfected MCF-7 cells were incubated for 20 min at 25 °C with 2 μg of poly(dI/dC) with or without unlabeled specific or nonspecific DNA competitor or C/EBPα antibody in binding buffer (4 mm Tris-HCl (pH 7.9), 1 mm EDTA (pH 8.0), 60 mm KCl, 12% glycerol, 12 mm HEPES, 1 mm dithiothreitol). This was further incubated with 0.5 ng of the labeled oligonucleotide probe (∼100,000 cpm) and incubation for 30 min at 25 °C. The samples were separated by electrophoresis on a 6% nondenaturing polyacrylamide gel that had been pre-electrophoresed for 30 min at 100 V/cm at 4 °C in 45 mm Tris-45 mm boric acid-1 mm EDTA. Electrophoresis was conducted for 2.5 h under identical conditions. The gel was dried and exposed to x-ray film at -80 °C with intensifying screens.
siRNA Transfections—The following are the sequences of the oligonucleotides used to knockdown C/EBPα expression. C/EBPα siRNA sequences are as follows: sense, 5′-GUCGGCCAGGAACUCGUCGUU-3′ and antisense, 3′-UUCAGCCGGUCCUUGAGCAGC-3′. Scrambled siRNA sequences are as follows: sense, 5′-GUAGUCCAUGGACCCGUAGUU and antisense, 3′-UUCAUCAGGUACCUGGGCAUC-3′. siRNA transfections were done using Oligofectamine™ (Invitrogen). Cells were transfected with C/EBPα siRNA following the manufacturer's instructions. One day prior to transfection, 5 × 104 cells/well were seeded in 6-well plates (corresponding to a density of 40% at the time of transfection) without antibiotics. The transfection mixture (containing 20 μm siRNA with Oligofectamine reagent) was added to the 6-well plate 20 min after mixture preparation. After 24–48 h of transfection the medium was changed to DMEM with 2% charcoal-stripped FBS. Transfected cells were treated with vehicle or 1,25(OH)2D3 for 24–72 h as described above. Cells were then harvested, and cell viability was determined by trypan blue exclusion. Similarly transfected and treated cells were also used for preparation of nuclear extracts for Western blotting.
Nuclear Extracts—For nuclear extract preparation from MCF-7 cells, cells were rinsed twice with phosphate-buffered saline at 4 °C, harvested by scraping, gently pelleted, washed, and lysed in hypotonic buffer containing 10 mm HEPES (pH 7.4), 1.5 mm MgCl2, 10 mm KCl, 0.5 mm dithiothreitol, phosphatase inhibitors (1 mm sodium orthovanadate, 10 mm sodium fluoride), protease inhibitors (0.5 mm phenylmethylsulfonyl fluoride, 1 mg of pepstatin A per ml, 2 mg of leupeptin per ml, 2 mg of aprotinin per ml), and 1% Triton X-100. Nuclei were pelleted at 3,500 × g for 5 min, and cytoplasmic supernatants were separated. Nuclei were resuspended in hypertonic buffer containing 0.42 m NaCl, 0.2 mm EDTA, 25% glycerol, and the phosphatase and protease inhibitors indicated above. Soluble nuclear proteins were released by 60 min of incubation at 4 °C, and insoluble material was separated by centrifugation at 12,000 × g for 5 min. The protein concentration of the supernatant was measured by using Bradford's method (26), and aliquots were stored at -80 °C.
Co-immunoprecipitation Assay—Co-immunoprecipitation assay was performed as described previously (14). Briefly, nuclear extracts were isolated using the NEPER nuclear extraction reagents kit from Pierce. 1 μg of primary antibody (C/EBPα, or Brm) was added to the nuclear extract in 500 μl of immunoprecipitation buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, and 1% Triton X 100) and incubated for 4 h to overnight with rotation at 4 °C. 25 μl of protein A-Sepharose 4 Fast Flow Beads (Amersham Biosciences) were added to the nuclear extract-antibody mix, and it was further incubated with rotation at 4 °C for 1 h. After centrifugation at 3000 rpm at 4 °C for 5 min, the supernatant was discarded and the immunoprecipitated complex was washed with immunoprecipitation buffer three times, eluted and separated on 4–20% SDS-PAGE gel (Bio-Rad). The Western blot was probed with Brm or C/EBPα antibody, respectively.
Chromatin Immunoprecipitation Assay—MCF-7 cells were cultured in DMEM supplemented with 10% FBS to 95% confluence and then treated with vehicle or 1,25(OH)2D3 in medium supplemented with 2% charcoal-stripped serum under the conditions and for the times indicated. Treated cells were used for the ChIP assay performed as described earlier (27, 28). In brief, treated cells were washed with phosphate-buffered saline and cross-link using 1% formaldehyde for 15 min. Glycine was added to a final concentration of 0.125 m to stop cross-linking. The cells were washed twice with ice-cold phosphate-buffered saline and collected by scraping. The cells were lysed for 20 min each first in buffer 1 (5 mm Pipes, pH 8.0, 85 mm KCl, 0.5% Nonidet P-40) and then in buffer 2 (1% SDS, 10 mm EDTA, 50 mm Tris-HCl, pH 8.1). The resulting chromatin pellet was sonicated to an average DNA size of 500-bp DNA (evaluated by 1% agarose gel electrophoresis) using a Fisher model 100 sonic dismembranator at a power setting of 1. The sonicated extract was centrifuged for 10 min at 13,000 rpm at 4 °C and then diluted into ChIP dilution buffer (16.7 mm Tris-HCl, pH 8.1, 150 mm NaCl, 0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA). Immunoprecipitations were performed at 4 °C overnight with the indicated antibody. After a 1-h incubation with salmon sperm DNA and bovine serum albumin-pretreated Zysorbin (Zymed Laboratories Inc., San Francisco, CA), the precipitates were collected by centrifugation. Precipitates were washed sequentially in buffer I (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.1, 150 mm NaCl), buffer II (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.1, 500 mm NaCl), buffer III (0.25 m LiCl, 1% Nonidet P-40, 1% deoxycholate, 1 mm EDTA, 10 mm Tris-HCl, pH 8.1), and twice in TE buffer (10 mm Tris, 1 mm EDTA). The protein-DNA was then eluted by using 1% SDS and 0.1 m NaHCO3 for 15 min twice. Cross-links were reversed by incubating at 65 °C overnight in elution buffer with 0.2 m NaCl. DNA fragments were purified using Qiagen QIAquick PCR purification kits, and PCR was performed using the primers designed to amplify fragments of human VDR promoter C/EBP motif (upper, 5′-GAGGCGAATAGCAATATCTTCC-3′; lower, 5′-GAGACCTGGAATTGTGGATGG-3′). PCR analysis was performed in the linear range of DNA amplification. 248-bp PCR products were resolved in 1% agarose gel and visualized using ethidium bromide staining. DNA obtained before precipitation was used as the input. 10% of input was used for PCR reaction.
