Abstract
The cytoplasmic amino terminus of HCN1, the primary full-length HCN isoform expressed in trout saccular hair cells, was found by yeast two-hybrid protocols to bind the cytoplasmic carboxyl-terminal domain of a protocadherin 15a-like protein. HCN1 was immunolocalized to discrete sites on saccular hair cell stereocilia, consistent with gradated distribution expected for tip link sites of protocadherin 15a. HCN1 message was also detected in cDNA libraries of rat cochlear inner and outer hair cells, and HCN1 protein was immunolocalized to cochlear hair cell stereocilia. As predicted by the trout hair cell model, the amino terminus of rat organ of Corti HCN1 was found by yeast two-hybrid analysis to bind the carboxyl terminus of protocadherin 15 CD3, a tip link protein implicated in mechanosensory transduction. Specific binding between HCN1 and protocadherin 15 CD3 was confirmed with pull-down assays and surface plasmon resonance analysis, both predicting dependence on Ca2+. In the presence of calcium chelators, binding between HCN1 and protocadherin 15 CD3 was characterized by a KD = 2.39 × 10-7 m. Ca2+ at 26.5-68.0 μm promoted binding, with KD = 5.26 × 10-8 m (at 61 μm Ca2+). Binding by deletion mutants of protocadherin 15 CD3 pointed to amino acids 158-179 (GenBank™ accession number XP_238200), with homology to the comparable region in trout hair cell protocadherin 15a-like protein, as necessary for binding to HCN1. Amino terminus binding of HCN1 to HCN1, hypothesized to underlie HCN1 channel formation, was also found to be Ca2+-dependent, although the binding was skewed toward a lower effective maximum [Ca2+] than for the HCN1 interaction with protocadherin 15 CD3. Competition may therefore exist in vivo between the two binding sites for HCN1, with binding of HCN1 to protocadherin 15 CD3 favored between 26.5 and 68 μm Ca2+. Taken together, the evidence supports a role for HCN1 in mechanosensory transduction of inner ear hair cells.
HCN12 is the primary full-length HCN isoform underlying Ih (hyperpolarization-activated, cyclic nucleotide-gated, nonselective cation channel current) in a model hair cell preparation from the trout sacccule (1). cAMP-gated Ih, possibly in addition to the mechanosensory-transduction current, sets the membrane potential for a subpopulation of saccular hair cells (2, 3). The membrane potential in the saccular hair cell subpopulation is sufficiently depolarized to activate voltage-gated calcium channels, permitting influx of calcium and secretion of hair cell transmitter (2). Given that saccular hair cells expressing IK1 in addition to Ih are more hyperpolarized, not supporting activation of the voltage-gated calcium channels, we predicted that spontaneous release of transmitter from the subpopulation of hair cells would constitute hair cell-generated spontaneous activity for the saccule (1). However, little has been previously reported on the morphological localization of the HCN1 isoform in hair cells or possible links to structural proteins that mechanistically would localize HCN1 in hair cells (for preliminary report, see Ref. 4). In general, little is known about protein-protein interactions for the HCN isoforms that would modulate Ih and/or the associated instantaneous current (5).
Protocadherin 15 is a proposed tip link protein involved in connecting shorter stereocilia to adjacent taller stereocilia in the stereociliary array of inner ear hair cells, facilitating the opening of the mechanosensory transduction channel in response to auditory and vestibular stimuli. The active tip link protein in Danio rerio is protocadherin 15a (6), characterized by splice variants in its carboxyl terminus. In the mammal, protocadherin 15 CD3 is hypothesized to be a tip link protein at insertion sites in the tips of the shorter stereocilia of the stereociliary array (7, 8).
EXPERIMENTAL PROCEDURES
Acquisition of a Model Hair Cell Preparation from the Trout Saccule and an Organ of Corti Subfraction from the Rat Cochlea—Hair cell layers were isolated from the trout saccule as previously described (9, 10), yielding a hair cell preparation of 1.5 × 106 saccular hair cells, free of intact supporting cells. An organ of Corti fraction was microdissected from rat cochlea (one-month-old ACI Black Agouti rats; Harlan Sprague-Dawley) as previously described and morphologically characterized (11).
Yeast Two-hybrid Analysis: Trout Hair Cell Layer—Yeast two-hybrid screening for the interacting proteins of trout hair cell HCN1 was performed (Matchmaker kit; Clontech, Mountain View, CA) using the cytoplasmic amino terminus of trout saccular hair cell HCN1 (aa 1-118) as bait (see Fig. 1). PCR was carried out on trout hair cell cDNA with upstream primer 5′-GCCGAATTCATGGAAGATAAATCAAATTCGTTC-3′ and downstream primer 5′-TGCGGATCCATAGGGATGAATGATCCA-3′ (restriction sites underlined). The corresponding amplification product was purified, restriction-digested with EcoRI and BamHI enzymes and inserted into a similarly digested pGBKT7 vector to produce a fusion construct with the GAL4 DNA-binding domain. The fusion products were proven to be transcriptionally inactive in appropriate nutrient-deficient media, a necessary condition for unequivocal interpretation of results.
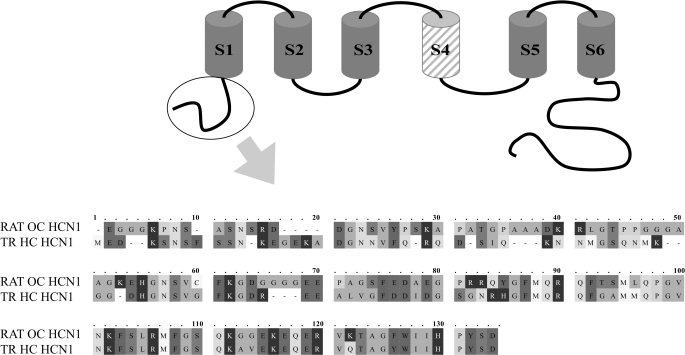
FIGURE 1.
Alignment (with OMIGA 2.0) of the amino terminus of rat organ of Corti HCN1 and trout saccular hair cell HCN1 (GenBank™ accession number AAQ04053), both used as bait in yeast two-hybrid protocols, pull-down assays and SPR analyses. The cytoplasmic amino terminus sequence is encircled in a schematic depiction of HCN1 with S1-S6 transmembrane regions and S4 (hatched) representing the voltage sensor. For rat organ of Corti HCN1, aa 11-18 display EF hand-like characteristics. Amino acids 11 and 17 are designated as having low α-helical character, whereas aa 12-16 have loop character (PROF predictions (47)). SRD (aa 13-15) and DGN (aa 16-18) have been cited as Ca2+-binding motifs (48).
A trout hair cell cDNA library was created from the isolated hair cell layer (1, 4) for prey construct according to instructions (Matchmaker kit; Clontech). Hair cell message was reverse-transcribed with oligo(dT) and random hexamer primers, with an equal proportion of each cDNA used in constructing the library. Transformants of host strain AH 109 were plated on SD/-Leu medium, and the colonies were collected after 5 days.
Cells containing bait constructs in strain Y187 were mixed with the cells containing prey constructs in AH 109 to screen for interacting proteins. The cells were allowed to mate in 2× YPD medium overnight at 30 °C and plated on a selection medium SD/-Trp/-Leu/-His/-Ade. After 4-5 days, the larger colonies were used to identify, by PCR, the prey interacting with bait. Purified PCR products were sequenced, and the sequences were identified using BLAST (NCBI).
Yeast Two-hybrid Analysis: Rat Organ of Corti—Binding between HCN1 and protocadherin 15 CD3 was analyzed with yeast two-hybrid protocols. The HCN1 amino-terminal cytoplasmic region (HCN1-N) was PCR-amplified from a rat organ of Corti cDNA preparation, and the sequence was verified and cloned into the bait vector pGBKT7. Similarly, the carboxyl terminus of protocadherin 15 CD3 in rat organ of Corti was targeted in PCR using specific primers (XM_238200) (nucleotides 295-831) (upper primer, 5′-TGGGGATCCATGATCAAGTTGGTGGTCGAT-3′; lower primer, 5′-GAATTCTCAGAGTTTTGTCATTGGTAT-3′) and cloned in the prey vector pGADT7. For the yeast two-hybrid assay, ∼500 ng of each vector was employed to cotransform AH 109 yeast strain using the standard protocol (Clontech yeast two-hybrid manual), and the transformants were plated on nutrient-deficient selection medium (SD/-Trp/-Leu) for 3-5 days. The colonies were picked and streaked on higher stringency medium (SD/-Trp/-His/-Leu/-Ade + 3-AT). Negative controls contained either the bait or the prey, along with corresponding empty vector.
