Abstract
Brd4 is a chromatin adaptor containing tandem bromodomains binding to acetylated histone H3 and H4. Although Brd4 has been implicated in the transcriptional control of papillomavirus-encoded E2 protein, it is unclear how Brd4 regulates E2 function and whether the involvement of Brd4 in transactivation and transrepression is common to different types of E2 proteins. Using DNase I footprinting performed with in vitro reconstituted human papillomavirus (HPV) chromatin and nucleosome-free DNA templates, we found that Brd4 facilitates E2 binding to its cognate sequences in chromatin depending on bromodomains and the E2-interacting region of Brd4. Moreover, the coactivator and corepressor function of Brd4 requires at least one intact bromodomain and is mediated by its direct association with E2 proteins encoded by cancer-inducing high risk HPV-16 and HPV-18, wart-causing low risk HPV-11, and bovine papillomavirus type 1, in part through enhancing the protein stability of E2 that is normally degraded via the ubiquitin-dependent proteasome pathway. Our findings indicate that a chromatin adaptor can bridge and enhance the binding of a sequence-specific transcription factor to chromatin and further promote the stability of a labile transcription factor via direct protein-protein interaction.
E2 encoded by human papillomaviruses (HPVs)2 is a multifunctional protein regulating viral DNA replication, genome segregation, transcription, cell cycle control, and senescence (1). Its primary function relies on the sequence-specific recognition of a 12-nucleotide palindrome, ACCN6GGT, located at the upstream regulatory region of HPVs and animal papillomaviruses, such as bovine papillomavirus type 1 (BPV-1). The sequence context and the location of E2-binding sites (E2BSs) as well as the nature of E2 proteins all contribute to E2 activity in viral gene regulation. The transcriptional activity of E2 appears to be commonly shared by different types of E2 proteins encoded, for example, by cervical cancer-inducing HPV types 16 (HPV-16) and 18 (HPV-18), genital wart-associated HPV type 11 (HPV-11), and BPV-1. In general, HPV-16 E2 (16E2) exhibits strongest transcriptional activity, followed by BPV-1 E2 (BE2), HPV-18 E2 (18E2), and HPV-11 E2 (11E2), and correlates well with their corresponding binding affinities to E2BSs derived from the promoter-proximal regions of naturally occurring HPV-11, HPV-16, and HPV-18 sequences (2). Because two of the four E2BSs in genital HPVs are flanked by an upstream Sp1-binding site and the downstream TATA box of the E6 early promoter, HPV E2 typically functions as a transcriptional repressor by excluding Sp1 and TFIID/TBP from binding to their cognate sequences and thus prevents the assembly of a transcriptional preinitiation complex (3–7). HPV E2 also exhibits transactivation activity functioning in a heterologous promoter context where multimerized E2BSs are situated away from the TATA box (8), as seen in several natural BPV-1 promoter regions (9, 10). Like many other cellular transcription factors, E2 has a dual role in gene activation and repression.
Recently, several groups have independently identified cellular bromodomain-containing protein 4 (Brd4) as an E2-interacting protein involved in viral genome segregation (11–14) and transcriptional control (1, 14–18). Although Brd4 and other cellular proteins, such as ChlR1 (19), may serve as chromatin adaptors facilitating viral genome segregation during mitosis, Brd4 appears to play a more active role in cell cycle progression (20) and cancer development (21, 22), largely through its ability to modulate gene transcription by recruiting different transcription components to selective target genes. The association of Brd4 with Mediator and positive transcription elongation factor b (P-TEFb) likely accounts for the coactivating function of Brd4 in Tat-independent stimulation of the human immunodeficiency virus type 1 promoter (17, 23, 24). Whether Brd4 is similarly implicated in transcriptional activation by HPV and BPV-1 E2 proteins is somewhat unclear, because only circumstantial evidence based primarily on the inhibition of E2-dependent reporter activity by overexpression of an E2-interacting domain containing the C-terminal 300 amino acids of Brd4 was provided (14–16). Because this C-terminal motif (CTM) of Brd4 is conserved among different species of Brd4 and some members of the BET family proteins, including mammalian Brdt and Drosophila Fsh (17), inhibition of E2 transactivation by overexpression of a CTM domain in the cell may be due to squelching of a CTM-interacting cellular protein needed for E2-dependent activation, not necessarily reflecting a true requirement for Brd4. Likewise, although the repressing activity of Brd4 has been convincingly demonstrated by in vitro reconstituted HPV chromatin-dependent transcription and cell-based experiments with 11E2 (1), it remains undetermined whether the corepressor activity of Brd4 is common to E2 proteins encoded by cancer-inducing HPVs and animal papillomaviruses.
To define the molecular mechanism by which Brd4 enhances E2-dependent repression of the HPV promoter and to determine whether Brd4 indeed plays a dual role in E2-dependent activation and repression seemingly common to different types of E2 proteins, we performed chromatin and DNA footprinting analysis as well as functional complementation experiments by introducing wild-type or mutant Brd4 back to a stable Brd4 knockdown human cell line. We found that the presence of Brd4 significantly enhances site-specific recognition of E2 to both DNA and chromatin with a strict dependence on the CTM but only a selective requirement of Brd4 bromodomains for E2 binding to chromatin but not DNA. Moreover, Brd4 was shown to be a universal coactivator and corepressor for different types of E2 proteins. Interestingly, direct association of Brd4 with E2 significantly enhances the stability of the labile E2 protein, which is normally undetectable in the cell lysate. These activities of Brd4 collectively contribute to E2-regulated transcription and institute the notion that chromatin adaptors, such as Brd4, not only function as nucleosome-binding factors but also play an active role in recruiting sequence-specific transcription factors to their chromatin target sites, thereby modulating gene activity via multiple tiers of regulatory mechanisms.
EXPERIMENTAL PROCEDURES
Plasmid Constructions—The full-length human Brd4 (hBrd4) cDNA encoding amino acids 1–1362 was cloned into a baculovirus transfer vector by a two-step cloning process. First, the coding region for amino acids 1–1043 was amplified by PCR using pcDNA4C-Brd4-FL (11) with an NdeI site-containing upstream primer and a BamHI site-containing downstream primer and cloned into pF:TBP-11d (25), after swapping with the TBP insert between NdeI and BamHI sites, to generate pF:hBrd4(1–1043)-11d. The FLAG-tagged N-terminal 1043-amino acid coding sequence was then isolated from pF:hBrd4(1–1043)-11d and subcloned into pVL1392 (Invitrogen) between XbaI and BamHI sites to create pVL-F:hBrd4(1–1043). An EcoRI-NotI fragment encoding amino acids 645–1362 of hBrd4 was isolated from pcDNA4C-Brd4-FL and cloned into pVL1393 (Invitrogen) at the same enzyme-cutting sites to generate pVL-F:hBrd4(EcoRI-NotI), in which the 3′ part of the coding region (amino acids 731–1362), along with the vector sequence, was isolated between SmaI and XhoI and ligated to the SmaI-XhoI fragment of pVL-F:hBrd4(1–1043) containing the coding sequence for the FLAG-tagged N-terminal 731 amino acids of Brd4 to create pVL-F:hBrd4(FL). The mammalian expression plasmid, pcDNA3-F:hBrd4(FL), was generated by cloning the NotI-flanked FLAG-tagged hBrd4-coding sequence into NotI-cleaved and calf intestine phosphatase-treated pcDNA3 (Invitrogen).