Statistical Analysis—Results are expressed as means ± S.E., and significance was determined by analysis by Student's t test for two-group comparison or by analysis of variance for multiple-group comparison.
RESULTS
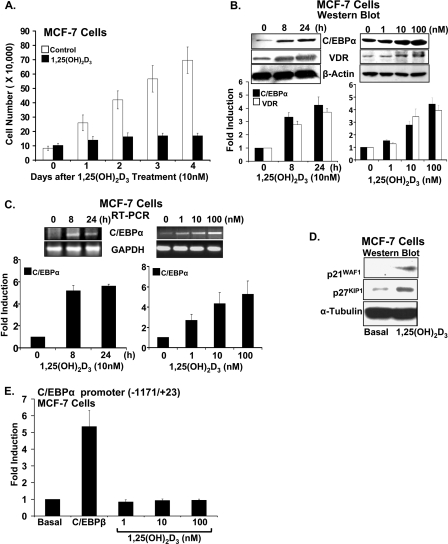
C/EBPα Expression Is Induced by 1,25(OH)2D3 in MCF-7 Breast Cancer Cells—Shown in Fig. 1A is the 1,25(OH)2D3-induced inhibition of MCF-7 cell proliferation. Because C/EBPα has been reported to have a growth inhibitory role in breast cancer (22), we tested the possibility that 1,25(OH)2D3 may regulate the expression of C/EBPα in breast cancer cells. When MCF-7 breast cancer cells were treated with 1,25(OH)2D3 (10 nm for 8–24 h), Western blot analysis, using nuclear extracts from the vehicle or 1,25(OH)2D3-treated cells, showed that 1,25(OH)2D3 induced C/EBPα expression in MCF-7 breast cancer cells (Fig. 1B, left panel; maximum induction, 4.2-fold). The induction of C/EBPα by 1,25(OH)2D3 in MCF-7 cells was accompanied by an increase in the expression of VDR (maximum 3.7-fold at 24 h) (Fig. 1B, left panel). A dose response of the stimulation by 1,25(OH)2D3 (1–100 nm for 24 h), shown in Fig. 1B (right panel), similarly indicates that induction of C/EBPα by 1,25(OH)2D3 is accompanied by an increase in VDR. C/EBPβ is not induced by 1,25(OH)2D3 in MCF-7 cells (not shown). Induction of C/EBPα mRNA by 1,25(OH)2D3 in MCF-7 cells is shown in Fig. 1C. 1,25(OH)2D3 also enhanced the expression of p21WAF1/Cip1 and p27Kip1 in MCF-7 cells (Fig. 1D). To examine a possible mechanism of regulation of C/EBPα by 1,25(OH)2D3 in MCF-7 breast cancer cells, MCF-7 cells were transfected with the rat C/EBPα promoter (-1171/+23) and treated with 1,25(OH)2D3. 1,25(OH)2D3 failed to show any effect on the activity of the C/EBPα promoter (Fig. 1E). The C/EBPα promoter was also unresponsive to 1,25(OH)2D3 when VDR-transfected COS-7 cells were used (not shown). As a positive control, MCF-7 cells were transfected with C/EBPβ expression vector. C/EBPβ significantly induced C/EBPα transcription 5.5-fold (p < 0.01 compared with cells transfected with vector alone; Fig. 1E). The unresponsiveness of the C/EBPα promoter to 1,25(OH)2D3 (similar to what we observed using the C/EBPβ promoter and transfection in osteoblastic cells (14)) suggests the possibility that C/EBPα is regulated by 1,25(OH)2D3 at other sites yet to be defined or that 1,25(OH)2D3 may be regulating C/EBPα at a post transcriptional level.
FIGURE 1.
C/EBPα is induced by 1,25(OH)2d3 in MCF-7 breast cancer cells. A, proliferation of MCF-7 cells is significantly decreased in the presence of 1,25(OH)2d3 (10 nm) at all times examined (1–4 days, p < 0.05 compared with vehicle). Data represent the mean ± S.E. of three independent experiments. B, left panel top, representative Western blot. Western blot was performed using nuclear extracts from MCF-7 cells treated with vehicle or 1,25(OH)2d3 (10 nm for 8–24 h) and probed with C/EBPα, VDR, and β-actin antibodies. Left panel bottom, graphic representation of densitometric scans of Western blots. Data represent the mean ± S.E. of three independent experiments. C/EBPα (42 kDa) and VDR (48 kDa) are significantly induced by 1,25(OH)2d3 at 8 and 24 h (p < 0.05 compared with 0 time). Right panel top, 1,25(OH)2d3 dose response; representative Western blot. Nuclear extracts from MCF-7 cells treated with vehicle or increasing concentrations of 1,25(OH)2d3 (1–100 nm) for 24 h were used. Right panel bottom, graphic representation of densitometric scans of Western blots. C/EBPα and VDR are significantly induced at 10 and 100 nm 1,25(OH)2d3 (p < 0.05 compared with vehicle control). Data represent the mean ± S.E. of three independent experiments. C, top panels: representative RT-PCR analyses of mRNA from MCF-7 cells for C/EBPα. Cells were treated with 1,25(OH)2d3 as in Fig. 1B. Bottom panels: quantitation of C/EBPα mRNA expression. Data represent the mean ± S.E. of three independent experiments. C/EBPα mRNA is significantly induced by 1,25(OH)2d3 at 8 and 24 h (left panel, p < 0.05 compared with 0 time). Right panel: C/EBPα mRNA is significantly induced at 1, 10, and 100 nm 1,25(OH)2d3 (p < 0.05 compared with vehicle control). D, Western blot analysis of MCF-7 cells treated with vehicle or 1,25(OH)2d3 (10 nm) for 24 h using p21, p27, and α-tubulin antibodies. The Western blot is representative of results obtained from at least two additional experiments. E, 1,25(OH)2d3 does not affect the activity of the C/EBPα promoter. MCF-7 cells transfected with the C/EBPα promoter (-1171/+23) (65) were co-transfected with the C/EBPβ expression plasmid (0.25 μg) or treated with 1,25(OH)2d3 (1–100 nm). The data represent the mean ± S.E. of three separate experiments. The induction of C/EBPα promoter activity by C/EBPβ (as previously reported (65)) is significant at p < 0.05 compared with cells transfected with vector alone. No significant change in promoter activity was observed after 1,25(OH)2d3 treatment (p > 0.5).
