Abstract
Chondrocyte fate determination and maintenance requires Sox9, an intrinsic transcription factor, but is inhibited by Wnt/β-catenin signaling activated by extrinsic Wnt ligands. Here we explored the underlying molecular mechanism by which Sox9 antagonizes the Wnt/β-catenin signaling in chondrocyte differentiation. We found that Sox9 employed two distinct mechanisms to inhibit Wnt/β-catenin signaling: the Sox9 N terminus is necessary and sufficient to promote β-catenin degradation, whereas the C terminus is required to inhibit β-catenin transcriptional activity without affecting its stability. Sox9 binds to β-catenin and components of the β-catenin “destruction complex,” glycogen synthase kinase 3 and β-transducin repeat containing protein, to promote their nuclear localization. Independent of its DNA binding ability, nuclear localization of Sox9 is both necessary and sufficient to enhance β-catenin phosphorylation and its subsequent degradation. Thus, one mechanism whereby Sox9 regulates chondrogenesis is to promote efficient β-catenin phosphorylation in the nucleus. This mechanism may be broadly employed by other intrinsic cell fate determining transcription factors to promptly turn off extrinsic inhibitory Wnt signaling mediated by β-catenin.
Differentiation of chondrocytes from mesenchymal progenitors is an early critical event in endochondral ossification, a major bone-forming process in vertebrate embryos (1). Chondrocyte fate determination and maintenance are regulated by both intrinsic and extrinsic factors such as Sox9 and Wnt/β-catenin signaling, respectively. Sox9 is an SRY-box (Sox) containing gene required for chondrocyte differentiation (3, 4). Heterozygous SOX9 mutations cause the human disease campomelic dysplasia (CD),2 a form of dwarfism characterized by extreme cartilage and bone malformation and sex reversal (5, 6). The Wnt/β-catenin pathway, which plays a critical role in regulating many cell proliferation and fate determination processes in embryonic development and oncogenesis (reviewed in Refs. 7 and 8), potently inhibits chondrocyte differentiation and Sox9 expression during skeletal development (9, 10). Because Wnt ligand expression is detected around the chondrogenic mesenchymal condensation, but Wnt signaling activity is dramatically down-regulated in the differentiating chondrocytes in which Sox9 is expressed (11), it is likely that one mechanism by which Sox9 promotes chondrocyte differentiation and maintains chondrocyte characters is to inhibit the antichondrogenic Wnt/β-catenin signaling activity. However, the molecular mechanism by which Sox9 promotes β-catenin degradation is still poorly understood.
In the Wnt/β-catenin pathway, β-catenin protein degradation is regulated by Wnt signaling, which controls β-catenin phosphorylation by casein kinase Iα (CKIα) and glycogen synthase kinase 3 (GSK3) in the destruction complex assembled by Axin (7). In the absence of Wnt signals, β-catenin is phosphorylated in the destruction complex and then degraded in proteosomes. Activation of Wnt signaling leads to inhibition of GSK3-mediated β-catenin phosphorylation and thus β-catenin is stabilized. According to the current model, β-catenin degradation occurs in the cytoplasm. β-Catenin, stabilized by Wnt signaling, then enters the nucleus where it activates downstream gene expression by binding to LEF/TCF transcription factors.
Sox9 is a transcription factor that contains three highly conserved domains: the high mobility group (HMG) domain that binds and bends DNA in a sequence specific manner (12-14), the C-terminal PQS transactivation domain and the PQA domain, both required for maximum transcriptional activity of Sox9 (15, 16). Sox9 has two nuclear localization signals (NLS) and a nuclear export signal (NES) located in the HMG domain (17, 18). Sox9 protein is localized either exclusively in the nucleus (i.e. differentiated chondrocytes) or in both nucleus and cytoplasm (i.e. early differentiating chondrocytes). In addition to cartilage, Sox9 is expressed in other tissues such as neural crests (19, 20), pancreatic progenitors (21, 22), and intestinal epithelial cells (23). It has been reported that in the developing cartilage and intestinal epithelium, Sox9 inhibits the Wnt/β-catenin signaling activities (24, 25). Sox9 is also expressed in the gonad where it is required for male sex determination (26). Like what has been observed in Sox9 loss of function mutants, activation of β-catenin in otherwise normal XY mice effectively disrupts the male program and results in male-to-female sex reversal (27).
Here we demonstrate that Sox9 inhibits Wnt signaling by two distinct mechanisms, which requires different domains of Sox9. We showed that the N terminus of Sox9 including the HMG domain promoted β-catenin degradation, whereas the C-terminal transactivation domain inhibited the transcriptional activity of β-catenin, but it was not required for β-catenin degradation. In addition, we found that Sox9 bound to components of the β-catenin destruction complex and relocated them into the nucleus. Sox9 nuclear localization, not the HMG domain per se, was both necessary and sufficient to induce nuclear localization of the β-catenin destruction complex components and enhance β-catenin phosphorylation for its subsequent degradation. Furthermore, our results suggest that more efficient β-catenin phosphorylation and degradation in the nucleus may be a general mechanism whereby transcription factors effectively antagonizing inhibitory Wnt/β-catenin signaling in cell fate determination and maintenance.
EXPERIMENTAL PROCEDURES
Plasmids, Viruses, and Antibodies—Mouse Sox9 cDNA (provided by Dr. Yoshi Yamada) was tagged with FLAG and subcloned into modified pIRES-hrGFP-1 (pIRES), expression vector (Stratagene) where GFP was removed. The Sox9 mutants were generated by standard PCR-based mutagenesis procedures (Stratagene) and subcloned into modified pIRES-hrGFP-1 vector in-frame with the FLAG tag. To generate NLS fusion, the NLS 5′-PKKKRKVE-3′ from the SV40 T antigen (28) were added to the C-terminal end of the Sox9 mutant proteins. The coding region of rat ck1α and human β-CATENIN were HA-tagged and subcloned into pHM6 (2). The wild type human β-CATENIN was Myc-tagged and inserted into the pcDNA-3A (Invitrogen) expression vector. ΔNβ-CATENIN, provided by Dr.F. McCormick (University of California, San Francisco, CA) was subcloned into the pIRES vector. The Sox9, Wnt14, GFP, and CRE-adenovirus were generated according to the manufacturer's instructions (Clontech) and concentrated viral stocks were produced by SAIC (SAIC, Frederick, MD). β-Catenin was fused in-frame to the GST sequences of the pGEX-4T1 bacterial vector (Amersham Bioscience). Recombinant GST-tagged β-catenin was expressed in BL21 Escherichiacoli cells and purified using glutathione-Sepharose columns (Amersham Biosciences). Anti-Sox9 (H-90, Santa Cruz), anti-Axin (Zymed Laboratories Inc.), anti-β-catenin (C19220, BD Transduction Laboratories), anti-tubulin (ICN), anti-GSK-3β (Cell signaling and Abcam), anti-FLAG (M2, Sigma), anti-phospho-specific-β-catenin (Thr41/Ser45 and Ser33/37) (Cell Signaling), anti-HA (cl3F10, Roche Applied Science), anti-Myc tag (9B11, Cell Signaling), anti-TCF4 (6H5-3, Upstate), anti-actin (Sigma), and anti-LRP5 (Santa Cruz) antibodies were used in Western blots, IP, or immunohistochemistry.
Cell Culture, Transfection, Adenoviral Infection, and Luciferase Assay—CHO, HEK293, COS-1, NCI-H28, SW48, SW480, SNU475, and L-cells were obtained from American Type Culture Collection. Primary mouse chondrocytes were prepared according to a previously published protocol (29). Cells were seeded the day before transfection and transfected with the indicated plasmids using Lipofectamine PLUS as specified by the manufacturer (Invitrogen). In some cases, transfection was performed using nucleofection technology (Amaxa GmbH). For luciferase assays, cells were transfected with Super-TOPFLASH or FOPFLASH plasmids, Renilla Luciferase plasmid (phRL-null, Promega), and the indicated plasmids. The total amount of transfected DNA was kept constant by adding empty vector DNA. Luciferase activity was measured according to the Dual Luciferase Reporter Assay System (Promega). The results are shown as relative luciferase activity. The histograms are presented as the average ± S.D. from three independent transfections. All measurements were made using Lumat LB9507 Luminometer (EG&G Berthold). Adenoviral infection of cells was performed at 1 × 109 plaque-forming units/ml for 3-4 h, and the results were analyzed 24-72 h later.
Co-immunoprecipitation and Immunoblotting Assay—In co-immunoprecipitation assays, cells were lysed in lysis buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% Triton X-100, and protease inhibitors (Calbiochem), and Halt™ phosphatase inhibitor mixture (Pierce)). Equal amounts of cell lysates were incubated with the indicated antibodies for 2 h and Protein-G Plus (Santa Cruz) was added and incubated for overnight at 4 °C. Immunoprecipitates were washed four times in lysis buffer, resolved by NuPAGE, and subjected to standard Western immunoblot analysis with the specific antibodies.