The mammalian expression plasmid pcDNA3-F:hBrd4(1-1223) was constructed by replacing the coding sequence for amino acids 1057–1362 between BstEII and XhoI sites with the coding region for 1057–1223, which was prepared by BstEII and XhoI digestion of a DNA fragment amplified from pcDNA3-F:hBrd4(FL) with an upstream primer annealing to amino acid position 1033 and a downstream XhoI site-containing primer hybridizing to amino acid position 1223. A BglII-XbaI fragment containing the coding sequence for FLAG-tagged hBrd4 amino acids 1–1223 was then isolated from pcDNA3-F:hBrd4(1–1223) and cloned into pVL1392 between BglII and XbaI sites to generate pVL-F:hBrd4(1–1223).
To generate domain-specific deletion mutants of hBrd4, an intermediate plasmid pF:hBrd4(1–722)-7 was first created by cloning the coding sequence for the N-terminal 722 amino acids of hBrd4, amplified from pcDNA4C-Brd4-FL with an NdeI site-containing upstream primer and a BamHI site-containing downstream primer, into pFLAG(S)-7 (26) between NdeI and BamHI sites. Inverse PCR was then employed to generate domain-specific deletions of BDI, BDII, and ET, respectively, in pF:hBrd4(1–722)-7 by using 5′-phosphorylated primer pairs flanking the deleted region for outward PCR amplification. The amplified products were then digested with DpnI at 37 °C for 1 h to remove the parental template before self-ligation at 16 °C overnight. The resulting clones, after bacterial transformation and DNA sequencing, were named pF:hBrd4(1–722)ΔBDI-7, pF:hBrd4(1–722)ΔBDII-7, and pF:hBrd4(1–722)ΔET-7, respectively. The ΔBDI/II deletion plasmid pF:hBrd4(1–722)ΔBDI/II-7 was similarly constructed by inverse PCR using pF:hBrd4(1–722)ΔBDI-7 as template with 5′-phosphorylated primer pairs flanking the BDII region.
To generate pVL-F:hBrd4ΔBDI, pVL-F:hBrd4ΔBDII, and pVL-F:hBrd4ΔBDI/II for protein expression and purification in insect cells, the BglII-EcoRI fragment containing the coding region for FLAG-tagged N-terminal 645 amino acids of hBrd4 with the deleted bromodomain(s) was released from pF:hBrd4(1–722)ΔBDI-7, pF:hBrd4(1–722)ΔBDII-7, and pF:hBrd4(1–722)ΔBDI/II-7, respectively, and cloned into BamHI/NotI-cleaved pVL1393, along with the EcoRI-NotI fragment containing the coding region for amino acids 645–1362 prepared from pVL-F:hBrd4(FL), for three-piece ligation. Plasmids pcDNA3-F:hBrd4ΔBDI, pcDNA3-F:hBrd4ΔBDII, and pcDNA3-F:hBrd4ΔBDI/II were similarly constructed by three-piece ligation, except using BamHI/NotI-linearized pcDNA3.
To construct pVL-F:hBrd4ΔET, a SacI-cleaved fragment spanning the coding region for amino acids 514–706 with the deleted ET domain was first used to replace the corresponding wild-type sequence in pF:hBrd4(1–1043)-11d to generate pF:hBrd4(1–1043)ΔET-11d, in which the XbaI-SmaI fragment containing the coding sequence for FLAG-tagged N-terminal 731 amino acids with the deleted ET domain was used to replace the corresponding wild-type sequence in pVL-F: hBrd4(FL). The plasmid pcDNA3-F:hBrd4ΔET was then created by subcloning the FLAG-tagged hBrd4ΔET insert from pVL-F:hBrd4ΔET into pcDNA3 between EcoRI and NotI sites.
Baculovirus expression plasmids pVL-F:BE2, pVL-F:16E2, and pVL-F:18E2 were constructed by cloning the FLAG-tagged E2 insert, amplified by PCR, respectively, from pF:BE2–11d, pF:16E2–11d, and pF:18E2–11d (2) with a NotI site-containing upstream primer (5′-AGAATTCGCGGCCGCCATGGACTACAAAGACGT-3′) and a BamHI site-containing downstream primer, into pVL1392 between NotI and BamHI sites. Mammalian expression plasmids pCMV-F:11E2, pCMV-F: 16E2, and pCMV-F:18E2 were similarly constructed by cloning the FLAG-tagged 11E2, 16E2, and 18E2 cDNA, amplified from pF:11E2–11d (2), pF:16E2–11d, and pF:18E2–11d, respectively, into NotI/BamHI-linearized pFLAG-CMV-2 (Sigma). The plasmid pCMV-F:BE2 was generated by first isolating the FLAG-tagged BE2 insert from pF:BE2–11d between BglII and EcoRI sites and then cloned into BamHI/EcoRI-linearized and calf intestine phosphatase-treated pcDNA3. The plasmid pVL-F°:E2, used for 11E2 expression and purification, has been described (27).
Protein Purification—FLAG-tagged full-length Brd4 and domain-specific deletion mutants, ΔBDI, ΔBDII, ΔBDI/II, ΔET, and 1–1223, were purified from insect Sf9 cells infected with baculoviruses harboring pVL-F:hBrd4(FL), pVL-F: hBrd4ΔBDI, pVL-F:hBrd4ΔBDII, pVL-F:hBrd4ΔBDI/II, pVL-F:hBrd4ΔET, and pVL-F:hBrd4(1–1223), respectively, following the published protocol (27). FLAG-tagged 11E2, 16E2, 18E2, and BE2 were similarly purified from Sf9 cells infected with recombinant baculoviruses carrying pVL-F°:E2, pVL-F: 16E2, pVL-F:18E2, and pVL-F:BE2, respectively. Purification of recombinant human AP-1 (28), p300, hNAP-1, Drosophila ACF, and HeLa core histones (29) has been described.