Western Blot for Saccular Hair Cell HCN1—The model saccular hair cell preparation (1.5 × 106 hair cells) was homogenized in PBS buffer containing 0.1% Tween 20, 0.1% CHAPS, and protease inhibitor mixture (Roche Applied Science or Sigma) and centrifuged at 20,900 × g for 20 min at 4 °C. The supernatant was incubated with 1× SDS-PAGE gel loading buffer with NuPAGE sample reducing agent (Invitrogen) (10 min at 70 °C), and the mixture was centrifuged at 12,000 × g for 10 min and loaded onto a 4-12% gel in an Invitrogen mini-cell apparatus. After electrophoresis, the proteins were electrotransferred to a nylon membrane. The membrane was blocked with PBST buffer containing 1% nonfat milk (PBSTM) for 1 h and then incubated overnight at room temperature with the primary antibody (1:1,250) in PBSTM (plus 2% serum corresponding to the secondary antibody). The custom rabbit polyclonal primary antibody, (purified by protein A chromatography, Maine Biotechnology Services), targeted the specific peptide LHKASHALQSGSLSRDVRH found in the carboxyl terminus of full-length HCN1 sequence (1). The membranes were washed and incubated for 1.5 h in the secondary antibody conjugated with horseradish peroxidase, and the proteins were detected using Western Lightning chemiluminescence (PerkinElmer Life Sciences).
Immunohistochemical Analysis—Saccular sacs were removed from the cranial cavity of 200-g rainbow trout (Oncorhynchus mykiss) and placed in fixative containing 4% paraformaldehyde, 0.1% glutaraldehyde in 0.1 m phosphate buffer, pH 7.4, on ice, for isolation of the saccular macula fraction, which included the sensory and nonsensory epithelium, otolithic membrane, basal lamina, and a connective tissue layer with afferent and efferent nerve fibers (9). Following 2-4 h in fixative, the saccular macular tissues were rinsed overnight in phosphate buffer at 4 °C, dehydrated in a graded ethanol series, and embedded in paraffin.
The isolation of the rat cochlea for immunohistochemical analysis has been previously described (12). In brief, the bony cochleas from 2-month-old ACI Black Agouti rats were perfused from basal to apical coil with the fixative consisting of 4% paraformaldehyde, 0.1% glutaraldehyde in 0.1 m phosphate buffer, pH 7.4, at 4 °C (13). Following fixation for 4-6 h, the bony cochleas were decalcified in 0.12 m EDTA, 0.1 m phosphate, pH 7.4, for 5-7 days at 4 °C. The cochlear tissues were dehydrated in a graded ethanol series and embedded in paraffin.
Four-μm deparaffinized sections of trout saccule and rat cochlea were sequentially incubated in 0.1% sodium borohydride (PBS plus 5 mm glycine) for 45 min, 3% H2O2 for 5 min (for avidin-biotin peroxidase method), 2% normal serum (corresponding to the species in which the secondary antibody was made) for 45 min, all at room temperature. The primary antibody was added for 12-16 h at 4 °C. The Vector ABC Elite protocol was used with 3,3′-diaminobenzidine as chromogen (BioGenex, San Ramon, CA). Alternatively, immunofluorescence was determined with secondary antibodies (Molecular Probes) conjugated to Alexa Fluor 488. The primary antibody for trout saccular hair cell HCN1 was a custom rabbit polyclonal (see “Western Blot for Saccular Hair Cell HCN1”) used at 1:1,250 dilution. Rat cochlear HCN1 was immunolocalized with affinity-purified rabbit polyclonal antibody at 1:100 (Alomone APC-056), targeting the peptide (C)KP NSASNSRDDGNSVYPSK corresponding to amino acids 6-24 of rat HCN1 (GenBank™ accession number AAF62173). The amino terminus of rat HCN1 expressed as a fusion protein was immunolabeled with this antibody on Western blot (see Fig. 8A).
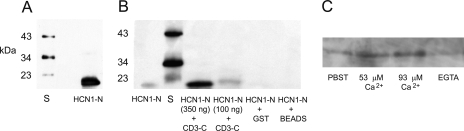
FIGURE 8.
GST pulldown assay of binding between the amino terminus of HCN1 and the carboxyl terminus of protocadherin 15CD3, both produced from rat organ of Corti cDNA. A, Western blot detection of the amino terminus fusion protein. First lane, standards of 23, 34, and 43 kDa (Santa Cruz). Second lane, prominent band identifies amino terminus of HCN1 fusion protein, predicted mass of 23 kDa, detected with affinity-purified rabbit polyclonal antibody at 1:200 (APC-056, Alomone Labs, Israel), targeting amino acids 6-24 of rat HCN1. The predicted mass of 23 kDa arises from 19 kDa HCN1 +∼4-kDa vector protein. B, HCN1-N pulldown with GST-protocadherin 15 CD3-C fusion protein in PBST. First lane, purified HCN1-N fusion product used in the pull-down assay, detected with an antipolyhistidine monoclonal antibody. Second lane (S), protein standards, molecular masses 23, 34, and 43 kDa. Third lane, binding of HCN1-N fusion protein (∼350 ng) with GST-protocadherin 15 CD3-C cleared lysate, pulled down with glutathione-Sepharose, and detected with antipolyhistidine monoclonal antibody. Fourth lane, binding of HCN1-N fusion protein (∼100 ng) with GST-protocadherin 15 CD3-C lysate. Fifth lane, HCN1-N (∼350 ng) incubated with GST bacterial lysate as a negative control. Sixth lane, rat HCN1-N fusion protein mixed with Sepharose beads under the same conditions as above, representing another negative control. C, HCN1-N pulldown with CD3-C in PBST, 53 μm Ca2+, 93 μm Ca2+, or EGTA. 200 μl of HCN1-N lysate (corresponding to ∼0.8 mg of total bacterial protein) and 250 μl of GST-CD3-C lysate (representing ∼0.8 mg of total bacterial protein) were used for each pull-down reaction.
3,3′-Diaminobenzidine immunostaining was examined with a Leitz Diaplan microscope and photographed with an Olympus OM-4T camera, and the negatives were digitized. Immunofluorescence was examined with an Olympus BX60 microscope with fluorescein excitation (470-490 nm) and emission (500-800 nm) filters and photographed with a PM 20 camera system, and the negatives were digitized. In this investigation of localization of HCN1 in the trout saccule and rat cochlea, two types of negative controls were performed; the primary antibodies were either preabsorbed with their respective antigens or omitted.
Reverse Transcription-PCR for Rat Organ of Corti and PCR for Rat λ ZAP cDNA Libraries of Inner and Outer Hair Cells—The mRNA of microdissected organ of Corti subfractions was converted to cDNA via SuperScript reverse transcriptase (Invitrogen) with either random hexamer or oligo(dT)12-18 primers (11). Primers for PCR targeting rat HCN1 (GenBank™ accession number NM_053375) were 5′-CAGCAGCCGCAACAGCAACAA-3′ upstream (nucleotides 2328-2348), and 5′-TTTTCTGCATCTGGGTCTTTATTTAAGA-3′ downstream (nucleotides 2755-2782), predicted to yield a 455-bp amplification product. The expression of HCN1 message in cochlear hair cells was determined from application of rat-specific HCN1 primers in PCR to λ ZAP cDNA libraries (a cDNA library formed with a specifically modified λ phage vector, λ ZAP (Stratagene)) (14, 15). A first amplification with an HCN1 gene-specific primer upstream coupled with λ ZAP-T7 reverse primer downstream was followed by a second amplification with HCN1 gene-specific primers for the 455-bp PCR product. A second set of nested primers was applied to the 455-bp inner hair cell amplification product with 5′-CCACACCGAAAAATGAAGTGCA-3′ upstream (nucleotides 2419-2440) and 5′-CCTGCTCCCTGCCTGAAGGCCAGTG-3′ downstream (nucleotides 2609-2633), predicted to yield a 215-bp amplification product. All of the PCR products were sequenced-verified.