Protein Binding Assay—In vitro pull-down assays were performed as described (30). Briefly, in β-catenin binding experiments, HEK293 or COS cells were transfected with the indicated constructs and 24 h after transfection, cells were lysed with lysis buffer (50 mm Tris-HCl (pH 7.5), 300 mm NaCl, 0.5% Nonidet P-40 and protease inhibitors). Equal amounts of lysates were incubated for 3 h at 4 °C, with 20 μl (about 5 μg) of either purified GST or GST-β-catenin bound to agarose beads in lysis buffer adjusted to 20% glycerol and 1 mm dithiothreitol. Agarose beads were washed five times with lysis buffer containing 500 mm NaCl and bound proteins were eluted in LDS sample buffer (Invitrogen) and detected by Western blot.
Indirect Immunofluorescence—Cells were plated in four chambers slides (Lab-Tek), transfected with the indicated plasmids, and fixed with methanol or 3.7% formaldehyde, permeabilized with 0.25% Triton X-100 and processed for indirect immunofluorescence.
Treatment with Inhibitors—To block protein degradation, cells were treated with MG132 (Sigma) for 8 h before lysis. LiCl (Sigma) and 6-bromoindirubin-3′-oxime (BIO, Calbiochem) were used to inhibit GSK3β at 40 mm and 5 μm, respectively.
Micromass Cultures—Micromass cultures were prepared according to a procedure described previously (9). Briefly, the Sox9- or Wnt14-adenoviruses were added to the limb mesenchymal cell suspension (∼2.5 × 106 cells/ml) at 5 × 109 plaque-forming units/ml, and the cell suspension was gently rocked at 4 °C for 2 h. Ascorbic acid was added to induce chondrocyte differentiation 48 h after plating the cells. Cells were fixed and stained with Alcian blue 48 h after ascorbic acid treatment.
Immunoprecipitation and CKIα and GSK3β Immune Complex Kinase Assay—Transfected cells were fractionated as described (31). To assess Axin-associated activity of GSK3β or CKIα, protein complex containing Axin-Myc was immunoprecipitated using an anti-Myc antibody, and the kinase activity of the complex was assayed (32, 33). CKIα kinase reaction was carried out for 10 min at 37 °C in a 40-μl volume of kinase buffer (50 mm HEPES (pH 7.5), 10 mm MgCl2, 20 mm β-glycerophosphate, 5 mm NaF) containing the immunocomplex on agarose beads, supplemented with 100 μg/ml myelin basic protein (Sigma) and 10 μCi of [γ-32P]ATP (Amersham Biosciences). Supernatant was separated by NuPAGE and the gel was vacuum-dried and exposed to a film. The activity of GSK3β was measured as described (34) with some modification. Briefly, immunocomplex on agarose beads was incubated in 40 μl of kinase buffer (20 mm Tris (pH 7.5), 5 mm MgCl2, 1 mm dithiothreitol, 250 μm ATP, 10 μCi of [γ-32P]ATP), with phosphoglycogen synthase peptide-2 (62.5 mm) (Upstate Biothechnology) for 30 min at 30 °C.
Reverse Transcription-PCR—Total RNA was extracted from mouse primary chondrocytes using the TRIzol reagent (Invitrogen) and treated with RNase-free DNase (Roche). cDNA was synthesized with oligo(dT) primers. Semi-quantitative analysis was performed using 2100 Bioanalyzer (Agilent). The primers used in this experiment were: mouse Sox9 forward, 5′-CCA CGG AAC AGA CTC ACA TCT CTC-3′ and mouse Sox9 reverse, 5′-CTG CTC AGT TCA CCG ATG TCC ACG-3′; mouse glyceraldehyde-3-phosphate dehydrogenase forward, 5′-ACC ACA GTC CAT GCC ATC AC-3′ and mouse glyceraldehyde-3-phosphate dehydrogenase reverse, 5′-TCC ACC ACC CTG TTG CTG TA-3′.
Confocal Image Acquiring—Confocal images were acquired at room temperature using either a Zeiss LSM 510 NLO Meta system mounted on a Zeiss Axiovert 200M microscope or on a Zeiss LSM 510 UV system mounted on a Zeiss Axiovert 100M microscope (Carl Zeiss Inc., Thornwood, NY) both using an oil immersion Plan-Apochromat ×63/1.4 DIC objective lens. Excitation wavelengths of 488, 561 (3), or 543 nm (UV), 633, and 740 nm (3), or 364 nm (UV) were used for detection of secondary antibodies conjugated by Alexa Fluor 488, Rhodamine, Alexa Fluor 633 (Invitrogen), and DAPI (Vector), respectively. Fluorescent emissions were collected in band-pass filters 500-550, 575-615, and 641-705 nm collected in the Meta detector and 390-465 nm, respectively (UV system filters were comparable). All pinholes were set at 1.00 Airy units, which corresponds to an optical slice of 0.8 μm (excluding the DAPI channel where a multiphoton laser was used). All confocal images were collected using the Zeiss AIM software version 4.0 sp2 (UV system version 3.2 sp2) of frame size 512 pixels by 512 pixels, scan zoom of 1 and line averaged 4 times.
RESULTS
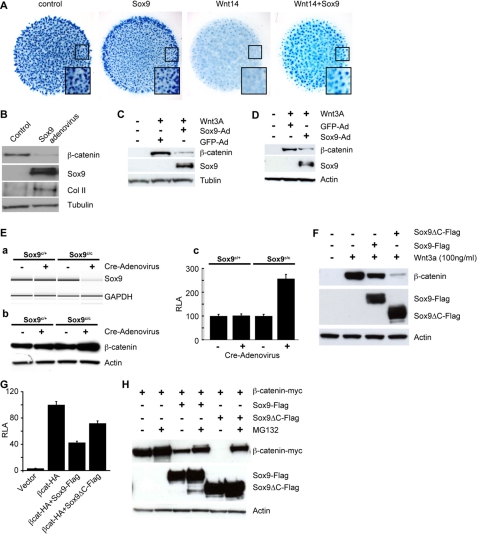
Sox9 Antagonizes Wnt/β-Catenin Signaling during Chondrocyte Differentiation by Promoting β-Catenin Degradation—We have shown previously that inactivation of Wnt/β-catenin signaling promotes chondrocyte differentiation from mesenchymal progenitors (9, 11). Because Sox9 promotes chondrocyte differentiation when ectopically expressed (35), we tested the hypothesis that one mechanism by which Sox9 promotes chondrogenesis is to antagonize Wnt/β-catenin signaling. We performed micromass cultures with the limb mesenchymal cells from 12.5 days post coitum mouse embryos. In the micromass cultures, chondrocytes differentiate from mesenchymal progenitors to form cartilage nodules, which are stained by Alcian blue. By infecting mesenchymal progenitors in the micromass cultures with Sox9- or Wnt14-adenovirus, we found that Sox9 expression increased cartilage nodule formation at the outer rim of micromass culture, whereas Wnt14, which signals through β-catenin (9), severely inhibited cartilage nodule formation (Ref. 9 and Fig. 1A). Sox9- and Wnt14-adenovirus co-infection rescued cartilage nodule formation inhibited by Wnt14 to a large extent (Fig. 1A). The weak prochondrogenic effect of Sox9 alone is consistent with a previous report, which showed that Sox9 needs other cofactor(s) to effectively enhance its transcription activity (35). When β-catenin protein levels in the micromass cultures were analyzed by Western blot, we found that enhanced cartilage nodule formation by Sox9 overexpression correlated with reduced β-catenin protein levels (Fig. 1B). In addition, Wnt3a treatment of L cells, which are fibroblast cells, resulted in elevated β-catenin protein levels, and such elevation was dampened by Sox9-adenovirus infection prior to Wnt3a treatment (Fig. 1C). In CHO cells Sox9 expression also led to reduced β-catenin accumulation promoted by Wnt3a treatment (Fig. 1D). These data indicate that Sox9 inhibits Wnt/β-catenin signaling, at least in part, by reducing β-catenin protein levels. These results also predict that β-catenin protein levels should be increased after Sox9 removal. To test this possibility primary chondrocytes from homozygous Sox9 conditional (Sox9c/c) (3) newborn mice were isolated and efficient Sox9 removal by Cre-adenovirus infection was confirmed by reverse transcriptase-polymerase chain reaction (Fig. 1E, panel a). β-Catenin protein levels were indeed increased in these Cre-induced Sox9-deficient primary chondrocytes (Fig. 1E, panel b). In addition, Wnt/β-catenin signaling activity measured by the TOPFLASH reporter (36) was also up-regulated in the Sox9-deficient primary chondrocytes (Fig. 1E, panel c). These data suggest that promoting β-catenin degradation is one of the mechanisms whereby Sox9 inhibits Wnt/β-catenin activity in primary mouse chondrocytes.
FIGURE 1.