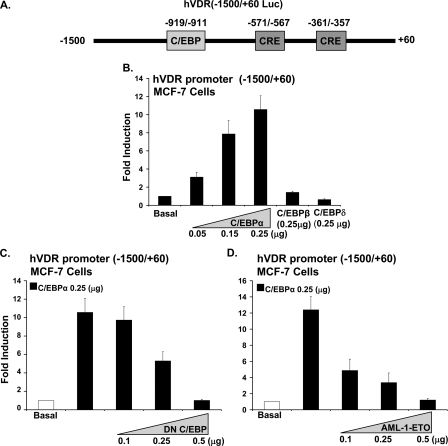
Enhancement of VDR Transcription by C/EBPα in MCF-7 Cells—Because the induction by 1,25(OH)2D3 of C/EBPα is accompanied by an increase in VDR (Fig. 1B) and because sequence analysis of the hVDR promoter revealed the presence of a putative C/EBP site at -919/-911 and two CRE sites at -571/-567 and -361/-357 (Fig. 2A (29)) (C/EBP family members have been reported to bind to a CRE or a C/EBP site (30)), the possibility that C/EBPα may have a role in the 1,25(OH)2D3-mediated enhancement of VDR in MCF-7 breast cancer cells was examined. MCF-7 cells were co-transfected with hVDR promoter (-1500/+60) and C/EBPα expression vector. A dose-dependent enhancement of hVDR transcription by C/EBPα was observed (maximum enhancement of 11 ± 1.2-fold at 0.25 μg of C/EBPα (Fig. 2B)). Unlike C/EBPα, C/EBPβ or C/EBPδ had no effect on VDR transcription (Fig. 2B). To further confirm the role of C/EBPα as an enhancer of the hVDR transcription, MCF-7 cells were transfected with the hVDR promoter (-1500/+60) in the presence of C/EBPα expression vector and dominant negative C/EBP (DN C/EBP). DN C/EBP, which lacks the transactivation domain sequence but retains the dimerization and DNA binding domain, suppressed the C/EBPα mediated enhancement of hVDR transcription in MCF-7 cells in a dose-dependent manner (Fig. 2C). AML-ETO has earlier been reported to block C/EBPα DNA binding and transactivation (31). Increasing concentrations of AML-ETO also suppressed the C/EBPα mediated activation of the hVDR promoter (Fig. 2D). These results indicate, for the first time, the involvement of C/EBPα in VDR transcription in MCF-7 cells.
FIGURE 2.
Enhancement of hVDR promoter transcription by C/EBPα in MCF-7 breast cancer cells. A, schematic of the luciferase construct of the human VDR promoter -1500/+60, including a putative C/EBP motif and two putative CRE sites. B, MCF-7 cells were co-transfected with hVDR promoter and increasing concentrations of C/EBPα (0.05 μg to 0.25 μg) or 0.25 μg of C/EBPβ or C/EBPδ. C/EBPα (0.05, 0.15, and 0.25 μg) results in a significant induction in hVDR promoter activity (p < 0.05 compared with basal). C and D, suppression of hVDR promoter transcription using DN C/EBP or AML-1/ETO. MCF-7 cells were co-transfected hVDR promoter, 0.25 μg of C/EBPα and increasing concentrations of DN C/EBP or of AML-1/ETO, a C/EBPα inhibitor. Empty vectors were used to keep the total DNA concentrations the same. In MCF-7 cells there was no effect of DN CEBP or AML-1/ETO at the concentrations used on basal levels of hVDR transcription. pRL-TF-Renilla luciferase was co-transfected as an internal control. Results of three or more separate experiments are presented (mean ± S.E.). Co-transfection with 0.25 μg or 0.5 μg of DN C/EBP or 0.1, 0.25, or 0.5 μg of AML-1/ETO resulted in a significant decrease in C/EBPα-induced VDR transcription (p < 0.05 compared with cells transfected with C/EBPα (0.25 μg) alone).
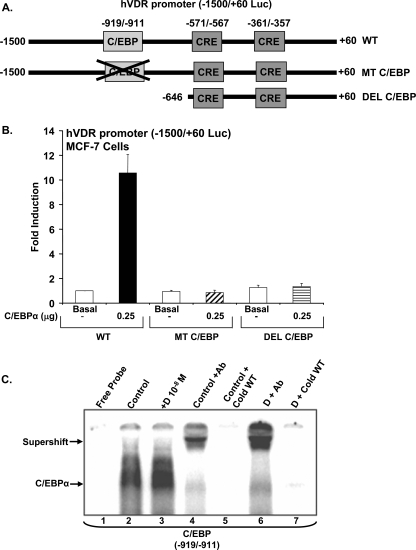
Identification of the C/EBPα Activation Domain in the VDR Promoter—Sequences in the hVDR promoter involved in transactivation by C/EBP were identified by deletion mutant analysis and site-directed mutagenesis. Deletion of the hVDR promoter to -646 (DEL C/EBP) eliminated the ability of C/EPBα to transactivate the hVDR promoter, suggesting the involvement of the site at -919/-911 in the induction by C/EBPα of hVDR transcription (Fig. 3, A and B). To investigate the specific contribution of this element to the induction of hVDR transcription by C/EBPα, a mutant hVDR promoter construct was generated with the C/EBP site at -919/-911 mutated (MT CEBP). Mutation of this site within the -1500/+60 promoter construct blocked the C/EBPα-mediated induction of transcription (Fig. 3B), suggesting that C/EBPα acts through this site to induce hVDR transcription.
FIGURE 3.
Detection of C/EBPα activation domain in the hVDR promoter. A, schematic of luciferase constructs of the hVDR promoter and mutation and deletions of the C/EBP site. hVDR promoter construct (-1500/+60) (WT); hVDR (-1500/+60) promoter with the putative C/EBP site mutated (MT C/EBP) and hVDR promoter -646/+60 with C/EBP putative site deleted (DEL C/EBP) are shown. B, luciferase assay determined in extracts of MCF-7 cells transfected using luciferase constructs of hVDR promoter shown in A with co-transfection of C/EBPα expression vector (0.25 μg) or vector alone (basal). Luciferase activity is represented as -fold induction over the control (mean ± S.E.; three to six observations per group). pRL-TK-Renilla luciferase was co-transfected as an internal control. C, identification of C/EBPα binding motif in the hVDR promoter by electrophoretic mobility shift assay. The 32P-labeled oligonucleotide probes containing wild-type sequence were incubated with 5 μg of nuclear protein from vehicle (lane 2) or 1,25(OH)2d3-treated (lane 3) MCF-7 cells transfected with the C/EBPα expression vector. The wild-type probe was incubated with C/EBPα antibody in the presence of 5 μg of vehicle treated (lane 4) or 1,25(OH)2d3-treated (lane 6) nuclear protein. The wild-type probe in presence of vehicle treated (lane 5) or 1,25(OH)2d3-treated (lane 7) nuclear protein was also incubated with a 100-fold molar excess of wild-type (WT) cold competitor oligonucleotide. Gel mobility shift data are representative of at least three experiments.