DNase I Footprinting with Chromatin and DNA Templates—HPV chromatin was assembled in vitro using p7072–70GLess/I+ DNA (7) with recombinant hNAP-1, Drosophila ACF, and HeLa core histones according to the published protocol (1). For DNase I footprinting, a 30-μl reaction containing 2 μl of HPV chromatin or mock-assembled DNA (i.e. without HeLa core histones, hNAP-1, and ACF), with or without 50 ng of hBrd4 and a different amount (10, 30, 100, or 300 ng) of 11E2 was incubated in transcription buffer (30) at 30 °C for 30 min. The incubated chromatin or DNA (12.5 μl) was digested with 0.25 unit (chromatin) or 0.025 unit (DNA) of DNase I (Invitrogen) at room temperature for 2 min. The reaction was terminated by adding 15 μl of DNase I stop solution containing 200 mm Tris-HCl (pH 7.5), 50 mm EDTA, 2% SDS, 200 μg/ml of proteinase K, and 250 μg/ml of glycogen and then treated with 20 μg of proteinase K at 60 °C for 30 min, followed by phenol/chloroform extraction. The soluble portion was precipitated with 200 μl of 5 m ammonium acetate, 2 μl of glycogen (10 mg/ml), and 1 ml of 100% ethanol at –20 °C overnight. The precipitated DNA was washed with 70% ethanol and air-dried. The pellet was resuspended in 10 μl of H2O. The resulting DNA was then amplified by asymmetric PCR at 95 °C for 1 min, 68 °C for 2 min, and 76 °C for 2 min for 2 cycles in a 25-μl reaction containing 10 μl of DNA, 0.4 unit of Vent DNA Polymerase (New England Biolabs), 0.1 pmol of the 32P-labeled Gless(AS)-31/55 primer (1), 0.2 m dNTP, 40 mm NaCl, 20 mm Tris-HCl (pH 8.0), 5 mm MgSO4, 0.01% gelatin (Sigma), and 0.1% Triton X-100. The PCR products were mixed with 125 μl of stop solution containing 10 mm Tris-HCl (pH 7.5), 4 mm EDTA, 260 mm sodium acetate (pH 5.2), and 20 μg of tRNA and then precipitated with 700 μl of 100% ethanol on dry ice for 10 min. The DNA fragments were spun, dried, resuspended in 6 μl of formamide dye (90% formamide, 1× TBE, and 0.02% bromophenol blue/xylene cyanol) and analyzed in a 6% polyacrylamide/7 m urea gel at 75 W for 2 h. The gel was transferred onto a chromatography paper (Fisher), dried, and exposed in a PhosphorImager screen. The signals were detected by Typhoon 9200 PhosphorImager (GE Healthcare).
In Vitro Transcription—HPV chromatin, assembled as described above, was used for order-of-addition transcription experiments according to the published scheme (1) with minor modifications. Briefly, a 30-μl reaction containing 6 μl of in vitro assembled chromatin, 30 μm acetyl-CoA, and 20 ng of p300 was incubated, in the absence or presence of 40 ng of AP-1, with 30 ng of hBrd4 and 1–15 ng of E2, unless otherwise indicated, in transcription buffer at 30 °C for 30 min. Eight μl of HeLa nuclear extract (8–10 mg/ml) and 10 ng of pMLΔ53 DNA were then added and incubated at 30 °C for another 30 min. Transcription was initiated by adding [α-32P]CTP and NTP mix and incubated for another hour. The reactions were terminated and processed as described previously (1). Relative transcription in each set of reactions was defined as the signal intensity quantified by PhosphorImager from the HPV chromatin relative to that performed in the presence of AP-1 without E2 and Brd4. Fold change in each set of experiments represents the level of relative transcription relative to that obtained in the absence of E2.
Luciferase Reporter Gene Assay—Human cervical cancer-derived C-33A cells containing a stably integrated Brd4 shRNA-expressing cassette (named #1–13; see Ref. 1) were maintained in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum. For E2 repression assays, #1–13 cells (∼80% confluency) grown in 24-well plates were transfected with 100 ng of pGL7072–161 (7), 50 ng of an E2 expression plasmid (pCMV-F:BE2, pCMV-F:11E2, pCMV-F:16E2, or pCMV-F:18E2), and 0.5 μg of pcDNA3-F:hBrd4(FL), pcDNA3-F:hBrd4ΔBDI/II, pcDNA3-F:hBrd4ΔBDI, pcDNA3-F:hBrd4ΔBDII, pcDNA3-F:hBrd4ΔET, or pcDNA3-F:hBrd4(1–1223) using FuGENE 6 (Roche Applied Science) according to the manufacturer's protocol. Four hours later, the medium was replaced with fresh medium containing 5 mm sodium butyrate and incubated for another 20 h. The cells were then harvested in 1× reporter lysis buffer (Promega) and mixed with assay buffer containing d-Luciferin (Pharmingen). Luciferase activity was measured with the POLARstar OPTIMA plate reader (BMG Labtechnologies). E2 activation assays were similarly performed as described above, except that 100 ng of the p2x2xE2BS-luc reporter (31) and 10 ng of an E2 expression plasmid were used. Sodium butyrate (5 mm) was added 24 h post-transfection. After another 24 h, the cells were harvested for luciferase assays. Fold repression or fold activation in each set of reactions was defined as the luciferase activity relative to that performed without Brd4 expression.
Protein Detection in Transiently Transfected Cells—For experiments described in Fig. 6C, #1–13 cells (∼80% confluency) grown in one 100-mm plate were transfected with 8 μg of a Brd4 expression plasmid and 1.5 μg of an E2 expression plasmid, both driven by the cytomegalovirus promoter, using FuGENE 6. 4 h later, sodium butyrate was added to a final concentration of 5 mm. After incubation for another 20 h, the cells were lysed in 200 μl of modified radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.5, 1% Nonidet P-40, 300 mm NaCl, 1 mm EDTA, and 10% glycerol). The lysates were cleared, following sonication and centrifugation at 4 °C, 13,000 rpm for 30 min and adjusted to 150 mm salt by mixing with an equal volume of modified radioimmune precipitation assay buffer without NaCl. Ten μl (bed volume) of anti-FLAG M2-agarose beads (Sigma) were then added for an overnight incubation with rotation at 4 °C. The immunoprecipitated E2 and Brd4 were analyzed by Western blotting with anti-FLAG M2 monoclonal antibody (Sigma).
FIGURE 6.