Sox9 promotes chondrocytes differentiation by antagonizing the Wnt/β-catenin signaling. A, micromass cultures using mouse limb mesenchymal cells were infected with the indicated adenovirus and stained with Alcian blue to show cartilage nodules. The boxed areas were enlarged and shown in the insets. B, the levels of total β-catenin protein in micromass cultures were analyzed by Western blot. Total protein level was normalized using Tubulin. C, L cells were infected with Sox9- or GFP-adenovirus and then treated with Wnt3a protein (100 ng/ml) for 1 h before harvesting. β-Catenin protein levels were analyzed by Western blot. D, CHO cells were infected with Sox9-adenovirus and then treated with Wnt3a (100 ng/ml) for 1 h. Total β-catenin protein levels were analyzed as in C. Actin was used as a loading control. E, mouse primary chondrocytes isolated from the Sox9c/c mice were infected with Cre-adenovirus to remove Sox9. Reduced Sox9 expression was confirmed by semi-quantitative reverse transcriptase-PCR in A. Glyceraldehyde-3-phosphate dehydrogenase (GADPH) was used to normalize the total RNA used in the reverse transcriptase-PCR. β-Catenin protein levels were analyzed by Western blot (B). Comparison of TOPFLASH activities with and without Cre-adenovirus infection is shown in C. F, CHO cells were transiently transfected with Sox9-Flag and Sox9ΔC-Flag and 24 h later, cells were treated with Wnt3a (100 ng/ml) for 1 h, lysed, and analyzed by Western blot. G, CHO cells were transiently transfected with the indicated plasmid DNA. Luciferase activity was measured 24 h after transfection. Standard deviations (S.D.) of three independent experiments are shown. H, CHO cells were transiently transfected with the indicated plasmid DNA. 24 h later, cells were treated with MG132 (25 μm/8 h) and analyzed by Western blot. Actin was used as a loading control.
Due to the difficulty of maintaining and efficiently transfecting the primary limb mesenchymal cells or primary chondrocytes, cell lines such as CHO, COS, and HEK293 that can be easily transfected, were chosen to investigate the molecular mechanisms by which Sox9 regulates the protein levels and activities of β-catenin. In all these cell lines, Sox9 reduced β-catenin protein levels like in the primary limb mesenchymal cells, primary chondrocytes, or L cells (Fig. 1F and data not shown). To dissect the molecular mechanism by which Sox9 promotes β-catenin degradation, a series of Sox9 deletion mutants were constructed (supplemental Fig. S1). Surprisingly, both the C-terminal deleted mutant (Sox9ΔC) and full-length Sox9 reduced β-catenin protein levels and inhibited its transcriptional activity in the TOPFLASH assay (Fig. 1, F and G). The Sox9ΔC induced even more pronounced β-catenin protein reduction than the full-length one. These results were different from previously published results showing that the C-terminal part of Sox9 was required for the degradation of a mutant β-catenin that could not be phosphorylated by GSK3 (24). Furthermore, treatment with MG132, a proteosome inhibitor, sustained β-catenin levels in the presence of Sox9 or Sox9ΔC (Fig. 1H), indicating that Sox9 acts through its N terminus, not its C terminus, to promote β-catenin degradation by proteosomes. However, our results supported that the Sox9 C terminus was required to inhibit β-catenin transcription activity as reported (24). Compared with Sox9, Sox9ΔC was more potent in inducing β-catenin protein degradation (Fig. 1F), but less active in inhibiting β-catenin-mediated transcription (Fig. 1G). Thus, Sox9 inhibits Wnt/β-catenin signaling through two parallel and independent mechanisms: it uses the N terminus to promote β-catenin protein degradation and the C terminus to inhibit transcription activity of β-catenin/TCF complex.
Sox9 Promotes β-Catenin Degradation by Enhancing Its Phosphorylation—Because β-catenin phosphorylation by CKIα and GSK3 and its subsequent association with βTrCP leads to β-catenin degradation (38-46), we tested whether β-catenin degradation promoted by Sox9 requires GSK3 and βTrCP. First, we found that Sox9 expression led to increased β-catenin phosphorylation by CKIα (at Ser45) and GSK3 (at Ser33/37) (Fig. 2A). The relative amount of β-catenin phosphorylated by GSK3 and CKIα versus the total β-catenin protein levels was significantly increased in Sox9 expressing cells compared with that in the control cells (Fig. 2A). In addition, when β-catenin protein degradation was blocked by MG132 treatment, more β-catenin phosphorylation by GSK3 was also observed in the accumulated β-catenin protein in Sox9 expressing cells (Fig. 2B). We then inhibited GSK3 activity by LiCl or BIO (47-49) and found that these inhibitors significantly compromised the ability of Sox9 and Sox9ΔC to promote β-catenin degradation (Fig. 2C). Sox9 and Sox9ΔC still exhibited residual activities to promote β-catenin degradation in the presence of LiCl or BIO, possibly due to incomplete inhibition of GSK3. These results indicate that Sox9-promoted β-catenin degradation requires GSK3 activity. Second, a dominant negative mutant of βTrCP (ΔβTrCP), which interacts with phosphorylated β-catenin but is unable to form an SCFβTrCP-ubiquitin ligase complex (50), reduced Sox9-promoted β-catenin degradation (Fig. 2D, lanes 4 and 6). Third, Sox9 was unable to promote degradation of a deleted form of β-catenin (ΔNβ-catenin) (51) that could not be phosphorylated by CKIα or GSK3 (Fig. 2E). This was further supported by our observation that Sox9 was not able to promote the degradation of a stabilized mutant β-catenin that contains a Ser33 to Tyr missense mutation in the SW48 cells (supplemental Fig. S2). In addition, expression of Sox9 in a colon cancer cell line, SW480, which contains a truncated APC protein lacking the Axin binding domain (52), failed to promote β-catenin degradation (supplemental Fig. S2). Finally, expression of Sox9 in an Axin-/- cell line, SNU475 (53), failed to promote β-catenin phosphorylation by GSK3 and degradation (supplemental Fig. S2). Taken together, these results indicate that Sox9 promotes β-catenin degradation by enhancing its phosphorylation in the degradation complex assembled by Axin.
FIGURE 2.
Sox9 promotes β-catenin phosphorylation by CKIα and GSK3β. A, CHO cells were transiently transfected with β-CATENIN and Sox9. Comparable amounts of total β-catenin protein in control and Sox9-expressing cells was loaded on the gel and analyzed by Western blot using antibody against β-catenin. The membrane was then stripped and re-probed with phospho-β-catenin-specific antibodies (anti-pS45 and anti-pS33/37). B, CHO cells were transiently transfected with β-CATENIN-HA and Sox9-Flag and treated with MG132 (25 μm) for 8 h before harvesting. β-Catenin phosphorylation was compared by Western blot in equal amount of immunoprecipitated total β-catenin. C, transiently transfected CHO cells were treated with LiCl (25 mm) or BIO (1 μm) as indicated for 12 h before harvesting. The level of β-catenin protein was analyzed with anti-Myc antibody. D, CHO cells were transiently transfected with the indicated plasmids and analyzed by Western blot. Actin was used as a loading control. E, CHO cells were transiently transfected with ΔNβ-CATENIN-myc and Sox9-Flag and analyzed by Western blot. Tubulin was used as a loading control.
Nuclear Localization of Sox9 Is Required to Promote
β-Catenin Degradation—Sox9 was detected in the nucleus in
CHO cells and when coexpressed with β-catenin, it led to β-catenin
nuclear localization in addition to reduced β-catenin protein levels
(Fig. 3, A-C).
Interestingly, Sox9ΔC that lacks the C terminus shown to bind
β-catenin (24) also
promoted β-catenin nuclear localization
(Fig. 3C). However,
when co-expressed with ΔNβ-catenin, Sox9ΔC failed to
down-regulate its transcription activity, which was inhibited by Sox9 and the
Sox9ΔN (supplemental Fig. S1) that contains the intact Sox9 C terminus
shown to inhibit β-catenin transcription activity
(24)
(Fig. 3D). Moreover,
Sox9ΔC actually increased its transcriptional activity. This is because
Sox9ΔC still promoted nuclear trafficking of this stabilized
ΔNβ-catenin (Fig.
3E). These data further support our previous conclusion
(Fig. 1G) that
Sox9ΔC lacks the C terminus to inhibit the transcription activity of
β-catenin. These results also suggest that the N-terminal part of Sox9
binds β-catenin too. Indeed, we found by GST pull down assays that
β-catenin can form a complex with Sox9ΔC similarly to Sox9 or an
N-terminal-deleted Sox9 (Sox9ΔN), both have been shown to bind
β-catenin (24)
(Fig. 3F and
supplemental Fig. S1). These data further raised an interesting possibility
that Sox9 nuclear localization might be required for it to promote
β-catenin degradation. To test this hypothesis, we altered Sox9 nuclear
localization and tested the effect on β-catenin degradation. The Sox9 NLS
resides in the HMG domain
(18). Mutations of the NLS
(Sox9-NLS*) that impaired Sox9 nuclear localization
(18) significantly reduced the
ability of Sox9 to promote β-catenin nuclear localization and degradation
(Fig. 4, A and
B). Strikingly, when Sox9 nuclear localization was
restored by fusing the Sox9-NLS* C terminus with the NLS of the
SV40 large T antigen (NLS(TA)), the resulting Sox9-NLS* NLS(TA) was
localized in the nucleus and is capable of promoting β-catenin
degradation (Fig. 4, A and
B). Furthermore, we asked if the DNA binding ability of
the HMG domain, or only the nuclear localization function of HMG is required
for the activity of Sox9 to promote β-catenin degradation. We generated
two mutant forms of Sox9: the HMG-deleted form (Sox9^HMG) and the HMG-deleted
form with the NLS(TA) fused at the C terminus of Sox9
(Sox9 HMG-NLS(TA))
(supplemental Fig. S1). These two forms of Sox9 proteins are almost identical,
except that Sox9
HMG-NLS(TA))
(supplemental Fig. S1). These two forms of Sox9 proteins are almost identical,
except that Sox9 HMG was
localized in the cytoplasm, whereas
Sox9
HMG was
localized in the cytoplasm, whereas
Sox9 HMG-NLS(TA) was
localized in the nucleus (Fig.