Gel mobility shift assays were performed using synthetic oligonucleotide corresponding to the wild-type (-919/-911) C/EBP binding sequences and nuclear extracts from control and 1,25(OH)2D3-treated MCF-7 cells transfected with C/EBPα. An interaction of MCF-7 cell nuclear extract with this element was observed and binding was enhanced in the presence of 1,25(OH)2D3 (Fig. 3C, lanes 2 and 3). A supershift was observed in the presence of C/EBPα antibody, indicating the specificity of this interaction for C/EBPα (Fig. 3C, lanes 4 and 6). Binding was competed by excess cold oligonucleotide, confirming specificity (Fig. 3C, lanes 5 and 7). C/EBPα failed to bind to mutant radiolabeled oligonucleotide, and the oligonucleotide containing the mutated C/EBP sequences could not deplete the binding of C/EBPα to the labeled WT probe (not shown).
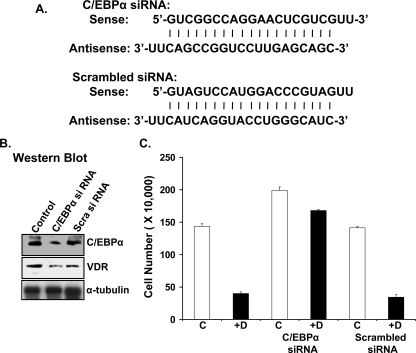
RNA Interference Knockdown of C/EBPα Reduces the Anti-proliferative Effect of 1,25(OH)2D3 in MCF-7 Cells—MCF-7 cells were transfected with C/EBPα siRNA or scrambled siRNA (Fig. 4A). C/EBPα siRNA transfection reduced the expression of C/EBPα as well as VDR protein in MCF-7 cells (Fig. 4B). Treatment of MCF-7 cells or control transfected MCF-7 cells (scrambled siRNA) with 10 nm 1,25(OH)2D3 decreased the number of the viable cells (70% decrease (Fig. 4C)). In contrast, 1,25(OH)2D3-treated C/EBPα antisense cells showed only a 20% decrease in the number of viable cells (Fig. 4C, C/EBPα siRNA). These findings suggest that C/EBPα plays a significant role in 1,25(OH)2D3-mediated antiproliferative effects in MCF-7 cells.
FIGURE 4.
Inhibition of C/EBPα expression using siRNA reduces the anti-proliferative effect of 1,25(OH)2d3 in MCF-7 cells. A, sequences of C/EBPα siRNA and scrambled siRNA used for transfection of MCF-7 cells. B, Western blot analysis of C/EBPα and VDR in control, C/EBPα siRNA-transfected, and scrambled siRNA-transfected MCF-7 cells. Two additional experiments yielded similar results. C, graphic representation of cell number of control, C/EBPα siRNA-transfected, and scrambled siRNA-transfected MCF-7 cells treated with vehicle or 10 nm 1,25(OH)2d3 as described under “Experimental Procedures.” Results represent the mean ± S.E. of three separate experiments.
Effect of C/EBPα Expression in MDA-MB-231 Cells—In MDA-MB-231 breast cancer cells, which are estrogen receptor-negative, C/EBPα could not be detected in the presence or absence of 1,25(OH)2D3 (Fig. 5A, upper panel, lanes 1 and 2; 1,25(OH)2D3 treatment, 10 nm for 24 h). Transient transfection of C/EBPα in MDA-MB-231 cells had no effect on hVDR promoter activity (not shown). We then transfected MDA-MB-231 cells with C/EBPα and neo+ plasmid and selected on G418 for 2 weeks. Expression of C/EBPα is shown in Fig. 5A (upper panel, lanes 3 and 4). C/EBPα expression resulted in enhanced basal as well as 1,25(OH)2D3-induced VDR levels in MDA-MB-231 cells (Fig. 5A, middle panel). hVDR promoter activity was induced 3.2 ± 0.25-fold in C/EBPα-expressing MDA-MB-231 cells (Fig. 5B, p < 0.05 compared with control, vector-transfected cells). We next examined the effect of 1,25(OH)2D3 on the proliferation of MDA-MB-231 cells in the presence or absence of C/EBPα. Although 1,25(OH)2D3 did not significantly affect the growth of MDA-MB-231 cells, C/EBPα was found to have a growth inhibitory role and 1,25(OH)2D3 significantly enhanced the inhibition of growth in C/EBPα-transfected MDA-MB-231 cells (Fig. 5, C and D). Thus, C/EBPα is needed to observe an antiproliferative effect of 1,25(OH)2D3 in MDA-MB-231 cells, further suggesting a role for C/EBPα in the antiproliferative effect of 1,25(OH)2D3 in breast cancer cells.
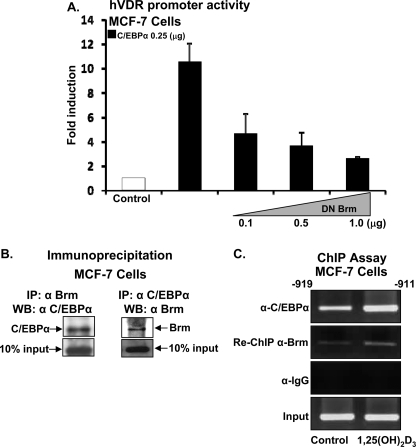
Functional Cooperation between C/EBPα and SWI/SNF Complex Members—Because the SWI/SNF complex, which remodels chromatin using the energy of ATP hydrolysis, was shown to be critical for C/EBPα-induced growth arrest in other cell types (32), a possible role of SWI/SNF in C/EBPα-induced VDR transcription was examined. Each SWI/SNF complex contains one of two homologous ATPase, Brahma (Brm) and Brahma/mutated gene (Brg-1). In MCF-7 cells, mutated Brm (with the ATPase site mutated, which acts as a dominant negative inhibitor) was found to inhibit the stimulatory effect of C/EBPα on hVDR transcription in a dose-dependent manner (Fig. 6A), suggesting cooperation between Brm and C/EBPα in the regulation of VDR transcription in MCF-7 cells. Co-immunoprecipitation experiments were performed to evaluate the possible interaction between C/EBPα and members of SWI/SNF complex. Nuclear extracts were prepared using MCF-7 cells, and immunoprecipitation assays were performed using Brm antibody and Western blot with C/EBPα antibody (Fig. 6B, left panel) or using C/EBPα antibody for immunoprecipitation and Brm antibody for Western blot (Fig. 6B, right panel). No signal was observed when lysed cells were immunoprecipitated with IgG (not shown). These findings indicate that C/EBPα can bind to Brm in MCF-7 cells and suggest functional cooperation of Brm with C/EBPα by direct protein-protein interaction.
FIGURE 6.