Brd4 functions as a general corepressor for different types of E2 proteins requiring a bromodomain and the CTM. A, schematic representation of the HPV-11 reporter construct. The numbers below the line indicate the positions of nucleotides in the HPV-11 genome. The transcription start site (+1) corresponds to nucleotide 93 in HPV-11 (7). B, levels of Brd4 in C-33A and #1–13 cells with or without exogenous Brd4 expression. Transfection was performed as described under “Experimental Procedures.” C, a bromodomain and the CTM are both crucial for Brd4-enhanced E2 repression. Transfection was performed in #1–13 cells with an HPV-11 reporter, an E2 expression plasmid, and a FL or domain-deleted Brd4 expression plasmid as described under “Experimental Procedures.” The bar graphs represent two independent experiments performed in duplicate with error bars indicating the standard deviations. Student's t test was used for statistic analysis by comparing fold repression between each mutant and the FL protein.
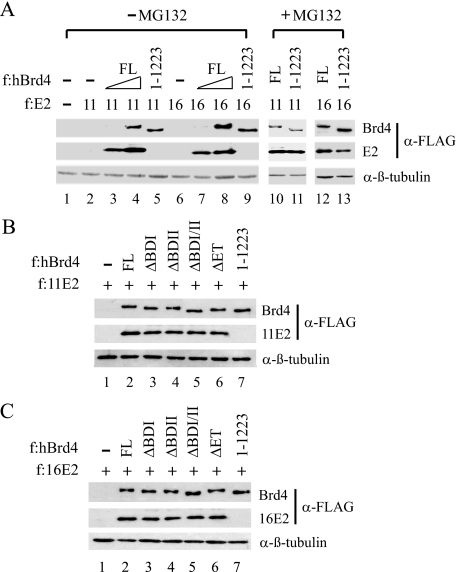
For experiments described in Fig. 8, #1–13 cells grown in a 150-mm plate at 60% confluency were transfected with 4 μg of pCMV-F:11E2 or pCMV-F:16E2, in the absence or presence of 12 μg of a wild-type or mutant Brd4 expression plasmid, using FuGENE 6. 42 h post-transfection, the cells were either left untreated or treated with DMSO or 40 μm MG132 for 6 h and then lysed in 200 μl of radioimmune precipitation assay buffer and analyzed by Western blotting with anti-FLAG M2 monoclonal antibody or with anti-β-tubulin antibodies (Santa Cruz).
FIGURE 8.
Brd4 stabilizes E2 through CTM-mediated interaction. Transfection was performed in #1–13 cells with an FLAG-tagged (f:) 11E2 or 16E2 expression plasmid, with (+) or without (–) a cotransfected FLAG-tagged FL or domain-deleted Brd4 expression plasmid. The cells were treated with the proteasome inhibitor MG132 (+) or control DMSO (–) in A or left untreated (B and C). Western blotting was conducted with anti-FLAG (for Brd4 and E2 detection) or β-tubulin antibodies.
RESULTS
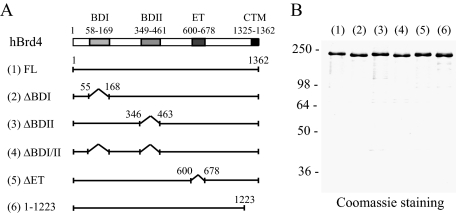
A Bromodomain and the CTM Are Both Crucial for Brd4-enhanced E2 Binding to Chromatin—To explore our working hypothesis that chromatin adaptor Brd4 binding to acetylated histone H3 and H4 helps stabilize E2 binding to its cognate sequences in chromatin (17) and to define the domains of Brd4 involved in this process, we first purified FLAG-tagged wild-type Brd4 (∼200 kDa) and several domain-specific deletion mutants from insect cells using a baculovirus expression system. Bromodomains I and II, implicated in binding to acetylated lysine residues, were removed individually or together, along with the deletion of an evolutionarily conserved extraterminal (ET) domain, whose function remains undefined, or the E2-interacting CTM (Fig. 1A). The FLAG epitope-coding sequence was introduced at the N terminus of the respective construct to facilitate protein purification via one-step immunoaffinity purification. Each protein was successfully purified from baculovirus-infected insect Sf9 cells with a small amount of degradation products inevitably detected because of the large size of the expressed protein (Fig. 1B).
FIGURE 1.
Construction and purification of wild-type and mutant human Brd4 (hBrd4). A, schematic representation of protein domains present in wild-type and mutant Brd4 proteins. The numbers indicate the positions of amino acid residues or the boundaries of respective protein domains, including BDI, BDII, ET domain, and the CTM. Each protein has an N-terminal FLAG tag sequence to facilitate protein purification. B, Coomassie Blue staining of purified human Brd4 proteins. Recombinant FLAG-tagged FL Brd4 and its internally truncated (Δ) or C-terminally deleted mutants were respectively purified from baculovirus-infected insect cells as described under “Experimental Procedures.” Protein size markers (in kDa) are indicated on the left.
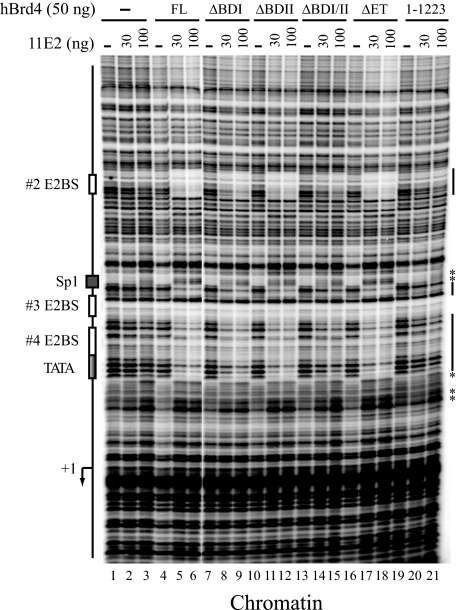
These proteins were then used for DNase I footprinting performed with HPV-11 chromatin containing the HPV-11 upstream regulatory region spanning nucleotides 7072–7933/1–70 that include four E2BSs and the TATA box of the E6 promoter linked to a 377-nucleotide G-less cassette. The chromatin template, prepared by incubating the HPV-11 DNA with purified HeLa core histones, human NAP-1 histone chaperone, and Drosophila ACF chromatin assembly/remodeling factor, faithfully reproduces the positions of nucleosomes assembled on the HPV genome typically seen in vivo (1). As shown in Fig. 2, purified 11E2 alone failed to bind stably to HPV-11 chromatin, because no protection of E2BSs were observed (lanes 2 and 3). However, in the presence of Brd4, which did not bind stably to chromatin by itself (lane 4), enhanced protection of #2, #3, and #4 E2BSs were readily detected on HPV chromatin (lanes 5 and 6 versus lanes 2 and 3), indicating that Brd4 indeed enhances E2 binding to its cognate sequences in chromatin. Deletion of one bromodomain (ΔBDI or ΔBDII) did not prevent E2 binding to E2BSs (lanes 8, 9, 11, and 12). However, removing both bromodomains significantly diminished Brd4-enhanced E2 protection (lanes 14 and 15), consistent with in vivo chromatin immunoprecipitation data showing at least one bromodomain is essential for Brd4 binding to chromatin (1). Deletion of the ET domain did not have any effect on E2 footprinting (lanes 17 and 18), indicating that the ET domain is not necessary for Brd4 enhancement of E2 binding to chromatin. Not surprisingly, interaction with E2 is crucial for Brd4 function, because removal of the CTM dramatically abolished Brd4-enhanced E2 footprinting on HPV chromatin (lanes 20 and 21). It is noted that the hypersensitive sites induced by E2 binding to #3 and #4 E2BSs were not completely eliminated in ΔBDI/II (lanes 14 and 15, asterisk). Perhaps this is caused by Brd4-induced conformational changes via CTM interaction with E2 that somehow unmask the DNA-binding domain of E2 (see below), thereby slightly enhancing E2 cooperativity for binding to juxtaposed E2BSs.