4C), but unable to bind DNA.
Sox9
HMG-NLS(TA) was
localized in the nucleus (Fig.
4C), but unable to bind DNA.
Sox9 HMG failed to promote
β-catenin degradation (Fig.
4D), but addition of the NLS(TA) not only restored its
nuclear localization, but also its ability to promote β-catenin
degradation (Fig. 4, C and
D). In addition,
Sox9
HMG failed to promote
β-catenin degradation (Fig.
4D), but addition of the NLS(TA) not only restored its
nuclear localization, but also its ability to promote β-catenin
degradation (Fig. 4, C and
D). In addition,
Sox9 HMG-NLS(TA) exhibited
similar activities as wild type Sox9 to inhibit β-catenin transcriptional
activity indicated by the TOPFLASH luciferase assay
(Fig. 4E).
Sox9
HMG-NLS(TA) exhibited
similar activities as wild type Sox9 to inhibit β-catenin transcriptional
activity indicated by the TOPFLASH luciferase assay
(Fig. 4E).
Sox9 HMG also weakly
inhibited β-catenin transcriptional activity, possibly by sequestrating
β-catenin in the cytoplasm (Fig.
4E). Taken together, these results demonstrate that it is
Sox9 nuclear localization, rather than its ability to bind DNA, that is
required to promote β-catenin degradation.
HMG also weakly
inhibited β-catenin transcriptional activity, possibly by sequestrating
β-catenin in the cytoplasm (Fig.
4E). Taken together, these results demonstrate that it is
Sox9 nuclear localization, rather than its ability to bind DNA, that is
required to promote β-catenin degradation.
FIGURE 3.
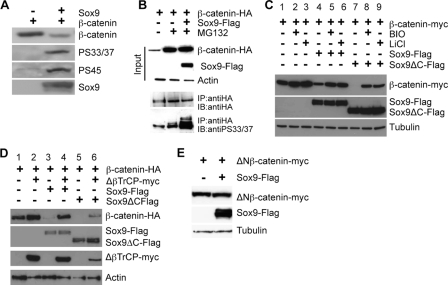
Sox9 promotes nuclear translocation of β-catenin. A-C, CHO cells were transfected with β-CATENIN-HA alone (A) or β-CATENIN-HA and Sox9-Flag (B) or β-CATENIN-HA and Sox9ΔC-Flag (C). 24 h later, subcellular localization of exogenous β-catenin (green) and Sox9 (red) were detected by anti-HA and anti-FLAG antibody, respectively. The nucleus is stained by DAPI (blue). D, CHO cells were transiently transfected with the indicated DNA constructs and TOPFLASH luciferase activities and ΔNβ-catenin protein levels were analyzed. E, CHO cells were transfected with the indicated plasmid and ΔNβ-catenin was stained with anti-Myc (green) and Sox9 was stained with anti-FLAG (red) antibodies. The nucleus is stained with DAPI (blue). F, CHO cells were transiently transfected with a vector or the indicated plasmids, lysed, and equal amounts of cell lysates were incubated with GST- or GST-coupled-β-catenin beads as described under “Experimental Procedures.” Proteins released from the beads were analyzed by Western blot. RLA, relative luciferase activity.
FIGURE 4.
Sox9 nuclear localization is both necessary and sufficient to
promote β-catenin degradation. A and B, CHO
cells were transiently transfected with β-CATENIN-HA and a
cytoplasmic mutant, Sox9-NLS*-Flag (top panel) or
a nuclear mutant, Sox9-NLS*-NLS(TA)-Flag
(bottom panel). Cells were stained with anti-HA (green) and
anti-FLAG (red) antibodies (A) or analyzed by Western blot
(B). Tubulin was used as a loading control. C and
D, CHO cells were transiently transfected with β-CATENIN-HA and
with a cytoplasmic mutant,
Sox9 HMG-Flag
(top panel), or a nuclear mutant,
Sox9
HMG-Flag
(top panel), or a nuclear mutant,
Sox9 HMG-NLS(TA)(bottom
panel). Immunofluorescent staining and Western blot analysis was
performed 24 h later as in A and B. Lanes 1 and 2
were put together with lanes 3 and 4 by deleting irrelevant
lanes in between on the same membrane. E, CHO cells were transiently
transfected with the indicated plasmids and TOPFLASH activity was measured 24
h later. IB, immunoblot.
HMG-NLS(TA)(bottom
panel). Immunofluorescent staining and Western blot analysis was
performed 24 h later as in A and B. Lanes 1 and 2
were put together with lanes 3 and 4 by deleting irrelevant
lanes in between on the same membrane. E, CHO cells were transiently
transfected with the indicated plasmids and TOPFLASH activity was measured 24
h later. IB, immunoblot.
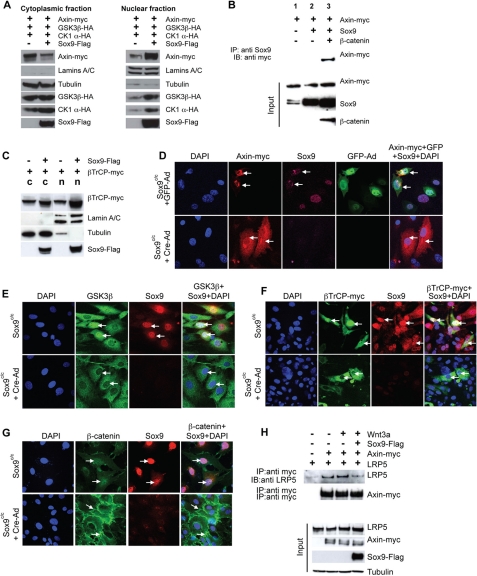
Sox9 Relocates the β-Catenin Destruction Complex to the Nucleus—Because β-catenin degradation promoted by Sox9 still depended on its phosphorylation by GSK3, which binds the scaffold protein Axin (44), it is likely that critical components of the β-catenin phosphorylation destruction complex were also relocated to the nucleus upon Sox9 expression. To test this hypothesis, we examined whether cytosolic and nuclear distribution of components in the Axin complex was altered by Sox9 expression. Sox9 expression resulted in increased nuclear Axin levels at the expense of cytosolic Axin (Fig. 5A), suggesting that Sox9 induced a translocation of Axin from the cytoplasm to the nucleus. This was further confirmed by fluorescent cytochemistry (supplemental Fig. S3A). Intriguingly, Sox9 itself did not bind Axin in CHO cells (Fig. 5B), indicating that Sox9 does not bring Axin to the nucleus through direct binding. When β-catenin was also expressed, Sox9 was associated with Axin (Fig. 5B). However, Sox9 is required to promote Axin nuclear localization. In wild type primary chondrocytes that express high levels of Sox9, Axin was detected mainly in the nucleus, whereas in the Sox9-/- chondrocytes, Axin was detected in both cytoplasm and nucleus (Fig. 5D). In cell fractionation experiments, Sox9 expression also led to increased GSK3β and CKIα levels in the nucleus (Fig. 5A). However, the cytoplasmic levels of GSK3β and CKIα were either unchanged or slightly increased (Fig. 5A). In addition, βTrCP, which binds β-catenin phosphorylated by GSK3, was distributed in both the cytoplasm and nucleus in cell fractionation experiments in CHO cells (Fig. 5C). After coexpression with Sox9, more βTrCP protein was found in the nucleus (Fig. 5C), suggesting that Sox9 also induced βTrCP nuclear localization. Consistent with this, fluorescent cytochemistry showed that in CHO cells, Sox9 expression promoted nuclear localization of GSK3 and βTrCP (supplemental Fig. S3, B and C). Furthermore, we found by both fluorescent cytochemistry and cell fractionation assays that in the Sox9c/c chondrocytes, when Sox9 was removed by Cre-adenovirus infection, GSK3 and βTrCP lost their nuclear localization and were mainly localized in the cytoplasm (Figs. 5, E and F, and supplemental S4). In addition, more β-catenin protein, although the total level was low, was found to be in the nucleus rather than the cytoplasm of primary Sox9c/c chondrocytes (Figs. 5G and supplemental S4). When Sox9 was removed in these cells, β-catenin protein levels increase substantially and most β-catenin protein was either associated with cell membrane or in the cytoplasm (Figs. 5G and supplemental S4). These results indicate that Sox9 recruits Axin and its associated β-catenin destruction complex to the nucleus to promote β-catenin degradation. Because our analysis had indicated that Sox9 was able to down-regulate β-catenin in the presence of Wnt signals (Fig. 1, C and D), Sox9 in the nucleus may promote β-catenin degradation by inhibiting the plasma membrane sequestration of the destruction complex during active Wnt signaling. Indeed, in the presence of Sox9, the amount of Axin-bound LRP5 was reduced (Fig. 5H).