Functional cooperation between C/EBPα and SWI/SNF complex. A, MCF-7 cells were co-transfected with the hVDR promoter luciferase construct -1500/+60 and C/EBPα expression plasmid (0.25 μg) in the presence or absence of increasing concentrations of dominant negative BRM. Co-transfection with DN Brm resulted in a significant decrease in VDR promoter activity at all concentrations used (p < 0.05 compared with cells transfected with C/EBPα (0.25 μg) alone). B, co-immunoprecipitation of C/EBPα and Brm. Left panel: MCF-7 cells were lysed and immunoprecipitated with BRM antibody and Western blots were performed using C/EBPα antibody. Right panel: cells were lysed and immunoprecipitated with C/EBPα antibody, and Brm was detected in the immunoprecipitate using Brm antibody. These findings are representative of three separate experiments. Enhanced interaction was not observed when immunoprecipitation was done in the presence of 10 nm 1,25(OH)2d3 (not shown). C, C/EBPα and Brm are recruited to the VDR promoter in intact cells. MCF-7 cells were treated with vehicle or 1,25(OH)2d3 (10 nm) for 24 h, cells were cross-linked, and cell lysates were subjected to immunoprecipitation first with C/EBPα antibody and then with Brm antibody. DNA was isolated and PCR, using specific primers designed against the C/EBP site on the hVDR promoter, was performed. PCR was carried out in the linear range of DNA amplification. Immunoprecipitation with IgG was used as control (IgG panel). These experiments are representative of three separate experiments performed under the similar condition.
Similar experiments were also performed using Brg-1 antiserum. However, immunoprecipitation assays failed to show an interaction between C/EBPα and Brg-1 (data not shown).
C/EBPα Interacts with the hVDR Promoter in MCF-7 Breast Cancer Cells—To determine whether C/EBPα and Brm bind to the same site in the hVDR promoter, a ChIP assay was performed in MCF-7 cells. The ChIP assay was done as previously described (27, 28). Cells were cross-linked with formaldehyde, chromatin pellets were sonicated, and immunoprecipitations were performed at 4 °C overnight using antibodies against C/EBPα and Brm. DNA fragments were purified and subjected to PCR using primers designed to amplify the C/EBPα binding site containing region of hVDR promoter. Primers designed to amplify more upstream regions of the hVDR promoter (-1457/-1189), used as a negative control, resulted in no amplification (data not shown). ChIP/re-ChIP analysis shows that C/EBPα and Brm bind simultaneously to the hVDR promoter (Fig. 6C). Treatment of MCF-7 cells with 1,25(OH)2D3 resulted in enhanced recruitment of C/EBPα and Brm to the VDR promoter. No amplicons were detected when IgG was used in place of C/EBPα antibody (Fig. 6C, IgG panel). Similar re-ChIP experiments using Brg-1 antibody failed to indicate simultaneous binding of C/EBPα and Brg-1 to the same site (-919/-911) in the VDR promoter (not shown).
DISCUSSION
Although a major function of 1,25(OH)2D3 is to maintain calcium homeostasis, 1,25(OH)2D3 has also been identified as a factor that negatively regulates the growth of a number of malignant cells in vitro and in vivo, including breast cancer cells. However, little is known about the molecular mechanisms and target genes mediating the antiproliferative effects of 1,25(OH)2D3. In this study we demonstrate that 1,25(OH)2D3 enhances the expression of C/EBPα in the ER-expressing breast cancer cell line MCF-7. C/EBPα could not be detected in the presence or absence of 1,25(OH)2D3 in the ER negative cell line MDA-MB-231, which is poorly responsive to the growth inhibitory actions of 1,25(OH)2D3 (33). However, antiproliferative effects of 1,25(OH)2D3 were observed in C/EBPα-transfected MDA-MB-231 cells. In addition, knockdown of C/EBPα by RNA interference results in a significant reduction in the ability of 1,25(OH)2D3 to inhibit the growth of MCF-7 cells, indicating that C/EBPα mediates, at least in part, the responsiveness of these cells to 1,25(OH)2D3. C/EBPα and Brm, a component of the SWI/SNF chromatin-remodeling complex, were found to cooperate in the up-regulation of VDR transcription in MCF-7 cells. Because the levels of VDR correlate with the antiproliferative effects of 1,25(OH)2D3 in breast cancer cells (34, 35), our findings indicate for the first time that induction of C/EBPα by 1,25(OH)2D3 and the enhancement of VDR transcription by C/EBPα may provide an important mechanism whereby 1,25(OH)2D3 inhibits growth of breast cancer cells.
C/EBPα but not C/EBPβ was found to be induced by 1,25(OH)2D3 and to affect VDR-mediated transcription in MCF-7 cells. C/EBPα regulates differentiation, causes growth arrest, and is down-regulated during proliferation (16, 36, 37). Mutations in C/EBPα are observed in subtypes of acute myelogenous leukemia (38). Hepatocytes from C/EBPα-deficient mice display increased proliferative activity (19), and C/EBPα is reduced in hepatocellular carcinoma (39) as well as in lung (40), skin (41), and breast cancer (22). In contrast, C/EBPβ has been reported to play a role in promoting proliferation (42). It is required for normal function of the mammary gland, including expression of milk proteins (17), and it has been reported to cooperate with Ras to induce transformation (43). Thus, these previously observed functions for C/EBPα and C/EBPβ are consistent with an induction in breast cancer by 1,25(OH)2D3 of C/EBPα and not C/EBPβ. The estrogen receptor (ER) modulator, 4-hydroxytamoxifen, has similarly been found to induce C/EBPα (44). It was suggested that C/EBPα may play a central role in the 4-hydroxytamoxifen-ER-induced apoptosis observed in breast cancer cells (44). Dexamethasone induces C/EBPα in glucocorticoid-sensitive BDS1 hepatoma cells and has been shown to be required for glucocorticoid-mediated cell cycle arrest of the hepatoma cells (45). Our study has shown that C/EBPα (but not C/EBPβ or C/EBPδ) induces VDR transcription in breast cancer cells, thus providing a model where C/EBPα may be a central element in VDR-mediated growth arrest. Increased VDR in breast cancer cells would result in an enhanced effect on VDR target genes, for example induction of p21WAF1/Cip1 and inhibition of the estrogen receptor (46–48).
It should be noted, however, that induction of C/EBPα by 1,25(OH)2D3 may also mediate growth arrest, in part, by additional effects of C/EBPα that are not mediated by up-regulation of VDR. C/EBPα alone has been reported to have a direct effect on p21WAF1/Cip1 transcription (49). C/EBPα also regulates p21WAF1/Cip1 post transcriptionally by binding to p21WAF1/Cip1 and blocking proteolytic degradation, resulting in stabilization of p21WAF1/Cip1 (49). In addition, C/EBPα has been reported to interact with and inhibit cyclin-dependent kinases (50) and to repress the activity of E2F, a transcription factor required for the transcription of several genes required for DNA synthesis (51). Thus, multiple, additional mechanisms may be involved in the C/EBPα-mediated anti-proliferative effects of 1,25(OH)2D3 in MCF-7 cells.