FIGURE 2.
Bromodomains and the CTM are both critical for Brd4-enhanced E2 binding to HPV chromatin. In vitro reconstituted HPV-11 chromatin, prepared as described under “Experimental Procedures,” was incubated with or without (–) HPV-11 E2 (11E2) and different forms of Brd4 proteins as indicated. Chromatin footprinting was conducted as described under “Experimental Procedures” with PCR-amplified labeled DNA fragments separated by denaturing polyacrylamide/urea gel electrophoresis and visualized following PhosphorImager analysis. The positions of protein factor-binding sites and the transcription start site (+1) are indicated on the left. The protected regions and hypersensitive sites (*) are marked on the right.
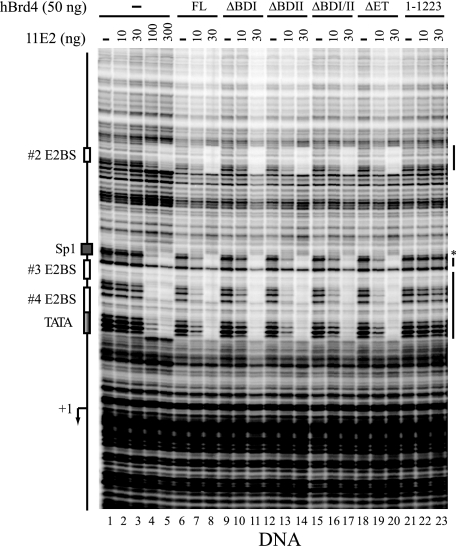
The CTM, but Not Bromodomains, Is Essential for Brd4-stabilized E2 Binding to Its Cognate Sequences in DNA—To examine whether Brd4 enhancement of E2 binding is unique to the chromatin template, we performed a similar footprinting analysis with the same DNA template without assembled nucleosomes. As shown in Fig. 3, E2 alone exhibited sequence-specific binding to DNA only at higher concentrations (lanes 1–5), indicating an inherently weak DNA binding activity of E2. Nevertheless, E2 binds more efficiently to DNA rather than chromatin, as a clear protection of E2BSs could be detected by 100 ng of E2 with the DNA but not with the chromatin template (compare Fig. 3, lane 4 with Fig. 2, lane 3). Somewhat surprisingly, we also observed enhanced E2 footprinting in the presence of Brd4 with the DNA template (lane 8 versus lane 3), suggesting that Brd4-enhanced E2 binding to its cognate sequences is not unique to the chromatin environment. Perhaps Brd4 association with the N-terminal domain of E2 induces a conformational change that allosterically enhances the C-terminal DNA binding activity of E2, consistent with the observation that the N-terminal domain of 18E2 negatively regulates its C-terminal DNA binding activity (6). Because Brd4 could stimulate site-specific binding of E2 to DNA, we further analyzed the domain requirement of Brd4 for E2 binding. Interestingly, we found that although the E2-interacting CTM was absolutely essential for this stimulation (lanes 22 and 23), the bromodomains were not needed at all for Brd4-enhanced E2 binding to DNA (lanes 11, 14, and 17). This suggests that bromodomains are indeed evolved for chromatin targeting by Brd4. As observed with the chromatin template, the ET domain was also dispensable for this protein-DNA interaction (lane 20).
FIGURE 3.
Bromodomains are dispensable for Brd4-enhanced E2 binding to HPV DNA. DNase I footprinting was performed as described under “Experimental Procedures.” The markings are the same as outlined in the legend to Fig. 2.
Brd4 Promotes E2-mediated Repression of HPV Chromatin Transcription by Different Types of E2 Proteins—The corepressor function of Brd4 was illustrated previously with 11E2 encoded by a benign HPV that causes genital warts (1). It is important to demonstrate whether this corepressor activity of Brd4 could likewise enhance transrepression of E2 encoded by oncogenic HPVs, such as 16E2 and 18E2, as well as by animal papillomaviruses, particularly BE2. To this end, we conducted in vitro transcription using an AP-1-dependent chromatin transcription system in which recombinant dimeric c-Jun/c-Fos was first incubated with HPV-11 chromatin, along with p300 histone acetyltransferase and acetyl-CoA, with or without E2 and Brd4. HeLa nuclear extract, which provides all the cellular components necessary for chromatin-dependent transcription, and a DNA control template containing the adenovirus major late core promoter (pMLΔ53) were then included, followed by the addition of [α-32P]CTP and other ribonucleoside triphosphates to initiate transcription. Transcripts of ∼380 and 280 nucleotides derived respectively from HPV chromatin and the internal control template were separated by polyacrylamide gel electrophoresis and quantified by PhosphorImager. As shown in Fig. 4A, dose-dependent repression of AP-1-stimulated HPV chromatin transcription by BE2 (lanes 3–5 versus lane 2), 16E2 (lanes 12–14 versus lane 11), and 18E2 (lanes 21–23 versus lane 20) was further enhanced by the presence of Brd4 (compare lanes 7–9 and 3–5, lanes 16–18 and 12–14, and lanes 25–27 and 21–23). This chromatin transcription experiment had been repeated several times with similar results. A line graph averaged from two representative experiments showing Brd4-enhanced repression of AP-1-dependent chromatin transcription by BE2, 16E2, and 18E2 was presented in Fig. 4B.
FIGURE 4.