GST Pull-down Assay for HCN1 and Protocadherin 15 CD3 of the Rat Organ of Corti—Rat HCN1-N was amplified from a rat organ of Corti cDNA preparation, using rat HCN1-specific upstream (5′-ATGGAAGGCGGCGGCAAGCCCAAC-3′) and downstream (5′-GGATGGATAATCCAGAAGCCTGC-3′) primers, to which BamHI and EcoRI restriction enzyme sites were added (TCGGATCC and GAATTC, respectively). The restriction-digested PCR products were ligated to similarly digested pRSET-A vector with a polyhistidine fusion tag. Carboxyl-terminal sequence of rat protocadherin 15 CD3 (CD3-C) (XM_238200) was similarly PCR-amplified with gene-specific upstream (5′-ATGATCAAGTTGGTGGTCGATAGGGA-3′) and downstream (5′-TCAGAGTTTTGTCATTGGTAT-3′) primers to which BamHI and Not1 sites (CTGGGATCC and TCGCGGCCGC, respectively) were added. The restriction-digested PCR products were ligated into similarly digested pGEX-6P-1 vector with GST fusion tag (Amersham Biosciences). Clones for HCN1-N and CD3-C were selected and sequence-verified, and fusion proteins were expressed in Escherichia coli BL21 (DE3) using 1 mm final concentration of isopropyl β-d-thiogalactopyranoside. The fusion protein for HCN1-N was affinity-purified with a nickel-nitrilotriacetic acid spin column (Qiagen) or TALON magnetic beads (Clontech), with purification checked by 4-12% PAGE. The protein was dialyzed overnight in a Spectra Dispodialyzer (Sigma) with four or five buffer changes in PBST (Sigma-Aldrich) containing 1× protease inhibitors (Sigma-Aldrich) at 4 °C, and concentration was estimated by a Quant-iT fluorescent assay kit in a Qubit™ fluorometer (Invitrogen).
Affinity-purified rat HCN1-N polypeptide (0.5 μg) was mixed with bacterial lysate containing GST-CD3-C fusion protein (20 μg) in PBST containing 1× protease inhibitor mixture for 3 h at room temperature. In a separate series of experiments, Ca2+ was included at low μm levels. Glutathione-Sepharose beads (50%), preincubated in PBST containing 1% bovine serum albumin, were added for 1 h of incubation, and the beads were collected by centrifugation and washed five times in PBST buffer. The immobilized proteins were eluted with protein gel-loading solution at 70 °C (10 min). The proteins recovered in the supernatant from centrifugation at 1,300 × g were separated by 4-12% PAGE and electroblotted to a polyvinylidene difluoride membrane. The membrane was incubated in PBSTM for 1 h at room temperature, with anti-polyhistidine monoclonal antibody (1:5,000, Invitrogen) for 7 h, washed in PBST five times for 5 min each time, and incubated for 1 h in goat anti-mouse secondary antibody (1:6,000 in PBSTM). After six washes of the membrane in PBST, the HCN1-N fusion product was detected with an ECL chemiluminesence kit (GE Healthcare). Two negative controls were used, one containing the HCN1-N fusion product and bacterial lysate with the GST product instead of the GST-CD3-C fusion product and another containing a bacterial lysate without GST or GST-CD3-C.
Surface Plasmon Resonance (SPR) Analysis of Binding between HCN1 and Protocadherin 15 CD3 Expressed in Rat Organ of Corti—SPR experiments were performed as described (16) with a Biacore 3000 instrument (Biacore, Piscataway, NJ). SPR is an optical technique for detecting molecular interactions in which either molecule is immobilized on a sensor chip. Binding of the mobile molecule (analyte) to the immobilized molecule (ligand) changes the refractive index of the film, which can be detected. Because the method strictly detects mass, labeling of the molecules is not required.
In the present investigation, pRSET-A fusion proteins were made from rat organ of Corti cDNA for HCN1-N (see previous section for GST pulldown assays), the carboxyl terminus of protocadherin 15 CD3 (CD3-C) and its deletion mutants. pRSET-B was used for the carboxyl terminus of protocadherin 15 CD1 (CD1-C) (GenBank™ accession number XM_001075929). Upstream and downstream gene-specific primers for rat CD3-C were the same as those described for the GST pulldown assay except with added restriction enzyme sites for BamHI and Bgl2 (TGGGGATCC and TGAAGATCT, respectively). Gene-specific primers for CD1-C were 5′-CTTCCTCCTCCTCCTCGACCA-3′ (upstream) and 5′-TTACAAGGACGTTGACTGACT-3′ (downstream), with restriction enzymes sites for Bgl2 and EcoRI added (TCGAGATCT and GAATTC, respectively). Fusion proteins were affinity-purified as described in the previous section.
Affinity-purified HCN1-N fusion polypeptide (ligand) was immobilized on a CM5 research grade sensor chip by amine coupling. The immobilization involved activation of carboxymethyl groups on a dextran-coated chip by reaction with N-hydroxysuccinimide, followed by covalent bonding of the ligand to the chip surface via amide linkages. Reference surfaces were prepared in the same manner but blocked with ethanolamine. Binding analysis was carried out by injecting the affinity-purified CD3-C fusion peptide (analyte) into the flow cells (ligand and reference cell), and the interaction (response units, Ru) between analyte and ligand was recorded. Equilibrium dissociation constants were calculated from the ratio of reverse to forward rate constants, determined graphically. The determination of Ca2+ dependence of binding was based upon the analysis of Ca2+ levels in experimental solutions by inductively coupled plasma mass spectroscopy.
RESULTS
A Protocadherin 15a-like Protein Binds to Trout Saccular Hair Cell HCN1, Determined by Yeast Two-hybrid Analysis—Yeast two-hybrid protocols were used to identify binding partners for HCN1, the primary HCN isoform underlying Ih in hair cells of the trout saccule (1). Amino terminus sequence in bait construct pGBKT7 was tested in mating protocols with a saccular hair cell cDNA library in prey construct pGADT7-Rec. Transformation efficiency for the prey library was 8.4 × 105/3 μg of vector, based upon the number of colonies that appeared on the SD/-Leu plates after 5 days. The mating efficiency was 28.4% (∼1,560 colonies). After selection in a quadruple knock-out medium, the prey in ∼125 colonies were sequenced for the amino-terminal HCN1 bait (HCN1-N), aa 1-118 (Fig. 1). A protocadherin 15a-like protein was identified as a protein-binding partner for HCN1 amino terminus bait (Fig. 2A and 3, A and B). Binding was confirmed by switching bait and prey, at increased stringency by inclusion of 3-AT (Fig. 2B).
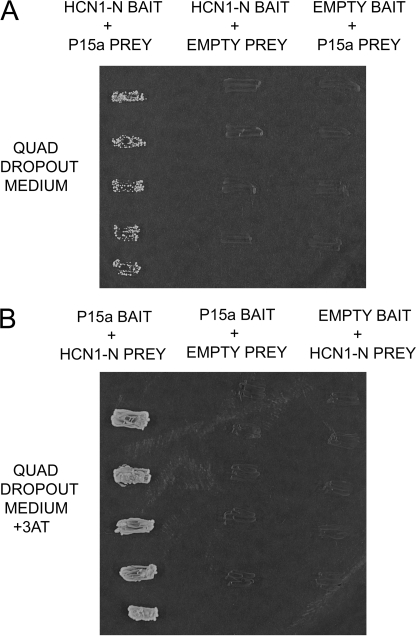
FIGURE 2.
Interaction of a protocadherin 15a-like sequence with trout saccular hair cell HCN1 amino-terminal cytoplasmic domain. A, first lane, binding of aa 1-118 of HCN1-N as bait to a protocadherin 15a-like protein as prey, determined on quadruple drop-out medium with yeast two-hybrid mating protocols. Second lane, negative control with HCN1-N as bait and empty prey vector. Third lane, negative control with empty bait vector paired with prey sequence. B, reversal of bait and prey. First lane, protocadherin 15a-like protein as bait and HCN1-N as prey, on high stringency quadruple drop-out medium (with 2 mm 3-AT). Second lane, negative control with protocadherin 15a as bait and empty prey vector. Third lane, negative control in which empty bait vector is paired with the HCN1-N prey sequence.
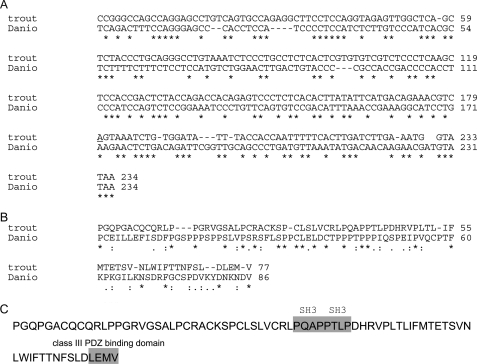
FIGURE 3.
Nucleotide and amino acid sequences of the trout saccular hair cell protocadherin 15a-like protein compared with corresponding sequences of D. rerio (GenBank™ accession number AY772390). A, comparison of nucleotide sequences of the carboxyl terminus of protocadherin 15a aligned with Clustal. B, comparison of amino acid sequences of the carboxyl terminus of protocadherin 15a for trout and D. rerio. C, indication of two SH3 binding sites, PQAP, PTLP, and PDZ3-interacting sequence LEMV in the trout saccular hair cell aa sequence.