Although we found that C/EBPα is regulated by 1,25(OH)2D3 in breast cancer cells, previous studies indicated that C/EBPβ and C/EBPδ but not C/EBPα are 1,25(OH)2D3 target genes (14, 52, 53). The different C/EBP isoforms show tissue/cell-specific regulation and function. C/EBPβ and C/EBPδ but not C/EBPα are induced by 1,25(OH)2D3 in osteoblastic cells and, together with VDR, have a role in regulating osteocalcin transcription (52, 53). In kidney cells C/EBPβ, but not C/EBPα, is induced by 1,25(OH)2D3 and can act as an enhancer of VDR-mediated transcription of 25-hydroxyvitamin D3-24-hydroxylase, the enzyme involved in the catabolism of 1,25(OH)2D3 (14). C/EBPβ has also been reported to have a role in regulating the transcription of 25-hydroxyvitamin-D3-1α-hydroxylase, the enzyme needed for the synthesis of 1,25(OH)2D3 (54, 55). However in MCF-7 cells, C/EBPα and not C/EBPβ induced VDR transcription. Collectively these studies provide increasing evidence that specific C/EBP isoforms may be key mediators of 1,25(OH)2D3 action in different cells. Because C/EBPα causes growth arrest, induced expression of this isoform would be important for mediating the anti-proliferative effects of 1,25(OH)2D3 in cancer cells. Thus, cell type specificity is important for the different cellular responses mediated by the specific isoforms. Tissue-specific coactivators may allow the tissue-specific regulation and function of C/EBP isoforms. For example, specific coactivators may be involved in the tissue-specific regulation of specific C/EBP isoforms by 1,25(OH2D3 and may allow the regulation of VDR transcription by C/EBPβ in osteoblastic cells and by C/EBPα in MCF-7 cells.
The VDR gene has been suggested to represent a target for chemoprevention in breast cancer (35). The importance of VDR for the anti-cancer effects of 1,25(OH)2D3 was definitively demonstrated in studies using VDR-null mice (VDR knock out). Cell lines derived from mammary tumors that developed in VDR knock out mice were completely resistant to 1,25(OH)2D3-mediated growth arrest (56). In addition, VDR knock out mice exhibited increased incidence of mammary hyperplasia, enhanced tumor development in epidermis and lymphoid tissue, and a higher percentage of hormone-independent tumors (57). Despite the importance of VDR in anti-cancer effects, we are only now beginning to understand the various factors that modulate VDR expression in cancer cells and the mechanisms involved. In human colon cancer cells, which are also growth-inhibited by 1,25(OH)2D3, the transcription factor SNAIL was found to repress the activity of the hVDR promoter (58). This repression was associated with a down-regulation of E-cadherin. The Wilms' tumor suppressor WT1, which induces p21, was found to activate the human and mouse VDR promoter (59, 60). In our study we show for the first time that, in breast cancer cells, the VDR gene is a target for transcriptional activation by C/EBPα. It is of interest that C/EBPα is present and is induced by 1,25(OH)2D3 in MCF-7 cells and not in ER-negative MDA-MB-231 cells. MCF-7 cells are more responsive to 1,25(OH)2D3 growth inhibition and express twice the amount of VDR compared with MDA-MB-231 cells (33, 34). Transfection of C/EBPα enhanced VDR levels in MDA-MB-231 cells (Fig. 5A). These findings suggest that transcriptional activation of VDR by C/EBPα may be one factor involved in the increased levels of VDR and increased responsiveness to 1,25(OH)2D3 of MCF-7 cells compared with MDA-MB-231 cells. Although our study suggests an indirect effect of 1,25(OH)2D3 through the induction of C/EBPα on stimulation of VDR gene expression, it should be noted that direct effects of 1,25(OH)2D3 on the VDR gene have been described by others. Recent studies examining the mouse VDR gene reported three VDR binding sites within introns downstream of the transcription start site that likely contribute to VDR autoregulation (61). Also, using T47D cells, 100 nm 1,25(OH)2D3 was found to induce a luciferase construct containing -800 to +31 of the exon 1c hVDR promoter ∼2-fold (62). This induction was not observed using the VDR promoter containing a mutated Sp1 site at -262. Thus it is likely that VDR regulation by 1,25(OH)2D3 may be both direct and indirect and that VDR transcription may be mediated by multiple tissue/cell type-specific factors.
In this study, Brm, an ATPase that is a component of the SWI/SNF chromatin-remodeling complex, was found to cooperate with C/EBPα in the regulation of hVDR transcription as indicated by inhibition of C/EBPα induction of hVDR promoter activity by DN Brm, interaction between C/EBPα and Brm, and reChIP analysis showing C/EBPα and Brm binding simultaneously to the hVDR promoter. Previous studies of adipocyte differentiation also reported an interaction between C/EBPα and Brm (63). In addition SWI/SNF recruitment by C/EBPα was shown to be important for the expression of adipocyte specific genes and to be an integral part of the C/EBPα-dependent process of adipocyte differentiation (63). Subsequently, studies examining the role of the nuclear hormone receptor peroxisome proliferator-activated receptor γ, which regulates adipogenesis, showed that the peroxisome proliferator-activated receptor γ promoter, similar to the VDR promoter, binds C/EBPα and recruits SWI/SNF enzymes (64). In addition to differentiation control by C/EBPα, a functional SWI/SNF complex has also been shown to be required for C/EBPα-mediated proliferation arrest (32). C/EBPα did not repress the proliferation of Brm-deficient C33A and SW13 cells (32). In addition, suppression of Brm expression by siRNA in BALB/c fibroblasts abrogated the antiproliferative effect of C/EBPα in these cells, although the expression of Brg-1 was not affected (32). These findings suggest, similar to our studies in which Brm but not Brg was recruited to the hVDR promoter, a specific role for Brm in C/EBPα-mediated regulation of genes involved in growth arrest. Thus our findings, similar to the findings related to both proliferation arrest and differentiation, support a molecular mechanism of SWI/SNF recruitment to mediate cell type specific functions of C/EBPα.
In summary, we found that 1,25(OH)2D3 stimulated growth arrest of MCF-7 breast cancer cells involves the 1,25(OH)2D3-induced expression of C/EBPα, functional cooperation between C/EBPα and the SWI/SNF complex to transactivate VDR, resulting in reduced proliferation of MCF-7 cells. Thus our results establish a critical role for C/EBPα, through regulation of VDR, in the 1,25(OH)2D3-mediated inhibition of proliferation of breast cancer cells.
This work was supported, in whole or in part, by National Institutes of Health Grant DK 38961 (to S. C.). This work was also supported by a grant from the American Hellenic Educational Progressive Association (to S. C. and R. W.) and by the New Jersey Medical School Cancer Center Grants Program (to S. C. and R. W.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: 1,25(OH)2d3, 1,25-dihydroxyvitamin D3; ER, estrogen receptor; VDR, vitamin D receptor; hVDR, human VDR; C/EBPα, CCAAT enhancer-binding protein α; FBS, fetal bovine serum; DMEM, Dulbecco's modified Eagle's medium; DN, dominant negative; CMV, cytomegalovirus; siRNA, small interference RNA; ChIP, chromatin immunoprecipitation.