Brd4 enhances repression of HPV chromatin transcription by different types of E2 proteins. A, inhibition of AP-1-dependent chromatin transcription. In vitro transcription was performed as described under “Experimental Procedures” with HPV chromatin and an internal control DNA template (pMLΔ53), in the absence (–) or presence (+) of AP-1, hBrd4, and different types and amounts of E2 proteins as indicated. The signals were quantified by PhosphorImager analysis. B, graph presentation of transcription signals. The numbers were averaged from two independent experiments with error bars indicating the standard deviation. Rel Txn, relative transcription.
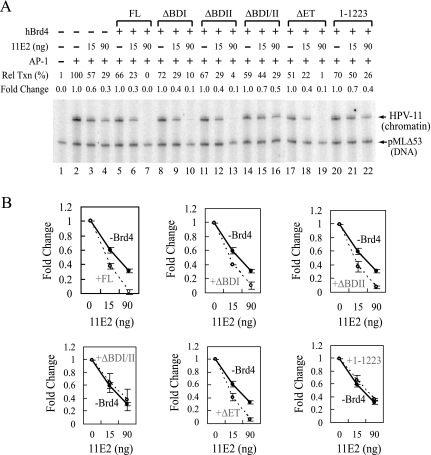
To define whether the requirement of a bromodomain and the E2-interacting CTM for enhanced E2 binding to chromatin reflects a functional role of Brd4 for E2-mediated inhibition of HPV chromatin transcription, we analyzed by in vitro chromatin transcription experiments different domain-specific deletion mutants of Brd4. As shown in Fig. 5A, Brd4 mutants deficient in both bromodomains (lanes 14–16) or the CTM (lanes 20–22) failed to support E2-mediated inhibition of HPV chromatin transcription (lane 7), whereas removal of the ET domain (lanes 17–19) or only one bromodomain (lanes 8–10 and 11–13) had no effect on E2 repressing activity, thus providing a functional verification of the involvement of the bromodomain and the CTM for E2 targeting to HPV chromatin as revealed by the chromatin footprinting assay (Fig. 2). A line graph averaged from two representative experiments was also presented in Fig. 5B.
FIGURE 5.
Brd4 enhances repression of HPV chromatin transcription in a bromodomain- and E2-dependent manner. A, at least a bromodomain and the E2-interacting region are necessary for Brd4-mediated E2 repression. In vitro transcription was the same as described in Fig. 4A, except that 50 ng of full-length hBrd4 (or a domain-specific deletion mutant) and 15 or 90 ng of 11E2 were used in the assay. Fold change is defined under “Experimental Procedures.” B, graph presentation of transcription signals. The numbers were averaged from two independent experiments with error bars indicating the standard deviation. Rel Txn, relative transcription.
A Bromodomain and the CTM Are Both Essential for Brd4-enhanced Repression by Different Types of E2 Proteins in Living Cells— Because Brd4 could enhance E2-mediated repression by different types of E2 proteins in chromatin-dependent transcription assays in vitro, we further examined whether the corepressor function of Brd4 could be detected in vivo and whether the bromodomain and the E2-interacting CTM of Brd4 are generally required for transrepression by different types of E2 proteins. To address this, we conducted an E2 repression assay by transfecting an HPV-11 upstream regulatory region-driven luciferase reporter (Fig. 6A), together with an expression plasmid for different types of E2 and either wild-type or a domain-specific deletion mutant of Brd4, into a human cervical carcinoma-derived C-33A cell line (#1–13) that harbors a stably integrated Brd4 shRNA-expressing cassette. An approximate 50-fold reduction of Brd4 protein was observed in #1–13 cells (Fig. 6B, lanes 1 and 2), in which the reduced level of Brd4 could be efficiently restored by exogenous Brd4 expression to a level slightly (∼70%) higher than the endogenous protein (lane 1 versus lane 3). Under this experimental condition, we compared different mutants of Brd4 with the wild-type protein for their ability to support E2-mediated inhibition of E6 promoter activity. As shown in Fig. 6C, an effective inhibition of E6 promoter activity by BE2, 11E2, 16E2, and 18E2 was observed when full-length, ΔET, ΔBDI, or ΔBDII, but not ΔBDI/II and CTM-deleted, Brd4 was cotransfected into #1–13 cells. This result indicates that Brd4 is indeed a general corepressor for different types of E2 proteins and, furthermore, a bromodomain and the CTM are both crucial for Brd4-mediated E2 repression of HPV E6 promoter activity. A slightly reduced repression was reproducibly seen with ΔBDI, ΔBDII, and ΔET for unknown reasons. When the levels of different Brd4 and E2 proteins were monitored in transfected cells, we found that although the amounts of various Brd4 mutants and E2 proteins remained mostly comparable in these assays, the expression levels of different types of E2 proteins appeared to be reduced when Brd4(1–1223) was coexpressed with E2 in the Brd4 knockdown cells (Fig. 6C, lanes 6, 12, 18, and 24). Clearly, the bromodomain and the CTM of Brd4 are both important for E2-inhibited HPV transcription, even though the protein stability of E2 is also influenced by its ability to associate with the CTM of Brd4 (see below).
Brd4 Also Functions as a Transcriptional Coactivator for Different Types of E2 Proteins with the Same Bromodomain and CTM Requirements as in Transrepression—Because most of the transcription cofactors display a dual role in gene activation and repression (33) and circumstantial evidence has implicated Brd4 in E2 transactivation (see Introduction), we were prompted to explore the coactivator function of Brd4 in E2-mediated activation. With the experimental system described above for the study of E2 transrepression, we performed a similar complementation experiment in Brd4 knockdown C-33A cells using a reporter construct containing multimerized E2BSs that are situated at the promoter-distal region (Fig. 7A). This enhancer configuration of E2BSs allows BE2, 11E2, 16E2, and 18E2 to activate reporter gene activity, again in a bromodomain- and CTM-dependent manner (Fig. 7B). Clearly, Brd4 is capable of stimulating E2-dependent activation by different types of E2 proteins, and this coactivating activity of Brd4 also depends on its ability to interact with E2 via the CTM and its ability to target chromatin via one of its bromodomains.
FIGURE 7.
Brd4 enhances activation by different types of E2 in a bromodomain- and CTM-dependent manner. A, schematic representation of the p2x2xE2BS-luc reporter. B, a bromodomain and the CTM are both necessary for Brd4-enhanced E2 transactivation. Transfection was performed in #1–13 cells with the p2x2xE2BS-luc reporter, an E2 expression plasmid, and a FL or domain-deleted Brd4 expression plasmid as described under “Experimental Procedures.” The bar graphs represent two independent experiments performed in duplicate with error bars indicating the standard deviations. Student's t test was used for statistic analysis by comparing fold activation between each mutant and the FL protein.