The nucleotide sequence for the trout saccular hair cell protocadherin 15a-like protein corresponded to the cytoplasmic carboxyl terminus of protocadherin 15a of D. rerio, with 46% nucleotide identity, 24% aa identity, and 41% aa similarity (Fig. 3A), the highest homology to any D. rerio protein. The trout sequence was in frame, ending with a stop codon corresponding in position to the stop codon for D. rerio protocadherin 15a. The trout protein contained two SH3-binding motifs, PQAP, PTLP, and one PDZ3-interacting sequence motif, LEMV (Fig. 3, B and C). The highest homology in contiguous amino acid sequence between the trout hair cell peptide and D. rerio protocadherin 15a was 37% identity and 50% similarity for aa 15-53 of 79. The corresponding homology of the trout saccular hair cell sequence to the carboxyl terminus sequence of D. rerio protocadherin 15b was 12% aa identity and 44% similarity. Binding between HCN1-N and the protocadherin 15a-like protein was unaffected by the elimination of the PDZ3-interacting sequence motif, LEMV, in the protocadherin15a-like protein (supplemental Fig. S1), indicating that this motif was not involved in the interaction. The carboxyl terminus of protocadherin 15a is the only cytoplasmic portion of this presumptive tip link protein, which would be available for intracellular protein-protein binding, specifically demonstrated for the protocadherin 15a-like protein and HCN1 expressed in hair cells of the trout saccule.
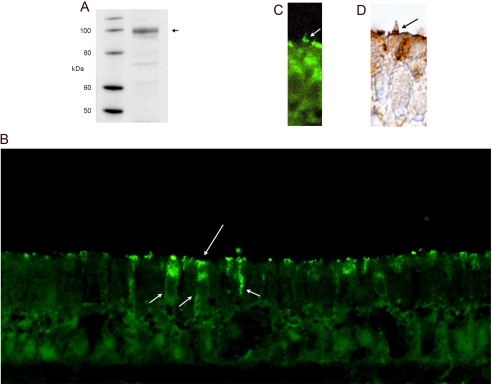
Immunolocalization of HCN1 in Hair Cells of the Trout Saccular Sensory Epithelium—A custom rabbit polyclonal antibody was raised against LHKASHALQSGSLSRDVRH found in the carboxyl terminus of HCN1 expressed in the model hair cell preparation from the trout saccule, for which the hair cell is the only intact cell type (1). As predicted, the rabbit polyclonal, purified by protein A chromatography (Maine Biotechnology Services), immunolabeled a protein in the trout saccular hair cell preparation on Western blot (Fig. 4A) with the approximate size of 104 kDa, the predicted mass for HCN1 (1) (GenBank™ accession number AF421883). Further, the rabbit polyclonal primary antibody immunolabeled saccular hair cells in the sensory epithelium detected with either immunofluorescence or 3,3′-diaminobenzidine (Fig. 4, B-D). Immunoreactivity was concentrated at apical, supranuclear sites of hair cells, extending to the lateral and basal cell membrane (Fig. 4B), which are sites of efferent input (17). In addition, HCN1 was immunolocalized to stereocilia, with discrete sites of expression that were gradated in their distribution (Fig. 4, C and D).
FIGURE 4.
Immunodetection of HCN1 protein in the trout saccular hair cells. A, Western blot of HCN1 protein in trout saccular hair cell layer. A custom rabbit polyclonal antibody (1:1,250), targeting a 19-aa peptide found in the carboxyl terminus of saccular hair cell HCN1, immunolabeled a protein of predicted molecular mass (104 kDa, arrow), based upon full-length HCN1 sequence (1) (GenBank™ accession number AF421883). B, immunofluorescence localization of HCN1 in the trout saccular sensory epithelium. Immunoreactivity for HCN1 was found in saccular hair cells at the apical membrane (long arrow) extending along the lateral cell membranes to basal sites of the hair cells (short arrows), the latter consistent in position to sites opposing efferent innervation. Sometimes, circular patterns were observed when the plane of sectioning of the epithelium was tilted, possibly corresponding to the edges of the cuticular plate. C, HCN1 was immunolocalized with immunofluorescence to the hair cell stereocilia (arrow), with foci of immunofluorescence present at gradated sites on the stereociliary array. D, immunoreactivity for HCN1 was also detected with 3,3′-diaminobenzidine, with discrete puncta present in stereociliary arrays (arrow).
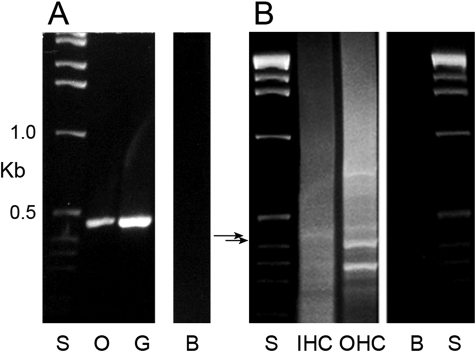
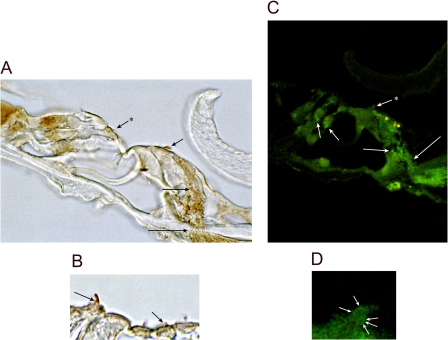
HCN1 Expression in Cochlear Inner and Outer Hair Cells—HCN1 message was detected in the organ-of-Corti microdissected cochlear subfraction cDNA and in λ ZAP cDNA libraries of inner and outer hair cells (Fig. 5). The outer hair cell ∼420-bp amplification product and inner hair cell 455-bp product, the latter further characterized by nested primers, yielded HCN1 rat-specific nucleotide sequence. HCN1 protein was immunolocalized with a rabbit polyclonal antibody targeting the amino terminus (6-24 aa) of rat HCN1 (Alomone) and immunolabeling a fusion protein of organ of Corti HCN1-N on Western blot (see Fig. 8A). HCN1 immunoreactivity was localized to nerve fibers approaching and passing the habenula perforata to the organ of Corti. These fibers traversed the organ of Corti to the base of inner hair cells and outer hair cells (Fig. 6, A and C) and in part comprised afferent dendrites, consistent with HCN1 mRNA expression in spiral ganglion afferent cell bodies (Fig. 5A). Immunoreactivity for HCN1 protein was also found to be associated with hair cell stereocilia, both of inner hair cells and outer hair cells (Fig. 6), with immunostaining eliminated by preabsorption of the primary antibody with specific antigen (not illustrated).
FIGURE 5.
HCN1 message expressed in rat organ of Corti and cochlear hair cells. A, amplification with PCR of a 455-bp cDNA from microdissected organ of Corti (lane O) and spiral ganglion (lane G) subfractions from the rat cochlea, with 1-kb standards (lane S) and negative control in which cDNA was omitted (lane B). B, HCN1 message in cochlear inner hair cells and outer hair cells. HCN1 message in λ-ZAP cDNA libraries of rat cochlear inner hair cells (lane IHC) and outer hair cells (lane OHC). The outer hair cell ∼420-bp amplification product (lower arrow) and inner hair cell 455-bp amplification product (upper arrow), the latter with application of nested primers, yielded rat HCN1 sequence. Lane S, standard; lane B, water blank.
FIGURE 6.
HCN1 immunoreactivity within the organ of Corti of the rat cochlea. A, HCN1 is immunolocalized to nerve fibers approaching and passing the habenula perforata (long arrow) en route to the base of the inner hair cell (intermediate length arrow). Immunoreactivity was also localized to stereocilia of the inner hair cell (short arrow) and outer hair cell (short arrow + asterisk). B, HCN1 immunoreactivity is associated with stereocilia of outer hair cells (short arrow), as well as inner hair cells (intermediate length arrow). C, HCN1-immunoreactive nerve fibers approach and cross the habenula perforata (long arrow) and follow pathways to sites close to the base of the inner hair cell (intermediate length arrow). Immunoreactivity is observed at the base of outer hair cells overlapping Deiters' cells (short arrows) and is associated with the inner hair cell stereociliary array (short arrow + asterisk; green fluorescence). Yellow fluorescence overlapping apical sites of the inner hair cell represents autofluorescence, likely from flavin adenine dinucleotide (49). D, foci of HCN1 immunoreactivity (arrows) associated with the stereociliary array of an inner hair cell.