References
- 1.Christakos, S., Dhawan, P., Liu, Y., Peng, X., and Porta, A. (2003) J. Cell Biochem. 88 695-705 [DOI] [PubMed] [Google Scholar]
- 2.Jacobson, E. A., James, K. A., Newmark, H. L., and Carroll, K. K. (1989) Cancer Res. 49 6300-6303 [PubMed] [Google Scholar]
- 3.Colston, K. W., Chander, S. K., Mackay, A. G., and Coombes, R. C. (1992) Biochem. Pharmacol. 44 693-702 [DOI] [PubMed] [Google Scholar]
- 4.Colston, K. W., Mackay, A. G., James, S. Y., Binderup, L., Chander, S., and Coombes, R. C. (1992) Biochem. Pharmacol. 44 2273-2280 [DOI] [PubMed] [Google Scholar]
- 5.Anzano, M. A., Smith, J. M., Uskokovic, M. R., Peer, C. W., Mullen, L. T., Letterio, J. J., Welsh, M. C., Shrader, M. W., Logsdon, D. L., and Driver, C. L., Brown, C. C., Roberts A. B., and Sporn, M. B. (1994) Cancer Res. 54 1653-1656 [PubMed] [Google Scholar]
- 6.Mehta, R., Hawthorne, M., Uselding, L., Albinescu, D., Moriarty, R., and Christov, K. (2000) J. Natl. Cancer Inst. 92 1836-1840 [DOI] [PubMed] [Google Scholar]
- 7.Colston, K. W., Berger, U., and Coombes, R. C. (1989) Lancet 1 188-191 [DOI] [PubMed] [Google Scholar]
- 8.Janowsky, E. C., Lester, G. E., Weinberg, C. R., Millikan, R. C., Schildkraut, J. M., Garrett, P. A., and Hulka, B. S. (1999) Public Health Nutr. 2 283-291 [DOI] [PubMed] [Google Scholar]
- 9.Mawer, E. B., Walls, J., Howell, A., Davies, M., Ratcliffe, W. A., and Bundred, N. J. (1997) J. Clin. Endocrinol. Metab. 82 118-122 [DOI] [PubMed] [Google Scholar]
- 10.Abe, J., Nakano, T., Nishii, Y., Matsumoto, T., Ogata, E., and Ikeda, K. (1991) Endocrinology 129 832-837 [DOI] [PubMed] [Google Scholar]
- 11.Chouvet, C., Vicard, E., Devonec, M., and Saez, S. (1986) J. Steroid Biochem. 24 373-376 [DOI] [PubMed] [Google Scholar]
- 12.Wang, Q., Lee, D., Sysounthone, V., Chandraratna, R. A. S., Christakos, S., Korah, R., and Wieder, R. (2001) Breast Cancer Res. Treat. 67 157-168 [DOI] [PubMed] [Google Scholar]
- 13.Wang, Q., Yang, W., Uytingco, M. S., Christakos, S., and Wieder, R. (2000) Cancer Res. 60 2040-2048 [PubMed] [Google Scholar]
- 14.Dhawan, P., Peng, X., Sutton, A. L., MacDonald, P. N., Croniger, C. M., Trautwein, C., Centrella, M., McCarthy, T. L., and Christakos, S. (2005) Mol. Cell Biol. 25 472-487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson, R. W. (1998) J. Biol. Chem. 273 28543. [DOI] [PubMed] [Google Scholar]
- 16.Ramji, D. P., and Foka, P. (2002) Biochem. J. 365 561-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson, G. W., Johnson, P. F., Hennighausen, L., and Sterneck, E. (1998) Genes Dev. 12 1907-1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seagroves, T. N., Krnacik, S., Raught, B., Gay, J., Burgess-Beusse, B., Darlington, G. J., and Rosen, J. M. (1998) Genes Dev. 12 1917-1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flodby, P., Barlow, C., Kylefjord, H., Ahrlund-Richter, L., and Xanthopoulos, K. G. (1996) J. Biol. Chem. 271 24753-24760 [DOI] [PubMed] [Google Scholar]
- 20.Freytag, S. O., Paielli, D. L., and Gilbert, J. D. (1994) Genes Dev. 8 1654-1663 [DOI] [PubMed] [Google Scholar]
- 21.Radomska, H. S., Huettner, C. S., Zhang, P., Cheng, T., Scadden, D. T., and Tenen, D. G. (1998) Mol. Cell Biol. 18 4301-4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gery, S., Tanosaki, S., Bose, S., Bose, N., Vadgama, J., and Koeffler, H. P. (2005) Clin. Cancer Res. 11 3184-3190 [DOI] [PubMed] [Google Scholar]
- 23.Huening, M., Yehia, G., Molina, C. A., and Christakos, S. (2002) Mol. Endocrinol. 16 2052-2064 [DOI] [PubMed] [Google Scholar]
- 24.Menendez-Hurtado, A., Santos, A., and Perez-Castillo, A. (2000) Endocrinology 141 4164-4170 [DOI] [PubMed] [Google Scholar]
- 25.Muchardt, C., Reyes, J. C., Bourachot, B., Leguoy, E., and Yaniv, M. (1996) EMBO J. 15 3394-3402 [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford, M. M. (1976) Anal. Biochem. 72 248-254 [DOI] [PubMed] [Google Scholar]
- 27.Liu, Y., Shen, Q., Malloy, P. J., Soliman, E., Peng, X., Kim, S., Pike, J. W., Feldman, D., and Christakos, S. (2005) J. Bone Miner Res. 20 1680-1691 [DOI] [PubMed] [Google Scholar]
- 28.Shen, Q., and Christakos, S. (2005) J. Biol. Chem. 280 40589-40598 [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto, K., Kesterson, R. A., Yamamoto, H., Taketani, Y., Nishiwaki, E., Tatsumi, S., Inoue, Y., Morita, K., Takeda, E., and Pike, J. W. (1997) Mol. Endocrinol. 11 1165-1179 [DOI] [PubMed] [Google Scholar]
- 30.Wilson, H. L., and Roesler, W. J. (2002) Mol. Cell Endocrinol. 188 15-20 [DOI] [PubMed] [Google Scholar]
- 31.Pabst, T., Mueller, B. U., Harakawa, N., Schoch, C., Haferlach, T., Behre, G., Hiddemann, W., Zhang, D. E., and Tenen, D. G. (2001) Nat. Med. 7 444-451 [DOI] [PubMed] [Google Scholar]
- 32.Muller, C., Calkhoven, C. F., Sha, X., and Leutz, A. (2004) J. Biol. Chem. 279 7353-7358 [DOI] [PubMed] [Google Scholar]
- 33.Elstner, E., Linker-Israeli, M., Said, J., Umiel, T., de Vos, S., Shintaku, I. P., Heber, D., Binderup, L., Uskokovic, M., and Koeffler, H. P. (1995) Cancer Res. 55 2822-2830 [PubMed] [Google Scholar]