Brd4 Stabilizes E2 Protein via Direct Protein-protein Interaction—Because E2 is a labile protein with an estimated half-life of ∼1 h and appears to be ubiquitinated at its N-terminal domain (34, 35), we wondered whether Brd4 association with the N-terminal domain of E2 may stabilize E2 protein by preventing proteasome-mediated degradation and thus accounting for the reduction of E2 in CTM-deleted cotransfection experiments (Fig. 6C). To explore this, we analyzed the expression levels of low risk 11E2 and high risk 16E2 in Brd4 knockdown C-33A cells, with or without coexpressed wild-type or CTM-deleted Brd4. As shown in Fig. 8A, 11E2 and 16E2 were undetectable in Brd4 knockdown cells (lanes 2 and 6). Interestingly, coexpression of wild-type Brd4 stabilized E2 expression to a level comparable with the addition of the proteasome inhibitor MG132 (Fig. 8A, compare lanes 2–4 with lane 10 and lanes 6–8 with lane 12). Deletion of the CTM failed to provide the stabilizing effect on 11E2 and 16E2 (Fig. 8A, compare lanes 4 and 5 and lanes 8 and 9), suggesting that direct protein-protein interaction is essential for Brd4 to stabilize E2 protein in the cell. Additional transfection of an HPV-11 upstream regulatory region-driven reporter plasmid or other DNA templates together with E2 and Brd4 gave the same results as shown in Fig. 8A (data not shown), indicating that the Brd4-stabilized E2 effect is not due to conformational changes induced by E2 binding to DNA. This E2-stabilizing effect is unique to the CTM, because coexpression of other domain-specific deletion mutants of Brd4, which all retain the ability to interact with E2, failed to destabilize 11E2 (Fig. 8B) or 16E2 (Fig. 8C). We thus conclude that direct association with Brd4 is critical for E2 stabilization in the cell.
DISCUSSION
In this report, we dissected the mechanisms by which chromatin adaptor Brd4 regulates E2-dependent transcription using in vitro reconstituted chromatin and DNA templates for DNase I footprinting analysis and also carrying out chromatin-dependent transcription as well as functional complementation assays with wild-type and domain-specific deletion mutants of Brd4 in stable Brd4 knockdown human C-33A cells. We uncovered several important aspects of Brd4 functions. First, Brd4 enhances E2 binding to both chromatin and DNA templates in a site-specific and E2-dependent manner. Without E2 association, Brd4 binds chromatin/DNA in a transient and more dynamic manner, indicating that cooperative interactions between a sequence-specific transcription factor and a chromatin adaptor are indeed critical for Brd4-regulated transcription as predicted in our previous model (17). Second, whereas bromodomains are crucial for Brd4-enhanced E2 binding to chromatin, they (i.e. BDI and BDII) are dispensable for Brd4-facilitated E2 binding to DNA. This finding suggests that BDI and BDII are evolved for chromatin targeting, and the enhanced sequence-specific binding of E2 is potentially caused by Brd4-induced conformational changes, perhaps via its interaction with the N-terminal domain of E2 that allosterically regulates E2 C-terminal DNA binding activity. Third, Brd4 is an authentic transcription cofactor playing a dual role in E2-mediated activation and repression, depending on the sequence context of E2BSs, similar to the regulatory properties of general transcription cofactors, such as TFIID, Mediator, p300, and upstream stimulatory activity (USA)-derived positive and negative cofactors (33). Fourth, Brd4 serves as a universal transcription cofactor for different types of E2 proteins encoded by high risk and low risk HPVs as well as by animal papillomaviruses, such as BPV-1. Fifth, Brd4 is an important cellular factor stabilizing E2 proteins in the cell, because the normally undetectable level of E2 proteins observed in Brd4 knockdown cells could only be stabilized by wild-type Brd4, but not by a Brd4 mutant unable to interact with E2. It is noted that the growth rate of our human Brd4 knockdown cells slows down with a generation time nearly doubling depending on the extent of knockdown during passage. This is consistent with a recent report indicating that murine Brd4 is critical for G1 progression in both mouse NIH3T3 cells and mouse embryonic fibroblasts (20).
The ability of Brd4 to stabilize E2 proteins in the cell provides an additional tier of regulation for E2-dependent activation and E2-dependent repression. Under normal circumstances, the effect of Brd4-stabilized E2 may not be observed because of the high amount of endogenous Brd4 present in regular cells. The use of Brd4 knockdown cells, in combination with the functional rescue by exogenously expressed Brd4 proteins, allows us to uncover the role of Brd4 in E2 stabilization. Conceivably, the protein half-life of 18E2 and BE2, previously estimated each to be ∼1 h and degraded through the ubiquitin-dependent proteasome pathway, will be influenced by the levels of Brd4 in the cell, because no E2 proteins are detectable in Brd4 knockdown cells (Fig. 8A). It is likely that Brd4 stabilizes E2 by blocking ubiquitination at the E2 N terminus, preventing proteasome binding, or inhibiting phosphorylation-regulated E2 stability occurring through the hinge region. This is an area requiring further investigation in the future.
It is also important to mention that, although protein stability may partly account for reduced E2 activation and repression by the CTM-deleted Brd4 mutant (Figs. 6 and 7), chromatin targeting mediated by at least one of the Brd4 bromodomains is essential for both E2-dependent transcription and E2 promoter occupancy (1). The dependence of the CTM for E2 transcription activity was observed in low amounts of E2 expression, in which E2 instability was readily detected (Fig. 6), and also in high amounts of E2 expression, when the levels of E2 remained undiminished with coexpressed CTM-deleted Brd4 (1). Thus, the reduced repression observed in cotransfected CTM-deleted Brd4 could not be entirely attributed to a declined level of E2 expression in Brd4 knockdown cells. Evidently, multiple mechanisms are employed by Brd4 to regulate E2-dependent transcription, including promoter-specific chromatin targeting, enhanced E2 binding to chromatin and DNA, and protein stabilization. Another point to note is that the effect of Brd4 on E2 transactivation and transrepression is not unique to viral transcription factors, because Brd4 is able to interact with multiple cellular transcription components involved in gene regulation, such as P-TEFb and Mediator (reviewed in Ref. 17). A recent report identifying P-TEFb as another CTM-binding factor (32), besides papillomaviral E2 proteins, raises a concern regarding data interpretation based mainly on the use of CTM-containing domains for deducing the function of Brd4 in E2-regulated biological processes. The multifunctional effects of Brd4 have undoubtedly presented a challenge for properly deciphering the molecular mechanisms underlying E2-regulated transcription.