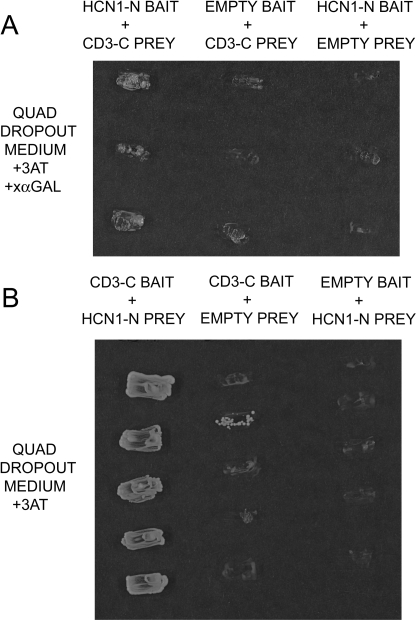
Interaction of the Amino Terminus of Rat Organ of Corti HCN1 with the Carboxyl Terminus of Organ of Corti Protocadherin 15 CD3 in Yeast Two-hybrid Analysis—Given expression of HCN1 mRNA in rat cochlear hair cells and immunolocalization of HCN1 protein to stereocilia of inner and outer hair cells, similar to immunolocalization of HCN1 protein to stereocilia of hair cells in the trout saccule, we investigated binding of HCN1 to protocadherin 15 CD3. Protocadherin 15 CD3 is thought to be a mammalian counterpart of protocadherin 15a of D. rerio, the latter implicated (above) as a binding partner for HCN1 in trout saccular hair cells. The amino-terminal peptide for rat organ of Corti HCN1 (HCN1-N), comparable with the amino-terminal segment of trout saccular hair cell HCN1 (Fig. 1), was found by yeast two-hybrid protocols to bind the carboxyl terminus of protocadherin 15 CD3 (CD3-C) expressed in the rat organ of Corti (Fig. 7). The segment of CD3-C used for analysis encompassed the trout protocadherin 15a-like carboxyl terminus amino acid sequence deduced as binding in yeast two-hybrid protocols and corresponded to aa 99-277 of rat protocadherin 15 CD3 (GenBank™ accession number XP_238200). Binding occurred with rat organ of Corti HCN1-N as bait and CD3-C as prey, or the reverse, with CD3-C as bait and HCN1-N as prey (Fig. 7), at high stringency (+3-AT). No growth was found in negative controls employing empty vectors in quadruple drop-out medium.
FIGURE 7.
HCN1 amino-terminal cytoplasmic domain interacts with protocadherin 15 CD3 carboxyl-terminal domain in yeast two-hybrid assay. A, rat organ of Corti HCN1-N as bait and rat organ of Corti CD3-C as prey in selection medium SD/-Trp/-Leu/-His/-Ade containing 2 mm 3-AT and α-galactoside. The first lane shows expression of the Gal-4 marker, which is expressed only when there is interaction between the bait and prey fusion peptides. The second and third lanes are negative controls in which the bait and prey sequences, respectively, are replaced by empty vector. B, reversal of bait and prey. First lane, CD3-C as bait and HCN1-N as prey in selection medium Sd/-Trp/-Leu/-His/-Ade containing 2 mm 3-AT. The second and third lanes are negative controls, CD3-C as bait and empty prey vector and HCN1-N as prey and empty bait vector, respectively.
GST Pulldown Assay of Binding between HCN1 and Protocadherin 15 CD3 Produced from Rat Organ of Corti cDNA—Binding observed with yeast two-hybrid analysis was confirmed with a pull-down assay (Fig. 8). Western blots indicated a predicted molecular mass for rat organ of Corti HCN1-N purified fusion protein of 23 kDa, detected with gene-specific antibody for rat HCN1 (Fig. 8A, second lane) or with an antipolyhistidine monoclonal antibody targeting the His tag on the fusion protein (Fig. 8B, first lane). Binding between rat organ of Corti CD3-C and rat organ of Corti HCN1-N was demonstrated (Fig. 8B, third lane (350 ng of HCN1-N) and fourth lane (100 ng of HCN1-N)). The binding was increased with inclusion of Ca2+ at low micromolar levels (Fig. 8C). Negative controls included HCN1-N incubated with either GST-bacterial lysate or with Sepharose beads under the same conditions as for the experimental samples.
SPR Analysis of HCN1 Binding to Protocadherin 15 CD3 for Organ of Corti Proteins—The affinity-purified amino terminus of rat organ of Corti HCN1 (HCN1-N) and the affinity-purified carboxyl terminus of rat organ of Corti protocadherin 15 CD3 (CD3-C) (Fig. 9A, second and fourth lanes, respectively) were found to bind in the presence of EDTA (Fig. 9B). With HCN1-N as ligand and CD3-C (0-32 nm) as analyte, the KD = 3.57 × 10-7 m. Comparable binding was demonstrated in the presence of EGTA, yielding a KD = 1.21 × 10-7 m.
FIGURE 9.
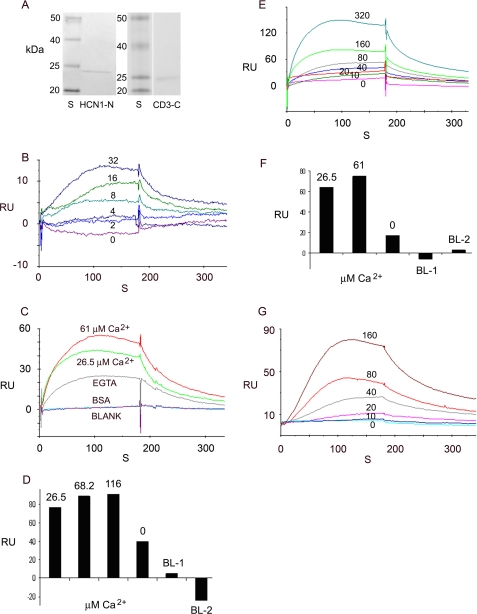
SPR analysis of binding interaction between HCN1 and protocadherin 15 CD3. A, affinity-purified fusion proteins. First lane, prestained protein standards on SDS-PAGE gel: 20, 25, 40, and 50 kDa (Bench-Mark, Invitrogen). Second lane, affinity-purified rat organ of Corti HCN1-N fusion protein at 23 kDa, stained with Coomassie Blue. Third lane, protein standards on SDS-PAGE gel. Fourth lane, affinity-purified rat organ of Corti protocadherin 15 CD3-C fusion protein at 24 kDa, stained with Coomassie Blue. B, binding between the amino terminus of rat organ of Corti HCN1 (HCN1-N) as ligand and the carboxyl terminus of rat organ of Corti protocadherin 15CD3 (CD3-C) as analyte as a function of CD3-C concentration in the presence of 3 mm EDTA. For sensorgram, the response units (RU) are indicated for 0 (violet), 2 (blue), 4 (gray-blue), 8 (turquoise), 16 (green), and 32 (purple) nm analyte. KD = 3.57 × 10-7 m. C, the binding of HCN1-N (ligand) to CD3-C (analyte) (50 nm) has aCa2+-dependent component. Response units are indicated for binding with CD3-C at 61 μm Ca2+ (red), 26.5 μm Ca2+ (green), or 1 mm EGTA (gray), compared with the response for bovine serum albumin (turquoise) or blank buffer (magenta). D, Ca2+ dependence of the response (RU) for interaction of CD3-C (analyte) with HCN1-N (ligand). The bar graph indicates the response for 150 nm CD3-C at 26.5 μm Ca2+, 68.2 μm Ca2+, 116 μm Ca2+, and 1 mm EGTA, compared with blank buffer (BL-1, BL-2). E, kinetics of binding between HCN1-N (ligand) and CD3-C (analyte) at 61 μm Ca2+:0 (pink), 10 (red), 20 (gray-green), 40 (blue), 80 (gray), 160 (green), and 320 (turquoise) nm analyte. KD = 2.3 × 10-8 m. F, bar graph depicting response with reversal of ligand and analyte. Ca2+-dependent binding of CD3-C (ligand) and HCN1-N (analyte) (100 nm) at 26.5 μm Ca2+, 61 μm Ca2+, and 1 mm EGTA, compared with responses for buffer with 1 mm EGTA + 1 mm Ca2+ (BL-1) or buffer alone (BL-2). G, kinetic series for binding between CD3-C (ligand) and HCN1-N (analyte) at 61 μm Ca2+ for 0 (turquoise), 10 (blue), 20 (pink), 40 (gray), 80 (red), and 160 (brown) nm analyte. KD = 3.41 × 10-8 m.
The specific binding of HCN1-N to CD3-C was enhanced by Ca2+, increasing by ∼90% at 26.5 μm Ca2+ relative to binding with 1 mm EGTA, with further increases in binding observed up to 68 μm Ca2+ (Fig. 9, C and D). Binding at 68 μm Ca2+ was significantly greater than binding at 26.5 μm Ca2+ (p < 0.05, paired-variate analysis, two-tailed t test). No binding was detected for bovine serum albumin and buffer negative controls. Binding as a function of CD3-C concentration (0-320 nm) in the presence of 61 μm Ca2+ (Fig. 9E) yielded a KD = 2.3 × 10-8 m, and in a separate series of experiments (0-160 nm CD3-C) at 61 μm Ca2+, a KD of 5.26 × 10-8 m (see Fig. 11D), consistent with Ca2+-dependent promotion of binding between HCN1-N and CD3-C. Similar results were obtained with SPR when ligand and analyte were reversed (CD3-C as ligand and HCN1-N as analyte); binding was again indicated between HCN1-N and CD3-C, which was further promoted by Ca2+ (61 μm) (Fig. 9F, 100 nm HCN1-N). The KD for interaction was 3.41 × 10-8 m determined from a kinetic series (Fig. 9G, 0-160 nm HCN1-N as analyte, 61 μm Ca2+).