FIGURE 5.
Sox9 induces nuclear accumulation of important components of β-catenin destruction complex. A, CHO cells were transfected with the indicated plasmids and cytoplasmic and nuclear fractions were analyzed by Western blot. Lamins A+C and tubulin were used as markers for nuclear and cytoplasmic fractions, respectively. B, 293 cells were transiently transfected with β-CATENIN, Axin-myc, and Sox9. Sox9 was precipitated from the cell lysates with anti-Sox9 antibody. Axin was detected with anti-Myc antibody. C, CHO cells were transiently transfected with βTrCP-myc and Sox9-Flag. Cells were fractionated and cytoplasmic and nuclear fractions were analyzed as in A. D, primary chondrocytes from Sox9c/c mice were nucleofected with Axin-myc and 24 h later were infected with GFP- (top panel) or Cre-adenovirus (bottom panel). Cells were stained with anti-Myc (red) and anti-Sox9 (purple) antibodies. The nucleus was stained by DAPI (blue). GFP was shown in green. E, primary chondrocytes from the Sox9c/c mice were infected with Cre-adenovirus. The subcellular localization of endogenous GSK3β was analyzed by immunofluorescence with anti-GSK3β antibody (green) before (top panel) and after Cre-adenovirus infection (bottom panel). Sox9 was stained with anti-Sox9 antibody (red). The nucleus was stained by DAPI (blue) and indicated by arrows. F, primary chondrocytes from Sox9c/c mice were transiently nucleofected with βTrCP-myc, then infected with Cre-adenovirus and analyzed as in D. βTrCP-Myc (green) was stained with anti-Myc antibody and Sox9 (red) was stained with anti-Sox9 antibody. G, primary chondrocytes from Sox9c/c mice were infected with Cre-adenovirus and analyzed as in E for subcellular localization of endogenous β-catenin with anti-β-catenin antibody (green). Sox9 (red) was stained with anti-Sox9 antibody. H, CHO cells were transiently transfected with the indicated plasmids. Cells were treated with Wnt3a (100 ng/ml) for 1 h, lysed, and Axin-Myc was immunoprecipitated with anti-Myc antibody. Equal amounts of immunoprecipitated Axin-Myc complex, were loaded on gel and analyzed with anti-LRP antibody.
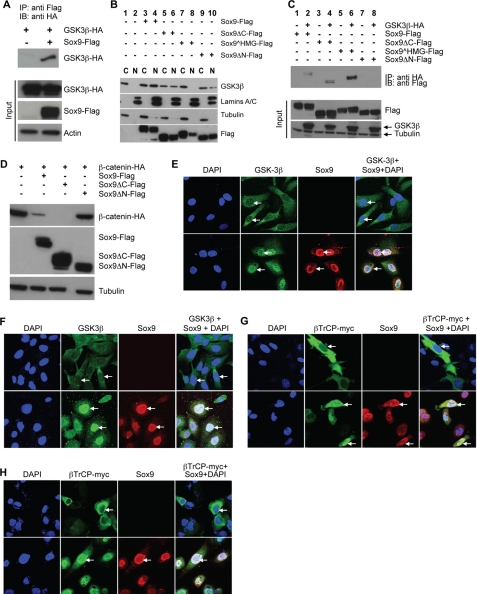
Sox9 Interacts with GSK3β and
βTrCP—Because Sox9 expression led to nuclear
accumulation of GSK3β, we asked if Sox9 interacts with GSK3β.
Sox9 co-immunoprecipitated with GSK3β, indicating that they
exist in the same protein complex (Fig.
6A). We then made serial Sox9 mutant constructs
with different interact with GSK3β. Sox9ΔC promoted GSK3β
nuclear localization (Fig.
6B, lanes 5 and 6). However,
Sox9 HMG, which is localized
mostly in the cytoplasm, failed to promote GSK3β nuclear localization
(Fig. 6B, lanes
7 and 8). Interestingly, Sox9 lacking the N terminus
(Sox9ΔN), but contains intact NLS, also has reduced activity in
promoting GSK3β nuclear localization, suggesting that Sox9 interaction
with GSK3β depends on this N-terminal part of Sox9 to a certain extent.
Indeed, Sox9ΔN did not co-immunoprecipitate with GSK3β
(Fig. 6C) and had
reduced ability in promoting β-catenin degradation
(Fig. 6D). In
contrast, deletion of the C terminus or the HMG domain did not affect
Sox9-GSK3β interaction (Fig.
6C). These results indicate that Sox9 uses its N terminus
to recruit GSK3β to the nucleus to enhance β-catenin degradation by
promoting its phosphorylation in the nucleus. With similar approaches, we
found that Sox9 used its C terminus to interact with βTrCP (supplemental
Fig. S5, A-C). We then asked whether interaction of Sox9 with
GSK3β or βTrCP requires β-catenin or Axin. To answer this
question, we employed the NCI-H28 cells that lack β-catenin and the
SNU475 cells that lack Axin. We found that in the NCI-H28 cells, Sox9 still
co-immunoprecipitated with βTrCP and GSK3β (supplemental Fig. S5,
D and E) and induced their nuclear translocations
(Fig. 6, E and
G). In the SNU475 cells, Sox9 also induced nuclear
translocation of βTrCP and GSK3β
(Fig. 6, F and
H). These data indicate that Sox9 interaction with
GSK-3β and βTrCP is independent of β-catenin and Axin. Taken
together, our findings suggest that β-catenin, GSK3β, βTrCP,
Axin, and Sox9 exist in one complex and Sox9 relocates this complex to the
nucleus.
HMG, which is localized
mostly in the cytoplasm, failed to promote GSK3β nuclear localization
(Fig. 6B, lanes
7 and 8). Interestingly, Sox9 lacking the N terminus
(Sox9ΔN), but contains intact NLS, also has reduced activity in
promoting GSK3β nuclear localization, suggesting that Sox9 interaction
with GSK3β depends on this N-terminal part of Sox9 to a certain extent.
Indeed, Sox9ΔN did not co-immunoprecipitate with GSK3β
(Fig. 6C) and had
reduced ability in promoting β-catenin degradation
(Fig. 6D). In
contrast, deletion of the C terminus or the HMG domain did not affect
Sox9-GSK3β interaction (Fig.
6C). These results indicate that Sox9 uses its N terminus
to recruit GSK3β to the nucleus to enhance β-catenin degradation by
promoting its phosphorylation in the nucleus. With similar approaches, we
found that Sox9 used its C terminus to interact with βTrCP (supplemental
Fig. S5, A-C). We then asked whether interaction of Sox9 with
GSK3β or βTrCP requires β-catenin or Axin. To answer this
question, we employed the NCI-H28 cells that lack β-catenin and the
SNU475 cells that lack Axin. We found that in the NCI-H28 cells, Sox9 still
co-immunoprecipitated with βTrCP and GSK3β (supplemental Fig. S5,
D and E) and induced their nuclear translocations
(Fig. 6, E and
G). In the SNU475 cells, Sox9 also induced nuclear
translocation of βTrCP and GSK3β
(Fig. 6, F and
H). These data indicate that Sox9 interaction with
GSK-3β and βTrCP is independent of β-catenin and Axin. Taken
together, our findings suggest that β-catenin, GSK3β, βTrCP,
Axin, and Sox9 exist in one complex and Sox9 relocates this complex to the
nucleus.
FIGURE 6.
Sox9 binds GSK3β and promotes its nuclear translocation.
A, GSK3β-HA was co-immunoprecipitated (co-IPed) with
Sox9-Flag when transiently co-expressed in CHO cells. B, CHO cells
were transiently transfected with a vector alone or the indicated plasmids.
Cells were fractionated and subcellular distribution of endogenous GSK3β
was analyzed by Western blot by anti-GSK3 antibody. Sox9 and its mutants were
detected by anti-FLAG antibody. Lamins A/C and tubulin served as markers for
nuclear and cytoplasmic fractions, respectively.
Sox9 HMG, although at a lower
level, was also detected in the nuclear fraction, possibly due to some
contamination by the cytoplasmic fraction. C, CHO cells were
transiently transfected with the indicated plasmids. GSK3 was
immunoprecipitated using anti-HA antibody. Sox9 was detected with anti-FLAG
antibody. D, CHO cells were transiently transfected with
β-CATENIN-HA and the indicated plasmids and analyzed by Western
blot. Tubulin served as loading control. E and F, NCI-H28
(E) or SNU475 (F) cells were infected with
Sox9-adenovirus and localization of endogenous GSK3β
(green) and Sox9 (red) were detected 24 h later. DAPI
stained the nucleus (blue). G and H, NCI-H28
(G) or SNU475 (H) cells were transfected with
βTrCP-myc and infected with Sox9-adenovirus when
indicated. Localization of βTrCP-myc (green) and Sox9
(red) was detected by immunofluorescence. DAPI stained the nucleus.
IB, immunoblot.