Acknowledgments
We thank Dr. Peter M. Howley for providing pcDNA4C-Brd4-FL and p2×2xE2BS-luc reporter constructs. We are also grateful to Dr. Shwu-Yuan Wu for many insightful discussions and critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants CA103867 and CA124760. This work is report CSCN 041 from the University of Texas Southwestern Medical Center Simmons Comprehensive Cancer Center. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HPV, human papillomavirus; Brd4, bromodomain-containing protein 4; BPV, bovine papillomavirus; E2BS, E2-binding site; CTM, C-terminal motif; h, human; FL, full-length; ET, extra-terminal.
References
- 1.Wu, S.-Y., Lee, A.-Y., Hou, S. Y., Kemper, J. K., Erdjument-Bromage, H., Tempst, P., and Chiang, C.-M. (2006) Genes Dev. 20 2383–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou, S. Y., Wu, S.-Y., and Chiang, C.-M. (2002) J. Biol. Chem. 277 45619–45629 [DOI] [PubMed] [Google Scholar]
- 3.Dostatni, N., Lambert, P. F., Sousa, R., Ham, J., Howley, P. M., and Yaniv, M. (1991) Genes Dev. 5 1657–1671 [DOI] [PubMed] [Google Scholar]
- 4.Dong, G., Broker, T. R., and Chow, L. T. (1994) J. Virol. 68 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan, S.-H., Leong, L. E.-C., Walker, P. A., and Bernard, H.-U. (1994) J. Virol. 68 6411–6420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demeret, C., Desaintes, C., Yaniv, M., and Thierry, F. (1997) J. Virol. 71 9343–9349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou, S. Y., Wu, S.-Y., Zhou, T., Thomas, M. C., and Chiang, C.-M. (2000) Mol. Cell. Biol. 20 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirochika, H., Hirochika, R., Broker, T. R., and Chow, L. T. (1988) Genes Dev. 2 54–67 [DOI] [PubMed] [Google Scholar]
- 9.Li, R., Knight, J., Bream, G., Stenlund, A., and Botchan, M. (1989) Genes Dev. 3 510–526 [DOI] [PubMed] [Google Scholar]
- 10.Thierry, F., Dostatni, N., Arnos, F., and Yaniv, M. (1990) Mol. Cell. Biol. 10 4431–4437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You, J., Croyle, J. L., Nishimura, A., Ozato, K., and Howley, P. M. (2004) Cell 117 349–360 [DOI] [PubMed] [Google Scholar]
- 12.Dao, L. D., Duffy, A., Van Tine, B. A., Wu, S.-Y., Chiang, C.-M., Broker, T. R., and Chow, L. T. (2006) J. Virol. 80 4792–4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbate, E. A., Voitenleitner, C., and Botchan, M. R. (2006) Mol. Cell 24 877–889 [DOI] [PubMed] [Google Scholar]
- 14.McPhillips, M. G., Oliveira, J. G., Spindler, J. E., Mitra, R., and McBride, A. A. (2006) J. Virol. 80 9530–9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilves, I., Maemets, K., Silla, T., Janikson, K., and Ustav, M. (2006) J. Virol. 80 3660–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schweiger, M. R., You, J., and Howley, P. M. (2006) J. Virol. 80 4276–4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu, S.-Y., and Chiang, C.-M. (2007) J. Biol. Chem. 282 13141–13145 [DOI] [PubMed] [Google Scholar]
- 18.Sénéchal, H., Poirier, G. G., Coulombe, B., Laimins, L. A., and Archambault, J. (2007) Virology 358 10–17 [DOI] [PubMed] [Google Scholar]
- 19.Parish, J. L., Bean, A. M., Park, R. B., and Androphy, E. J. (2006) Mol. Cell 24 867–876 [DOI] [PubMed] [Google Scholar]
- 20.Mochizuki, K., Nishiyama, A., Jang, M. K., Dey, A., Ghosh, A., Tamura, T., Natsume, H., Yao, H., and Ozato, K. (2008) J. Biol. Chem. 283 9040–9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawford, N. P. S., Alsarraj, J., Lukes, L., Walker, R. C., Officewala, J. S., Yang, H. H., Lee, M. P., Ozato, K., and Hunter, K. W. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 6380–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French, C. A., Ramirez, C. L., Kolmakova, J., Hickman, T. T., Cameron, M. J., Thyne, M. E., Kutok, J. L., Toretsky, J. A., Tadavarthy, A. K., Kees, U. R., Fletcher, J. A., and Aster, J. C. (2008) Oncogene 27 2237–2242 [DOI] [PubMed] [Google Scholar]
- 23.Jang, M. K., Mochizuki, K., Zhou, M., Jeong, H.-S., Brady, J. N., and Ozato, K. (2005) Mol. Cell 19 523–534 [DOI] [PubMed] [Google Scholar]
- 24.Yang, Z., Yik, J. H. N., Chen, R., He, N., Jang, M. K., Ozato, K., and Zhou, Q. (2005) Mol. Cell 19 535–545 [DOI] [PubMed] [Google Scholar]
- 25.Chiang, C.-M., Ge, H., Wang, Z., Hoffmann, A., and Roeder, R. G. (1993) EMBO J. 12 2749–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiang, C.-M., and Roeder, R. G. (1993) Peptide Res. 6 62–64 [PubMed] [Google Scholar]
- 27.Wu, S.-Y., Thomas, M. C., Hou, S. Y., Likhite, V., and Chiang, C.-M. (1999) J. Biol. Chem. 274 23480–23490 [DOI] [PubMed] [Google Scholar]
- 28.Wang, W.-M., Lee, A.-Y., and Chiang, C.-M. (2008) Protein Expr. Purif. 59 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas, M. C., and Chiang, C.-M. (2005) Mol. Cell 17 251–264 [DOI] [PubMed] [Google Scholar]
- 30.Wu, S.-Y., Zhou, T., and Chiang, C.-M. (2003) Mol. Cell. Biol. 23, 6229–6242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovelman, R., Bilter, G. K., Glezer, E., Tsou, A. Y., and Barbosa, M. S. (1996) J. Virol. 70 7549–7560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisgrove, D. A., Mahmoudi, T., Henklein, P., and Verdin, E. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 13690–13695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, M. C., and Chiang, C.-M. (2006) Crit. Rev. Biochem. Mol. Biol. 41 105–178 [DOI] [PubMed] [Google Scholar]
- 34.Bellanger, S., Demeret, C., Goyat, S., and Thierry, F. (2001) J. Virol. 75 7244–7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penrose, K. J., Garcia-Alai, M., de Prat-Gay, G., and McBride, A. A. (2004) J. Biol. Chem. 279 22430–22439 [DOI] [PubMed] [Google Scholar]