FIGURE 11.
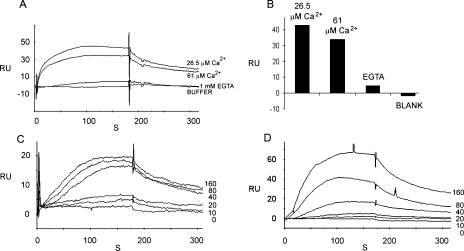
Rat organ of Corti HCN1 binds to itself via its amino terminus, and the binding is Ca2+-dependent. A, SPR sensorgram for HCN1-N (200 nm) as analyte, binding to HCN1-N as ligand, at 26.5 μm Ca2+, 61 μm Ca2+, and with EGTA (1 mm), compared with the response with buffer alone (26.5 μm Ca2+). B, bar graph representation in same order as for A. C, kinetic series of binding interactions for HCN1-N as analyte to HCN1-N as ligand (at 61 μm Ca2+) for 0, 10, 20, 40, 80, and 160 nm analyte. D, comparable kinetic series of binding interactions for CD3-C as analyte with HCN1-N as ligand (at 61 μm Ca2+) in a back-to-back comparison to C for 0, 10, 20, 40, 80, and 160 nm analyte.
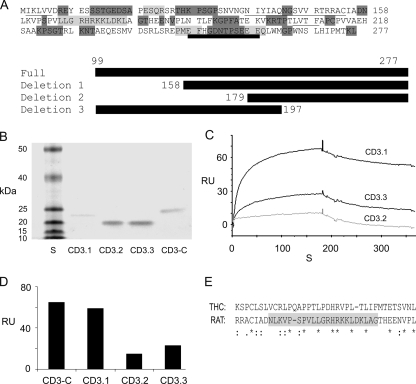
Deletion mutants for CD3-C (Fig. 10A) were designed, and the corresponding fusion proteins were affinity-purified (Fig. 10B). At 26.5 μm Ca2+, binding with HCN1-N was maximal with deletion mutant 1 (CD3.1) compared with deletion mutant 3 (CD3.3), with least binding occurring for deletion mutant 2 (CD3.2), all reflecting less binding than for the original CD3-C (Fig. 10, C and D). The differences in response, i.e. binding between CD3.1, CD3.3, and CD3.2 with HCN1-N, point to a 22-aa region in CD3-C (aa 158-179) (rat protocadherin 15 CD3, GenBank™ accession number XP_238200) as being important for binding to HCN1-N. This region in rat protocadherin 15 CD3, implicated as required for binding to rat HCN1, is 32% identical in amino acid sequence to the trout protocadherin 15a-like protein (Fig. 10E), higher than that found overall (13-18%) for rat CD3-C compared with either the trout protocadherin 15a-like protein or D. rerio protocadherin 15a.
FIGURE 10.
Deletion mutants of rat organ of Corti protocadherin CD3 implicate a 22-amino acid peptide within the carboxyl terminus as a requirement for binding. A, amino acid sequence for the carboxyl terminus segment of protocadherin 15 CD3 used as prey. The secondary structure is indicated: underlining, β-sheets; light gray shading, α-helix; dark gray shading, loop; thick underlining, aa 248-259 comprising a putative EF hand Ca2+-binding motif (50). There was 11% helix, 19% β-strand, and 70% loop sequence. The full-length sequence (CD3-C) and deletion mutants 1 (CD3.1), 2 (CD3.2), and 3 (CD3.3) expressed in pRSET-A vector are indicated by heavy black bars. B, Coomassie Blue staining of SDS-PAGE gel of affinity-purified mutants. S, standards: 10, 15, 20, 25, 40, and 50 kDa (Invitrogen). The molecular mass in kDa is indicated for CD3.1, CD3.2, CD3.3, and CD3-C. C, binding of deletion mutants of the protocadherin 15 CD3-C (CD3.1, CD3.3, and CD3.2, all as analytes at 120 nm) to HCN1-N at 26.5 μm Ca2+. D, comparison of SPR responses for CD3-C, CD3.1, CD3.2, and CD3.3. Overall, CD3-C versus CD3.1, 0.05 < p < 0.10, n = 5; CD3.1 versus CD3.2, p < 0.05, n = 7; CD3.1 versus CD3.3, 0.05 < p < 0.10, n = 7; CD3.3 versus CD3.2, p = 0.103, n = 6 (two-tailed paired-variate analysis). E, clustal alignment of amino acids in rat protocadherin 15CD3-C and trout protocadherin 15a. The 22-aa peptide region in the carboxyl terminus of rat protocadherin 15 CD3-C (highlighted in gray), implicated as important for binding to HCN1-N, has homology to protocadherin 15a-like protein expressed in trout saccular hair cells.
SPR Analysis of HCN1-N Binding to the Carboxyl Terminus of Protocadherin 15 CD1 from Rat Organ of Corti—Protocadherin 15 CD1 is a second protocadherin 15 protein implicated as having a role in the formation of hair cell tip links (7, 8). The carboxyl terminus of rat protocadherin 15 CD1, with limited homology to rat protocadherin 15 CD3 (12% identity, 47% similarity), exhibited low or no binding to the amino terminus of HCN1 at either 26.5 μm Ca2+ (see supplemental Fig. S2) or 61 μm Ca2+ (not illustrated).
SPR Analysis of HCN1-N Binding to HCN1-N Derived from Rat Organ of Corti—With SPR, evidence was obtained for Ca2+-dependent binding of HCN1-N as ligand to HCN1-N as analyte (Fig. 11, A-C). A maximum response was observed at 26.5 μm Ca2+ compared with that at 61 μm Ca2+ (Fig. 11, A and B). No response was observed in the absence of Ca2+ (plus EGTA). The response at ≥61 μm Ca2+ was lower than that at 26.5 μm Ca2+ (p < 0.05, paired-variate analysis, two-tailed t test). Responses for HCN1-N (analyte) and HCN1-N (ligand) were lower than responses for CD3-C (analyte) and HCN1-N (ligand) carried out in back-to-back experiments at 61 μm Ca2+ (Fig. 11, C and D). The KD for the interaction between HCN1-N and HCN1-N at 61 μm Ca2+ = 1.29 × 10-8 m.
DISCUSSION
HCN isoforms, members of the K+ channel superfamily, underlie Ih, a slowly activating, inwardly rectifying cation current. Ih has slight selectivity for K+ relative to Na+ but is usually dominated by Na+, the major monovalent cation present in extracellular fluids. The reversal potentials for Ih produced from heterologously expressed HCN1 are reported to be -32 and -19 mV for 5.4 and 30 mm extracellular K+, respectively, corresponding to PK/PNa of 4.2 and 3.2 (18). Ih in heterologous expression systems or in isolated neurons, cardiac myocytes, and dorsal root ganglion neurons can also admit Ca2+ with fractional Ca2+ current constituting ∼0.5-0.6% of net inward current (19, 20).
In addition to slowly activating currents, HCN mRNA also codes for Iinst, a voltage-independent instantaneous current, which occurs when HCN isoforms are individually expressed in mammalian cells (5, 21, 22, 23, 24, 25, 26, 27) or in Xenopus oocytes (28). Iinst correlates with the amount of If (in the heart, corresponding to Ih in the central nervous system), with both currents increasing in high potassium external solution (25). Whether the Iinst and Ih represent currents corresponding to different states of the same channel (25) or different populations of channels (29) is being investigated.
In acousticolateralis systems, Ih has been detected electrophysiologically in adult hair cells of vestibular end organs of both lower and higher vertebrates (2, 30-32) as well as in differentiating cells from the organ of Corti of the Immortomouse (33). In adult fish and frog saccules, a subpopulation of hair cells have Ih as their only inwardly rectifying cation current. It is these ovoid/spherical hair cells (unlike the cylindrical hair cells exhibiting both IK1 and Ih) that have membrane potentials sufficiently depolarized to permit activation of voltage-gated calcium channels, influx of calcium, and efflux of hair cell transmitter (2).