HMG, although at a lower
level, was also detected in the nuclear fraction, possibly due to some
contamination by the cytoplasmic fraction. C, CHO cells were
transiently transfected with the indicated plasmids. GSK3 was
immunoprecipitated using anti-HA antibody. Sox9 was detected with anti-FLAG
antibody. D, CHO cells were transiently transfected with
β-CATENIN-HA and the indicated plasmids and analyzed by Western
blot. Tubulin served as loading control. E and F, NCI-H28
(E) or SNU475 (F) cells were infected with
Sox9-adenovirus and localization of endogenous GSK3β
(green) and Sox9 (red) were detected 24 h later. DAPI
stained the nucleus (blue). G and H, NCI-H28
(G) or SNU475 (H) cells were transfected with
βTrCP-myc and infected with Sox9-adenovirus when
indicated. Localization of βTrCP-myc (green) and Sox9
(red) was detected by immunofluorescence. DAPI stained the nucleus.
IB, immunoblot.
Sox9 Increases Axin-bound Protein Levels and Activities of
CKIα and GSK3β in the Nucleus—We next
asked whether the nuclear β-catenin destruction complex recruited by Sox9
has different activities compared with the cytoplasmic one. Despite the
striking difference of
Sox9 HMG and
Sox9
HMG and
Sox9 HMG-NLS(TA) in their
subcellular localization and abilities to promote β-catenin degradation,
Sox9
HMG-NLS(TA) in their
subcellular localization and abilities to promote β-catenin degradation,
Sox9 HMG still bound
β-catenin, GSK3β, and βTrCP (supplemental Fig. S6). However,
Sox9
HMG still bound
β-catenin, GSK3β, and βTrCP (supplemental Fig. S6). However,
Sox9 HMG that cannot enter
the nucleus failed to promote β-catenin phosphorylation
(Fig. 7A). The
nuclearly localized
Sox9
HMG that cannot enter
the nucleus failed to promote β-catenin phosphorylation
(Fig. 7A). The
nuclearly localized
Sox9 HMG-NLS(TA) bound
β-catenin better than the cytoplasmically localized
Sox9
HMG-NLS(TA) bound
β-catenin better than the cytoplasmically localized
Sox9 HMG (supplemental Fig.
S6A). In addition, phosphorylation of β-catenin on
Ser45 and Ser33/37 was increased in the nuclear
fractions of the cells expressing either wild type or mutant Sox9 with intact
NLS (Sox9ΔC and
Sox9
HMG (supplemental Fig.
S6A). In addition, phosphorylation of β-catenin on
Ser45 and Ser33/37 was increased in the nuclear
fractions of the cells expressing either wild type or mutant Sox9 with intact
NLS (Sox9ΔC and
Sox9 HMG-NLS(TA))
(Fig. 7A). We then
tested whether GSK3β and CKIα could be more active once they were
in the nucleus. We found that Sox9 not only induced nuclear localization of
GSK3β and CKIα proteins, it also increased the amount of GSK3β
and CKIα bound to Axin only in the nuclear fractions of cells
(Fig. 7B), but not in
cytoplasmic fractions (Fig.
7C). We then tested directly if Sox9 alters GSK3β
and CKIα kinase activities in the β-catenin destruction complex.
GSK3β activity was measured based on in vitro phosphorylation of
a primed substrate, phosphoglycogen synthase peptide-2, and CKIα
activity was measured based on in vitro phosphorylation of myelin
basic protein (32,
33). GSK3β and CKIα
kinase activities in the destruction complex were increased in the nuclear
fractions of cell lysates from Sox9-expressing cells
(Fig. 7D) but not in
the cytoplasmic fractions of the same lysates
(Fig. 7E). Therefore,
Sox9 increased both the protein levels and activities of Axin-bound GSK3β
and CKIα only in the nucleus. Sox9 does not just simply recruit
GSK3β, CKIα, and Axin to the nucleus, it also actively enhances the
activities of GSK3β and CKIα in the destruction complex in the
nucleus.
HMG-NLS(TA))
(Fig. 7A). We then
tested whether GSK3β and CKIα could be more active once they were
in the nucleus. We found that Sox9 not only induced nuclear localization of
GSK3β and CKIα proteins, it also increased the amount of GSK3β
and CKIα bound to Axin only in the nuclear fractions of cells
(Fig. 7B), but not in
cytoplasmic fractions (Fig.
7C). We then tested directly if Sox9 alters GSK3β
and CKIα kinase activities in the β-catenin destruction complex.
GSK3β activity was measured based on in vitro phosphorylation of
a primed substrate, phosphoglycogen synthase peptide-2, and CKIα
activity was measured based on in vitro phosphorylation of myelin
basic protein (32,
33). GSK3β and CKIα
kinase activities in the destruction complex were increased in the nuclear
fractions of cell lysates from Sox9-expressing cells
(Fig. 7D) but not in
the cytoplasmic fractions of the same lysates
(Fig. 7E). Therefore,
Sox9 increased both the protein levels and activities of Axin-bound GSK3β
and CKIα only in the nucleus. Sox9 does not just simply recruit
GSK3β, CKIα, and Axin to the nucleus, it also actively enhances the
activities of GSK3β and CKIα in the destruction complex in the
nucleus.
FIGURE 7.
Nuclear activities of Axin-bound GSK3β and CKIα were increased in cells expressing Sox9. A, CHO cells were transiently transfected with β-CATENIN-HA and Sox9 mutants. β-Catenin degradation was inhibited by MG132. Cells were fractionated and nuclear extracts were analyzed by Western blot. The total amount of β-catenin was detected with anti-HA antibody. The membrane was stripped and re-probed with phospho-β-catenin-specific antibodies (Ser45 and Ser33/37). Lamins A/C and tubulin were used as markers for nuclear and cytoplasmic fractions, respectively. B and C, CHO cells were transiently transfected with Axin-myc, GSK3β-HA, and ckIα-HA, fractionated, and Axin-Myc was immunoprecipitated from the nuclear (B) and cytoplasmic (C) fractions. Equal amounts of immunoprecipitated Axin was loaded on the gel and analyzed with anti-HA antibody. The same membrane was also probed with anti-Myc antibody to confirm that an equal amount of Axin was loaded. D and E, equal amounts of GSK3β-HA or CKIα-HA bound to the immunoprecipitated Axin were subjected to in vitro kinase assays using phosphoglycogen synthase peptide-2 (P-GSP-2) as substrate for GSK3β and myelin basic protein for CKIα in the nuclear (D) or cytoplasmic (E) Axin immunocomplexes.
DISCUSSION
Here we report that one important mechanism by which Sox9 inhibits the anti-chondrogenic activity of the canonical Wnt signaling is to promote more efficient β-catenin phosphorylation by GSK3 in the nucleus, which then results in more rapid degradation of β-catenin. Sox9 could not promote degradation of mutant β-catenin that cannot be phosphorylated by GSK3 in our system. We demonstrated here that the C terminus of Sox9 is not required for Sox9 to degrade β-catenin, although it acts to inhibit β-catenin transcription activity, possibly by competing with LEF/TCF factors for binding β-catenin (24). Sox9ΔC lacks the C terminus that binds β-catenin, but it is even more potent in promoting β-catenin degradation than the full-length Sox9. However, Sox9ΔC exhibited weaker activity in inhibiting the transcriptional activity of β-catenin (Fig. 1, F and G). In addition, it actually increased TCF/LEF reporter activity mediated by a stabilized β-catenin as Sox9ΔC promoted its nuclear localization without being able to promote its degradation (Fig. 3, D and E).
Mutations in SOX9 are responsible for the genetic condition known
as CD, a semi-lethal osteochondrodysplasia, characterized by severe bone
malformation and sex reversal
(5,
6). All known missense point
mutations in SOX9 occur in the HMG domain, and with one exception,
all SOX9 nonsense and frame-shift mutations described so far in
CD/sex reversal patients lead to truncation of the trans-activation domain
(5,
6,
54,
55). It was suggested that CD
arises by mutations that interfere with DNA binding by Sox9 or truncating the
C-terminal trans-activation domain of Sox9, thereby the ability of Sox9 to
activate target genes during organ development was impaired
(15). The Sox9ΔC and
Sox9 HMG used in our study
resemble the mutations seen in CD patients, and our results demonstrated that
Sox9, in addition to functioning as a transcription factor by binding and
bending DNA, has a strong activity in regulating intracellular β-catenin
level by promoting its degradation. It is possible that besides directly
regulating expression of Sox9 target genes, the ability of Sox9 to regulate
the protein level and transcriptional activity of β-catenin may also
account in part for some defects observed in CD patients. Sox9 may also use
the same mechanism to regulate β-catenin activity in other developmental
processes such as intestinal stem cell proliferation and sex determination
(23,
27).
HMG used in our study
resemble the mutations seen in CD patients, and our results demonstrated that
Sox9, in addition to functioning as a transcription factor by binding and
bending DNA, has a strong activity in regulating intracellular β-catenin
level by promoting its degradation. It is possible that besides directly
regulating expression of Sox9 target genes, the ability of Sox9 to regulate
the protein level and transcriptional activity of β-catenin may also
account in part for some defects observed in CD patients. Sox9 may also use
the same mechanism to regulate β-catenin activity in other developmental
processes such as intestinal stem cell proliferation and sex determination
(23,
27).