- 34.Swami, S., Raghavachari, N., Muller, U. R., Bao, Y. P., and Feldman, D. (2003) Breast Cancer Res. Treat. 80 49-62 [DOI] [PubMed] [Google Scholar]
- 35.Welsh, J., Wietzke, J. A., Zinser, G. M., Byrne, B., Smith, K., and Narvaez, C. J. (2003) J. Nutr. 133 2425S-2433S [DOI] [PubMed] [Google Scholar]
- 36.Zhang, D. E., Zhang, P., Wang, N. D., Hetherington, C. J., Darlington, G. J., and Tenen, D. G. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 569-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darlington, G. J., Ross, S. E., and MacDougald, O. A. (1998) J. Biol. Chem. 273 30057-30060 [DOI] [PubMed] [Google Scholar]
- 38.Pabst, T., Mueller, B. U., Zhang, P., Radomska, H. S., Narravula, S., Schnittger, S., Behre, G., Hiddemann, W., and Tenen, D. G. (2001) Nat. Genet. 27 263-270 [DOI] [PubMed] [Google Scholar]
- 39.Xu, L., Hui, L., Wang, S., Gong, J., Jin, Y., Wang, Y., Ji, Y., Wu, X., Han, Z., and Hu, G. (2001) Cancer Res. 61 3176-3181 [PubMed] [Google Scholar]
- 40.Halmos, B., Huettner, C. S., Kocher, O., Ferenczi, K., Karp, D. D., and Tenen, D. G. (2002) Cancer Res. 62 528-534 [PubMed] [Google Scholar]
- 41.Shim, M., Powers, K. L., Ewing, S. J., Zhu, S., and Smart, R. C. (2005) Cancer Res. 65 861-867 [PubMed] [Google Scholar]
- 42.Greenbaum, L. E., Li, W., Cressman, D. E., Peng, Y., Ciliberto, G., Poli, V., and Taub, R. (1998) J. Clin. Invest. 102 996-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu, S., Yoon, K., Sterneck, E., Johnson, P. F., and Smart, R. C. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 207-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng, J., Yu, D. V., Zhou, J. H., and Shapiro, D. J. (2007) J. Biol. Chem. 282 30535-30543 [DOI] [PubMed] [Google Scholar]
- 45.Ramos, R. A., Nishio, Y., Maiyar, A. C., Simon, K. E., Ridder, C. C., Ge, Y., and Firestone, G. L. (1996) Mol. Cell Biol. 16 5288-5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.James, S. Y., Mackay, A. G., Binderup, L., and Colston, K. W. (1994) J. Endocrinol. 141 555-563 [DOI] [PubMed] [Google Scholar]
- 47.Stoica, A., Saceda, M., Fakhro, A., Solomon, H. B., Fenster, B. D., and Martin, M. B. (1999) J. Cell Biochem. 75 640-651 [PubMed] [Google Scholar]
- 48.Swami, S., Krishnan, A. V., and Feldman, D. (2000) Clin. Cancer Res. 6 3371-3379 [PubMed] [Google Scholar]
- 49.Timchenko, N. A., Wilde, M., Nakanishi, M., Smith, J. R., and Darlington, G. J. (1996) Genes Dev. 10 804-815 [DOI] [PubMed] [Google Scholar]
- 50.Wang, H., Iakova, P., Wilde, M., Welm, A., Goode, T., Roesler, W. J., and Timchenko, N. A. (2001) Mol. Cell 8 817-828 [DOI] [PubMed] [Google Scholar]
- 51.Slomiany, B. A., D'Arigo, K. L., Kelly, M. M., and Kurtz, D. T. (2000) Mol. Cell Biol. 20 5986-5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gutierrez, S., Javed, A., Tennant, D. K., van Rees, M., Montecino, M., Stein, G. S., Stein, J. L., and Lian, J. B. (2002) J. Biol. Chem. 277 1316-1323 [DOI] [PubMed] [Google Scholar]
- 53.Villagra, A., Cruzat, F., Carvallo, L., Paredes, R., Olate, J., van Wijnen, A. J., Stein, G. S., Lian, J. B., Stein, J. L., Imbalzano, A. N., and Montecino, M. (2006) J. Biol. Chem. 281 22695-22706 [DOI] [PubMed] [Google Scholar]
- 54.Zierold, C., Nehring, J. A., and DeLuca, H. F. (2007) Arch. Biochem. Biophys. 460 233-239 [DOI] [PubMed] [Google Scholar]
- 55.Zhong, Y., and Christakos, S. (2006) J. Bone Miner. Res. 21 S120 [Google Scholar]
- 56.Zinser, G. M., McEleney, K., and Welsh, J. (2003) Mol. Cell Endocrinol. 200 67-80 [DOI] [PubMed] [Google Scholar]
- 57.Zinser, G. M., Suckow, M., and Welsh, J. (2005) J. Steroid Biochem. Mol. Biol. 97 153-164 [DOI] [PubMed] [Google Scholar]
- 58.Palmer, H. G., Larriba, M. J., Garcia, J. M., Ordonez-Moran, P., Pena, C., Peiro, S., Puig, I., Rodriguez, R., de la Fuente, R., Bernad, A., Pollan, M., Bonilla, F., Gamallo, C., de Herreros, A. G., and Munoz, A. (2004) Nat. Med. 10 917-919 [DOI] [PubMed] [Google Scholar]
- 59.Maurer, U., Jehan, F., Englert, C., Hubinger, G., Weidmann, E., DeLuca, H. F., and Bergmann, L. (2001) J. Biol. Chem. 276 3727-3732 [DOI] [PubMed] [Google Scholar]
- 60.Lee, T. H., and Pelletier, J. (2001) Physiol. Genomics 7 187-200 [DOI] [PubMed] [Google Scholar]
- 61.Zella, L. A., Kim, S., Shevde, N. K., and Pike, J. W. (2007) J. Steroid Biochem. Mol. Biol. 103 435-439 [DOI] [PubMed] [Google Scholar]
- 62.Wietzke, J. A., Ward, E. C., Schneider, J., and Welsh, J. (2005) Mol. Cell Endocrinol. 230 59-68 [DOI] [PubMed] [Google Scholar]
- 63.Pedersen, T. A., Kowenz-Leutz, E., Leutz, A., and Nerlov, C. (2001) Genes Dev. 15 3208-3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salma, N., Xiao, H., Mueller, E., and Imbalzano, A. N. (2004) Mol. Cell Biol. 24 4651-4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim, J. W., Monila, H., Pandey, A., and Lane, M. D. (2007) Biochem. Biophys. Res. Commun. 354 517-521 [DOI] [PubMed] [Google Scholar]