We previously examined expression of mRNA for HCN isoforms in a model hair cell preparation from the trout saccule, obtaining evidence that HCN1 is the primary full-length HCN isoform expressed in saccular hair cells (1). Transcripts for HCN2 (GenBank™ accession numbers AY14881 and AY14882) and HCN4 are truncated. Ih in hair cells of the teleost saccule would therefore derive primarily from expressed HCN1, with this HCN1 channel responsible for hair cell-derived spontaneous activity in the saccule itself (discussed in Ref. 1). This role for Ih would occur at “physiological gradients of Na+ and K+” (34), 151 mm Na+ and 5 mm K+ in cranial fluid for rainbow trout (35). However, hair cells, the receptor cells in both vestibular and auditory sensory end organs, are morphologically polarized. In addition to being surrounded at basolateral sites by high Na+, low K+-containing physiological extracellular fluids, hair cells, apically via stereocilia (hair cell cytoplasmic extensions), contact endolymph, an extracellular fluid high in K+ and low in Na+ found in the inner ears of lower and higher vertebrates. Endolymph contains K+ at 72.9 mm for the saccule of rainbow trout, Salmo irideus, now O. mykiss (35), and 51.4 mm for the saccule of oyster toadfish, Opsanus tau (36). Measurements in the rat cochlea indicate that endolymph contains K+ at 177-171 mm, Na+ at 1.0-2.6 mm, and Cl- at 140-132 mm, across basal-apical cochlear turns (37). High extracellular K+ concentrations in endolymph would be predicted to push the reversal potential for Ih closer to 0 (25) for HCN1 channels localized to the hair cell stereocilia.
In the present investigation, yeast two-hybrid protocols were utilized to identify, in a model hair cell preparation from the trout saccule (9), binding partners for hair cell HCN1 (4). Sequencing of clones for prey, binding to the amino terminus of HCN1 as bait, revealed a protocadherin 15a-like protein, implicated as a tip link protein in D. rerio (6). Reversal of bait and prey and negative constructs with empty vectors all confirmed interaction of the amino cytoplasmic terminus of HCN1 with the carboxyl cytoplasmic terminus of the protocadherin 15a-like protein.
The identity of the hair cell HCN1-binding protein as a protocadherin 15a-like protein was based upon the overall level of identity between the trout and D. rerio sequences. Multiple splice variants for the carboxyl terminus of protocadherin 15 are known to exist in D. rerio, with protocadherin 15a and 15b exhibiting less identity to each other than D. rerio protocadherin 15a to the trout sequence. The trout sequence was in frame, ending with a stop codon, as did the D. rerio protocadherin 15a sequence. The finding that it was the carboxyl terminus of protocadherin 15a that was bound, the only cytoplasmic cell-embedded portion of protocadherin 15a available for protein-protein interaction, was also consistent. Specificity of interaction was indicated by positive results for reversal of bait and prey and negative cloning results for prey vector containing protocadherin 15a-like peptide in the absence of bait HCN1. The immunolocalization of HCN1 to hair cell stereocilia with custom antibody specifically targeting trout saccular hair cell HCN1, at sites similar to those of predicted tip link positions, was a further point of consistency supporting interaction between an HCN1 ion channel and a tip link protein in the teleost saccular hair cell.
With the determination that mRNA for HCN1 was expressed in cochlear hair cells and HCN1 protein could be immunolocalized to cochlear hair cell stereocilia, it appeared plausible that organ of Corti HCN1 would bind to protocadherin 15 as found for the model hair cell preparation from the trout saccule. Comparable protein-protein interaction between the amino terminus of HCN1 and carboxyl cytoplasmic terminus of protocadherin 15 CD3 was confirmed for the rat organ of Corti with yeast two-hybrid protocols, GST pulldown assays, and SPR. Similar results were obtained with reversal of binding partners, and no interaction was observed for negative controls.
Moreover, the binding rates and equilibrium binding constants in the mammalian model pointed to a dependence of the HCN1-protocadherin 15 CD3 interaction on calcium concentration, with the association rate, maximum response, and binding affinity increased in the presence of Ca2+. EF hand-like amino acid sequences representing putative binding sites for Ca2+ can be identified in the rat protocadherin 15 CD3 carboxyl terminus (Fig. 10), possibly contributing to the calcium dependence. Analysis of binding by affinity-purified mutants of the carboxyl terminus of rat organ of Corti protocadherin 15 CD3 points to a 22-aa region as important for binding, a region that displayed homology to the comparable region of protocadherin 15a-like protein expressed in trout saccular hair cells. In contrast to the demonstrated interaction between HCN1 and protocadherin 15 CD3, little to no binding occurred between the amino terminus of rat organ of Corti HCN1 and the carboxyl terminus of protocadherin 15 CD1, which lacks the 22-aa region of protocadherin 15 CD3.
SPR analysis indicated that rat organ of Corti HCN1 also binds to itself via its amino terminus, confirming previous reports and representing a mechanism for channel formation from individual HCN1 subunits. The HCN1-HCN1 amino terminus binding analyzed by SPR was calcium-dependent, correlating with the identification of Ca2+-binding motifs in the amino terminus (Fig. 1). The interaction between HCN1-N and HCN1-N differed from the interaction between HCN1-N and protocadherin 15 CD3 in that no binding was observed between HCN1 subunits in the absence of Ca2+. Further, maximal binding was shifted to lower concentrations of Ca2+ for HCN1 subunits binding to each other relative to HCN1 binding to protocadherin 15 CD3. The results would be consistent with a calcium-dependent competition between HCN1-HCN1 binding and HCN1-protocadherin 15CD3 binding, particularly from 26.5 to 68 μm Ca2+.
Given this demonstrated interaction between protocadherin 15 CD3, a hair cell tip link protein presumed to be involved in mechanosensory transduction, and HCN1, the question arises as to the possible function of the HCN1-containing channel in vivo. Ih itself is slow and would not be considered a candidate for the mechanosensory transduction channel on the basis of short response latencies of mechanosensory transduction in vertebrate hair cells (38), although activation of Ih by stretch stimulus has recently been demonstrated, with stretch accelerating both activation and deactivation (39). Iinst coded for by all of the HCN isoforms is both fast and voltage-independent and is blocked along with Ih by ZD7288 (29, 40). Another interesting aspect of the HCN instantaneous current is that it is enhanced by prior hyperpolarization pulses (27), with possible applicability to low frequency spontaneous oscillation of hair bundles associated with mechanosensory transduction (41). Further, the instantaneous current is detected at high extracellular K+ (25), as would be present extracellularly in endolymph. Interestingly, structural investigations have suggested that the hair cell mechanosensory transduction channels “may be located only on the tops of the shorter stereocilia” (42, 43), and it is the tops of shorter stereocilia that appear to represent the site of protocadherin 15 (CD3) localization, as opposed to cadherin 23, the other putative component of tip links with stereociliary insertion points localized midway along the taller stereocilia (8). Further, recent experiments point to the tips of the shorter stereocilia as sites for calcium entry, required in the adaptation phase of mechanosensory transduction, again consistent with colocalization of mechanosensory transduction channels and protocadherin 15 tip link insertion sites (44-46). Overall, biochemical evidence obtained in the present investigation strongly points to HCN1 as binding the tip link antigen protocadherin 15 CD3, implicating a role for HCN1 in hair cell mechanosensory transduction.
Supplementary Material
Acknowledgments
We thank Dr. Stanley Terlecky for the use of the SPR facility and Biacore 3000 instrument. We further acknowledge Dr. Ted Huston (Keck ICP-MS Laboratory, University of Michigan) for analyses of Ca2+ concentrations and helpful insight.
This work was supported, in whole or in part, by National Institutes of Health Grants DC04076 and DC000156. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: HCN1, hyperpolarization-activated, cyclic nucleotide-gated nonselective cation channel isoform 1; HCN1-N, HCN1 amino-terminal cytoplasmic region; protocadherin 15 CD3-C, carboxyl-terminal sequence of protocadherin 15 CD3; 3-AT, 3-amino-1,2,4-triazole; aa, amino acid(s); PBS, phosphate-buffered saline; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; GST, glutathione S-transferase; SPR, surface plasmon resonance.