Sox9 has been shown to inhibit the canonical Wnt signaling by promoting degradation of stabilized β-catenin that could not be phosphorylated by GSK3 (24). It was proposed that the C-terminal part of Sox9 is involved in this degradation, as it is required for direct Sox9-β-catenin binding in vitro. Here we show that the C-terminal deleted Sox9 can still form a complex with β-catenin in cell lysates and promote β-catenin degradation with the same efficiency as the full-length Sox9. However, binding between Sox9 and β-catenin is not sufficient to promote β-catenin degradation. Rather, nuclear localization of the β-catenin destruction complex promoted by Sox9 is required for enhanced β-catenin phosphorylation by GSK3 and subsequent degradation. We showed that Sox9 binds to major components of the destruction complex: β-catenin, GSK3β, and βTrCP. Although we did not detect a direct binding between Sox9 and Axin, Sox9 appears to retain Axin in the nucleus. In addition, we found that Sox9 and β-catenin appeared to degrade through different pathways. We did not observe enhanced Sox9 degradation when β-CATENIN was coexpressed (Figs. 5B, and supplemental S5, A and C). Furthermore, ΔβTrCP inhibited degradation of β-catenin, but not Sox9 (Fig. 2D). We noted that unlike the published results in colon epithelial cells (25), DNA binding ability of Sox9 mediated by HMG is not required for Sox9 to promote β-catenin degradation in cells that we have tested. Similar to our finding, the DNA binding ability of Sox17 is not required for promoting β-catenin degradation and inhibiting its transcriptional activity (56). One possibility for this difference may be that point mutations in the SOX9 HMG domain (25) do not just disrupt DNA binding, they may also compromise SOX9 nuclear localization as a result.
A major mechanism by which Wnt ligands regulate β-catenin degradation is through the control of GSK3-mediated β-catenin phosphorylation in the destruction complex containing Axin (39, 52, 57, 58). A small increase in the amount of GSK3β bound to Axin results in a significant decrease in β-catenin protein levels and signaling activity (57, 59). It is intriguing that Sox9 increased both protein levels and kinase activities of Axin-bound GSK3 and CKIα only in the nucleus. Although both cytoplasmic and nuclear forms of Sox9 bind β-catenin, GSK3, and βTrCP, only the nuclear forms showed stronger activity in promoting β-catenin degradation. One possibility is that Sox9 recruits a nuclear factor to the destruction complex to increase the kinase activities of GSK3 and CKIα and their binding affinities to Axin.
β-Catenin phosphorylation and subsequent degradation are thought to occur in the cytoplasm to prevent its nuclear translocation required for transcriptional activation of Wnt signaling target genes (60). Our data suggest that the nucleus is where β-catenin phosphorylation by GSK3 takes place more efficiently. As βTrCP is promoted to be localized in the nucleus by Sox9, it is also possible that β-catenin, like c-Myc, can be degraded in the nucleus (61). Efficient nuclear β-catenin phosphorylation and degradation may explain the phenomenon that most β-catenin protein is detected in the cytoplasm instead of the nucleus when wild type β-catenin is overexpressed.
It has been suggested that β-catenin may exist in different forms with distinct activities in the cell. During Wnt signaling, a form of β-catenin is generated that is more active in activating transcription by binding to TCFs (37). It is conceivable that whether β-catenin is sequestered in the destruction complex determines its transcription activity. During Wnt signaling, the destruction complex is sequestered by the plasma membrane through the binding of Axin to LRP6. In this case, β-catenin quickly accumulates in the cytoplasm and the β-catenin that enters the nucleus is monomeric and binds TCF well. However, in the absence of Wnt signaling, β-catenin in the nucleus may still be sequestered in the destruction complex, preventing it from activating downstream gene expression by binding to TCFs. Wnt ligands are important extrinsic factors that signal through β-catenin to regulate many cell proliferation and fate determination processes including stem cell self-renewal and differentiation. It is conceivable that rapid elimination of β-catenin in the nucleus by an intrinsic cell fate determining transcription factor will allow timely termination of Wnt signaling downstream gene expression that would impose inhibition on a cell fate determination process.
Supplementary Material
Acknowledgments
We thank the Yang lab for stimulating discussions. We are grateful to Drs. Yoshi Yamada, Frank Constantini, Xi He, Randy Moon, Zhijian J. Chen, and Berry Gumbiner for providing DNA plasmids, Dr. Roel Nusse for the Axin antibodies, and Dr. Benoit de Crombrugghe for the Sox9c/c mice. We thank Paul Leo and Stephen Wincovitch for help with confocal microscopy and Julia Fekecs for help with figure preparation.
This work was supported, in whole or in part, by the National Institutes of Health, NHGRI intramural research program. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S6.
Footnotes
The abbreviations used are: CD, campomelic dysplasia; CKIα, casein kinase Iα; GSK3, glycogen synthase kinase 3; LEF, lymphoid enhancer factor; TCF, T-cell factor; HMG, high mobility group; NLS, nuclear localization signal; NES, nuclear export signal; GFP, green fluorescent protein; HA, hemagglutinin; GST, glutathione S-transferase; IP, immunoprecipitate; CHO, Chinese hamster ovary; DAPI, 4′,6-diamidino-2-phenylindole; βTrCP, β-transducin repeat containing protein.
References
- 1.Karsenty, G. (2003) Nature 423, 316-318 [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg, H. M. (2003) Nature 423, 332-3364 [DOI] [PubMed] [Google Scholar]
- 3.Akiyama, H., Chaboissier, M. C., Martin, J. F., Schedl, A., and de Crombrugghe, B. (2002) Genes Dev. 16, 2813-2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi, W., Deng, J. M., Zhang, Z., Behringer, R. R., and de Crombrugghe, B. (1999) Nat. Genet. 22, 85-89 [DOI] [PubMed] [Google Scholar]
- 5.Foster, J. W., Dominguez-Steglich, M. A., Guioli, S., Kowk, G., Weller, P. A., Stevanovic, M., Weissenbach, J., Mansour, S., Young, I. D., Goodfellow, P. N., Brook, J. D., and Schafer, A. J. (1994) Nature 372, 525-530 [DOI] [PubMed] [Google Scholar]
- 6.Wagner, T., Wirth, J., Meyer, J., Zabel, B., Held, M., Zimmer, J., Pasantes, J., Bricarelli, F. D., Keutel, J., Hustert, E., Wolf, U., Tommerup, N., Schempp, W., and Scherer, G. (1994) Cell 79, 1111-1120 [DOI] [PubMed] [Google Scholar]
- 7.Clevers, H. (2006) Cell 127, 469-480 [DOI] [PubMed] [Google Scholar]
- 8.Logan, C. Y., and Nusse, R. (2004) Annu. Rev. Cell Dev. Biol. 20, 781-810 [DOI] [PubMed] [Google Scholar]
- 9.Guo, X., Day, T. F., Jiang, X., Garrett-Beal, L., Topol, L., and Yang, Y. (2004) Genes Dev. 18, 2404-2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann, C., and Tabin, C. J. (2001) Cell 104, 341-351 [DOI] [PubMed] [Google Scholar]
- 11.Day, T. F., Guo, X., Garrett-Beal, L., and Yang, Y. (2005) Dev. Cell 8, 739-750 [DOI] [PubMed] [Google Scholar]
- 12.Harley, V. R., and Goodfellow, P. N. (1994) Mol. Reprod. Dev. 39, 184-193 [DOI] [PubMed] [Google Scholar]
- 13.Harley, V. R., Jackson, D. I., Hextall, P. J., Hawkins, J. R., Berkovitz, G. D., Sockanathan, S., Lovell-Badge, R., and Goodfellow, P. N. (1992) Science 255, 453-456 [DOI] [PubMed] [Google Scholar]
- 14.Pontiggia, A., Rimini, R., Harley, V. R., Goodfellow, P. N., Lovell-Badge, R., and Bianchi, M. E. (1994) EMBO J. 13, 6115-6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDowall, S., Argentaro, A., Ranganathan, S., Weller, P., Mertin, S., Mansour, S., Tolmie, J., and Harley, V. (1999) J. Biol. Chem. 274, 24023-24030 [DOI] [PubMed] [Google Scholar]