References
- 1.Cho, W. J., Drescher, M. J., Hatfield, J. S., Bessert, D. A., Skoff, R. P., and Drescher, D. G. (2003) Neuroscience 118 525-534 [DOI] [PubMed] [Google Scholar]
- 2.Holt, J. R., and Eatock, R. A. (1995) J. Neurophysiol. 73 1484-1502 [DOI] [PubMed] [Google Scholar]
- 3.Farris, H. E., Wells, G. B., and Ricci A. J. (2006) J. Neurosci. 26 12526-12536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramakrishnan, N. A., Drescher, M. J., and Drescher, D. G. (2007) Assoc. Res. Otolaryngol. Abstr. 30 31 [Google Scholar]
- 5.Proenza, C., Angoli, D., Agranovich, E., Macri, V., and Accili, E. A. (2002) J. Biol. Chem. 277 5101-5109 [DOI] [PubMed] [Google Scholar]
- 6.Seiler, C., Finger-Baier, K. C., Rinner, O., Makhankov, Y. V., Schwarz, H., Neuhauss, S. C., and Nicolson, T. (2005) Development 132 615-623 [DOI] [PubMed] [Google Scholar]
- 7.Ahmed, Z. M., Goodyear, R., Riazuddin, S., Lagziel, A., Legan, P. K., Behra, M., Burgess, S. M., Lilley, K. S., Wilcox, E. R., Riazuddin, S., Griffith, A. J., Frolenkov, G. I., Belyantseva, I. A., Richardson, G. P., and Friedman, T. B. (2006) J. Neurosci. 26 7022-7034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazmierczak, P., Sakaguchi, H., Tokita, J., Wilson-Kubalek, E. M., Milligan, R. A., Müller, U., and Kachar, B. (2007) Nature 449 87-92 [DOI] [PubMed] [Google Scholar]
- 9.Drescher, D. G., Khan, K. M., Arden, R. L., Drescher, M. J., Kerr, T. P., and Hatfield, J. S. (1989) Brain Res. 485 225-235 [DOI] [PubMed] [Google Scholar]
- 10.Drescher, M. J., and Drescher, D. G. (1991) J. Neurochem. 56 658-664 [DOI] [PubMed] [Google Scholar]
- 11.Oh, C. K., Drescher, M. J., Hatfield, J. S., and Drescher, D. G. (1999) Brain Res. Mol. Brain Res. 70 135-140 [DOI] [PubMed] [Google Scholar]
- 12.Drescher, M. J., Drescher, D. G., Khan, K. M., Hatfield, J. S., Ramakrishnan, N. A., Abu-Hamdan, M. D., and Lemonnier, L. A. (2006) Neuroscience 142 139-164 [DOI] [PubMed] [Google Scholar]
- 13.Khan, K. M., Drescher, M. J., Hatfield, J. S., Khan, A.-M., and Drescher, D. G. (2002) Neuroscience 111 291-302 [DOI] [PubMed] [Google Scholar]
- 14.Barritt, L. C., Fritzsch, B., and Beisel, K. W. (1999) Mol. Brain Res. 68 42-54 [DOI] [PubMed] [Google Scholar]
- 15.Drescher, M. J., Khan, K. M., Beisel, K. W., Karadaghy, A. A., Hatfield, J. S., Kim, S. Y., Drescher, A. J., Lasak, J. M., Barretto, R. L., Shakir, A. H., and Drescher, D. G. (1997) Mol. Brain Res. 45 325-330 [DOI] [PubMed] [Google Scholar]
- 16.Drescher, D. G., Ramakrishnan, N. A., and Drescher, M. J.(2009) Methods Mol. Biol. 493 323-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drescher, D. G., Ramakrishnan, N. A., Drescher, M. J., Chun, W., Wang, X., Myers, S. F., Green, G. E., Sadrazodi, K., Karadaghy, A. A., Poopat, N., Karpenko, A. N., Khan, K. M., and Hatfield, J. S. (2004) Neuroscience 127 737-752 [DOI] [PubMed] [Google Scholar]
- 18.Ludwig, A., Zong, X., Jeglitsch, M., Hofmann, F., and Biel, M. (1998) Nature 393 587-591 [DOI] [PubMed] [Google Scholar]
- 19.Yu, X., Duan, K.-L., Shang, C.-F., Yu, H.-G., and Zhou, Z. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 1051-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu, X., Chen, X.-W., Zhou, P., Yao, L., Liu, T., Zhang, B., Li, Y., Zheng, H., Zheng, L.-H., Zhang, C. X., Bruce, I., Ge, J.-B., Wang, S.-Q., Hu, Z.-A., Yu, H.-G., and Zhou, Z. (2007) Am. J. Physiol. 292 C1147-C1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gauss, R., Seifert, R., and Kaupp, U. B. (1998) Nature 393 583-587 [DOI] [PubMed] [Google Scholar]
- 22.Ishii, T. M., Takano, M., Xie, L.-H., Noma, A., and Ohmori, H. (1999) J. Biol. Chem. 274 12835-12839 [DOI] [PubMed] [Google Scholar]
- 23.Seifert, R., Scholten, A., Gauss, R., Mincheva, A., Lichter, P., and Kaupp, U. B. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 9391-9396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decher, N., Bundis, F., Vajna, R., and Steinmeyer, K. (2003) Pflügers Arch. Eur. J. Physiol. 446 633-640 [DOI] [PubMed] [Google Scholar]
- 25.Macri, V., and Accili, E. A. (2004) J. Biol. Chem. 279 16832-16846 [DOI] [PubMed] [Google Scholar]
- 26.Azene, E. M., Xue, T., Marbán, E., Tomaselli, G. F., and Li, R. A. (2005) Cardiovasc. Res. 67 263-273 [DOI] [PubMed] [Google Scholar]
- 27.Mistrík, P., Pfeifer, A., and Biel, M. (2006) Pflügers Arch. Eur. J. Physiol. 452 718-727 [DOI] [PubMed] [Google Scholar]
- 28.Chen, J., Mitchson, J. S., Tristani-Firouzi, M., Lin, M., and Sanguinetti, M. C. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 11277-11282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Proenza, C., and Yellen, G. (2006) J. Gen. Physiol. 127 183-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugihara, I., and Furukawa, T. (1996) J. Physiol. 495 665-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masetto, S., and Correia, M. J. (1997) J. Neurophysiol. 78 1913-1927 [DOI] [PubMed] [Google Scholar]
- 32.Rüsch, A., Lysakowski, A., and Eatock, R. A. (1998) J. Neurosci. 18 7487-7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagger, D. J., Holley, M. C., and Ashmore, J. F. (1999) Pflügers Arch. Eur. J. Physiol. 438 8-14 [DOI] [PubMed] [Google Scholar]
- 34.Santoro, B., Liu, D. T., Yao, H., Bartsch, D., Kandel, E. R., Siegelbaum, S. A., and Tibbs, G. R. (1998) Cell 93 717-729 [DOI] [PubMed] [Google Scholar]
- 35.Enger, P. S. (1964) Comp. Biochem. Physiol. 11 131-137 [DOI] [PubMed] [Google Scholar]
- 36.Ghanem, T. A., Breneman, K. D., Rabbitt, R. D., and Brown, H. M. (2008) Biol. Bull. 214 83-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sterkers, O., Ferrary, E., and Amiel, C. (1984) Am. J. Physiol. 247 F602-F606 [DOI] [PubMed] [Google Scholar]
- 38.Corey, D. P., and Hudspeth, A. J. (1979) Biophys. J. 26 499-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin, W., Laitko, U., Juranka, P. F., and Morris, C. E. (2007) Biophys. J. 92 1559-1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin, K. S., Rothberg, B. S., and Yellen, G. (2001) J. Gen. Physiol. 117 91-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin, P., Bozovic, D., Choe, Y., and Hudspeth, A. J. (2003) J. Neurosci. 23 4533-4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricci, A. J., Kachar, B., Gale, J., and Van Netten, S. M. (2006) J. Membr. Biol. 209 71-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kachar, B., Parakkal, M., Kurc, M., Zhao, Y., and Gillespie P. G. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 13336-13341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harasztosi, C., and Gummer, A. W. (2008) Assoc. Res. Otolaryngol. Abstr. 31 654 [Google Scholar]
- 45.Srinivasan, A., Harasztosi, C., and Gummer, A. W. (2008) Assoc. Res. Otolaryngol. Abstr. 31 655 [Google Scholar]
- 46.Nam, J.-H., and Fettiplace, R. (2008) Assoc. Res. Otolaryngol. Abstr. 31 658 [Google Scholar]
- 47.Rost, B., and Sander, C. (1993) J. Mol. Biol. 232 584-599 [DOI] [PubMed] [Google Scholar]
- 48.De Brouwer, A. P. M., Pennings, R. J. E., Roeters, M., Van Hauwe, P., Astuto, L. M., Hoefsloot, L. H., Huygen, P. L. M., ven den Helm, B., Deutman, A. F., Bork, J. M., Kimberling, W. J., Cremers, F. P. M., Cremers, C. W. R. J., and Kremer, H. (2003) Hum. Genet. 112 156-163 [DOI] [PubMed] [Google Scholar]
- 49.Sewell, W. F., and Mroz, E. A. (1993) Hear. Res. 70 131-138 [DOI] [PubMed] [Google Scholar]
- 50.Marsden B. J., Shaw, G. S., and Sykes, B. D. (1990) Biochem. Cell Biol. 68 587-601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.