- 16.Sudbeck, P., Schmitz, M. L., Baeuerle, P. A., and Scherer, G. (1996) Nat. Genet. 13, 230-232 [DOI] [PubMed] [Google Scholar]
- 17.Gasca, S., Canizares, J., De Santa Barbara, P., Mejean, C., Poulat, F., Berta, P., and Boizet-Bonhoure, B. (2002) Proc. Natl. Acad. Sci. U. S. A. 99, 11199-11204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudbeck, P., and Scherer, G. (1997) J. Biol. Chem. 272, 27848-27852 [DOI] [PubMed] [Google Scholar]
- 19.Mori-Akiyama, Y., Akiyama, H., Rowitch, D. H., and de Crombrugghe, B. (2003) Proc. Natl. Acad. Sci. U. S. A. 100, 9360-9365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spokony, R. F., Aoki, Y., Saint-Germain, N., Magner-Fink, E., and Saint-Jeannet, J. P. (2002) Development 129, 421-432 [DOI] [PubMed] [Google Scholar]
- 21.Lynn, F. C., Smith, S. B., Wilson, M. E., Yang, K. Y., Nekrep, N., and German, M. S. (2007) Proc. Natl. Acad. Sci. U. S. A. 104, 10500-10505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seymour, P. A., Freude, K. K., Tran, M. N., Mayes, E. E., Jensen, J., Kist, R., Scherer, G., and Sander, M. (2007) Proc. Natl. Acad. Sci. U. S. A. 104, 1865-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blache, P., van de Wetering, M., Duluc, I., Domon, C., Berta, P., Freund, J. N., Clevers, H., and Jay, P. (2004) J. Cell Biol. 166, 37-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyama, H., Lyons, J. P., Mori-Akiyama, Y., Yang, X., Zhang, R., Zhang, Z., Deng, J. M., Taketo, M. M., Nakamura, T., Behringer, R. R., McCrea, P. D., and de Crombrugghe, B. (2004) Genes Dev. 18, 1072-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastide, P., Darido, C., Pannequin, J., Kist, R., Robine, S., Marty-Double, C., Bibeau, F., Scherer, G., Joubert, D., Hollande, F., Blache, P., and Jay, P. (2007) J. Cell Biol. 178, 635-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morais da Silva, S., Hacker, A., Harley, V., Goodfellow, P., Swain, A., and Lovell-Badge, R. (1996) Nat. Genet. 14, 62-68 [DOI] [PubMed] [Google Scholar]
- 27.Maatouk, D. M., Dinapoli, L., Alvers, A., Parker, K. L., Taketo, M. M., and Capel, B. (2008) Hum. Mol. Genet. 17, 2949-2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jans, D. A., Ackermann, M. J., Bischoff, J. R., Beach, D. H., and Peters, R. (1991) J. Cell Biol. 115, 1203-1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefebvre, V., Huang, W., Harley, V. R., Goodfellow, P. N., and de Crombrugghe, B. (1997) Mol. Cell. Biol. 17, 2336-2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinner, D., Rankin, S., Lee, M., and Zorn, A. M. (2004) Development 131, 3069-3080 [DOI] [PubMed] [Google Scholar]
- 31.Andrews, N. C., and Faller, D. V. (1991) Nucleic Acids Res. 19, 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Primot, A., Baratte, B., Gompel, M., Borgne, A., Liabeuf, S., Romette, J. L., Jho, E. H., Costantini, F., and Meijer, L. (2000) Protein Expression Purif. 20, 394-404 [DOI] [PubMed] [Google Scholar]
- 33.Reinhardt, J., Ferandin, Y., and Meijer, L. (2007) Protein Expression Purif. 54, 101-109 [DOI] [PubMed] [Google Scholar]
- 34.Vyas, D. R., Spangenburg, E. E., Abraha, T. W., Childs, T. E., and Booth, F. W. (2002) Am. J. Physiol. 283, C545-C551 [DOI] [PubMed] [Google Scholar]
- 35.Kawakami, Y., Tsuda, M., Takahashi, S., Taniguchi, N., Esteban, C. R., Zemmyo, M., Furumatsu, T., Lotz, M., Belmonte, J. C., and Asahara, H. (2005) Proc. Natl. Acad. Sci. U. S. A. 102, 2414-2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korinek, V., Barker, N., Morin, P. J., van Wichen, D., de Weger, R., Kinzler, K. W., Vogelstein, B., and Clevers, H. (1997) Science 275, 1784-1787 [DOI] [PubMed] [Google Scholar]
- 37.Gottardi, C. J., and Gumbiner, B. M. (2004) J. Cell Biol. 167, 339-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs, S. Y., Chen, A., Xiong, Y., Pan, Z. Q., and Ronai, Z. (1999) Oncogene 18, 2039-2046 [DOI] [PubMed] [Google Scholar]
- 39.Hart, M., Concordet, J. P., Lassot, I., Albert, I., del los Santos, R., Durand, H., Perret, C., Rubinfeld, B., Margottin, F., Benarous, R., and Polakis, P. (1999) Curr. Biol. 9, 207-210 [DOI] [PubMed] [Google Scholar]
- 40.Kitagawa, M., Hatakeyama, S., Shirane, M., Matsumoto, M., Ishida, N., Hattori, K., Nakamichi, I., Kikuchi, A., and Nakayama, K. (1999) EMBO J. 18, 2401-2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagna, G., Carnevali, F., Marchioni, M., and Hemmati-Brivanlou, A. (1999) Mech. Dev. 80, 101-106 [DOI] [PubMed] [Google Scholar]
- 42.Latres, E., Chiaur, D. S., and Pagano, M. (1999) Oncogene 18, 849-854 [DOI] [PubMed] [Google Scholar]
- 43.Liu, C., Kato, Y., Zhang, Z., Do, V. M., Yankner, B. A., and He, X. (1999) Proc. Natl. Acad. Sci. U. S. A. 96, 6273-6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, C., Li, Y., Semenov, M., Han, C., Baeg, G. H., Tan, Y., Zhang, Z., Lin, X., and He, X. (2002) Cell 108, 837-847 [DOI] [PubMed] [Google Scholar]
- 45.Winston, J. T., Strack, P., Beer-Romero, P., Chu, C. Y., Elledge, S. J., and Harper, J. W. (1999) Genes Dev. 13, 270-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yost, C., Torres, M., Miller, J. R., Huang, E., Kimelman, D., and Moon, R. T. (1996) Genes Dev. 10, 1443-1454 [DOI] [PubMed] [Google Scholar]
- 47.Klein, P. S., and Melton, D. A. (1996) Proc. Natl. Acad. Sci. U. S. A. 93, 8455-8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meijer, L., Skaltsounis, A. L., Magiatis, P., Polychronopoulos, P., Knockaert, M., Leost, M., Ryan, X. P., Vonica, C. A., Brivanlou, A., Dajani, R., Crovace, C., Tarricone, C., Musacchio, A., Roe, S. M., Pearl, L., and Green-gard, P. (2003) Chem. Biol. 10, 1255-1266 [DOI] [PubMed] [Google Scholar]
- 49.Stambolic, V., Ruel, L., and Woodgett, J. R. (1996) Curr. Biol. 6, 1664-1668 [DOI] [PubMed] [Google Scholar]
- 50.Spencer, E., Jiang, J., and Chen, Z. J. (1999) Genes Dev. 13, 284-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tetsu, O., and McCormick, F. (1999) Nature 398, 422-426 [DOI] [PubMed] [Google Scholar]
- 52.Kishida, S., Yamamoto, H., Ikeda, S., Kishida, M., Sakamoto, I., Koyama, S., and Kikuchi, A. (1998) J. Biol. Chem. 273, 10823-10826 [DOI] [PubMed] [Google Scholar]
- 53.Satoh, S., Daigo, Y., Furukawa, Y., Kato, T., Miwa, N., Nishiwaki, T., Kawasoe, T., Ishiguro, H., Fujita, M., Tokino, T., Sasaki, Y., Imaoka, S., Murata, M., Shimano, T., Yamaoka, Y., and Nakamura, Y. (2000) Nat. Genet. 24, 245-250 [DOI] [PubMed] [Google Scholar]
- 54.Meyer, J., Sudbeck, P., Held, M., Wagner, T., Schmitz, M. L., Bricarelli, F. D., Eggermont, E., Friedrich, U., Haas, O. A., Kobelt, A., Leroy, J. G., Van Maldergem, L., Michel, E., Mitulla, B., Pfeiffer, R. A., Schinzel, A., Schmidt, H., and Scherer, G. (1997) Hum. Mol. Genet. 6, 91-98 [DOI] [PubMed] [Google Scholar]
- 55.Kwok, C., Weller, P. A., Guioli, S., Foster, J. W., Mansour, S., Zuffardi, O., Punnett, H. H., Dominguez-Steglich, M. A., Brook, J. D., Young, I. D., Goodfellow, P. N., and Schafer, A. J. (1995) Am. J. Hum. Genet. 57, 1028-1036 [PMC free article] [PubMed] [Google Scholar]
- 56.Sinner, D., Kordich, J. J., Spence, J. R., Opoka, R., Rankin, S., Lin, S. C., Jonatan, D., Zorn, A. M., and Wells, J. M. (2007) Mol. Cell. Biol. 27, 7802-7815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikeda, S., Kishida, S., Yamamoto, H., Murai, H., Koyama, S., and Kikuchi, A. (1998) EMBO J. 17, 1371-1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maniatis, T. (1999) Genes Dev. 13, 505-510 [DOI] [PubMed] [Google Scholar]
- 59.Dajani, R., Fraser, E., Roe, S. M., Yeo, M., Good, V. M., Thompson, V., Dale, T. C., and Pearl, L. H. (2003) EMBO J. 22, 494-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peifer, M., and Polakis, P. (2000) Science 287, 1606-1609 [DOI] [PubMed] [Google Scholar]
- 61.Amati, B., and Sanchez-Arevalo Lobo, V. J. (2007) Nat. Cell Biol. 9, 729-731 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.