Abstract
17-Allylamino-17-demethoxy geldanamycin (17-AAG), an inhibitor of heat shock protein 90 (Hsp90) function, is being developed as antitumor drug in patients with breast cancer. However, water-insolubility and hepatotoxicity limit its use. The purpose of this study was to begin to address these issues by determining whether 17-AAG can be formulated in long-circulating (PEGylated), biocompatible and biodegradable sterically stabilized phospholipid nanomicelles (SSM) to which vasoactive intestinal peptide (VIP) was grafted as an active targeting moiety and, if so, whether these nanomicelles are cytotoxic to MCF-7 human breast cancer cells. We found that particle size of 17-AAG loaded in VIP surface-grafted SSM was 16±1 nm and drug content was 97±2% (300 μg/ml). Cytotoxicity of 17-AAG loaded in VIP surface-grafted SSM to MCF-7 cells was significantly higher than that of 17-AAG loaded in non-targeted SSM (p<0.05) and similar to that of 17-AAG dissolved in dimethylsulfoxide. Collectively, these data demonstrate that 17-AAG is solubilized at therapeutically relevant concentrations in actively targeted VIP surface-grafted SSM. Cytotoxicity of these nanomicelles to MCF-7 cells is retained implying high affinity VIP receptors overexpressed on these cells mediate, in part, their intracellular uptake thereby amplifying drug potency. We propose that 17-AAG loaded in VIP surface-grafted SSM should be further developed as an actively targeted nanomedicine for breast cancer.
Keywords: nanomedicine, cancer, MCF-7 cells, chemotherapy, water-insoluble drugs, 17-AAG, Hsp90, targeting, DSPE-PEG2000
Introduction
It is well established that heat shock protein 90 (Hsp90), a ubiquitous intracellular molecular chaperone involved in folding and activation of several signaling proteins, is over-expressed in various cancers, most notably breast cancer (Ferrarini et al., 1992; Workman 2004). This, in turn, promotes growth and survival of tumor cells (Whitesell et al., 1994; Hanahan et al., 2000). Accordingly, inhibition of the biologic function of Hsp90 represents an important target for cancer therapeutics, including breast cancer (Workman 2004; Maloney et al., 2002; Neckers et al., 2002; Belikoff et al., 2004; Modi et al., 2007).
To this end, 17-allylamino-17-demethoxy geldanamycin (17-AAG), a semi-synthetic derivative of the ansamycin antibiotic geldanamycin, binds to ATP binding site in the N-terminal domain of Hsp90 thereby mitigating its chaperone activity (Workman 2004; Whitesell et al., 1994; DeBoer et al., 1970). This, in turn, promotes proteasomal-mediated degradation of its client proteins and inhibits cancer cell proliferation (Workman 2004; Whitesell et al., 1994; Hanahan et al., 2002; DeBoer et al., 1970). However, clinical use of 17-AAG is hampered by its water-insolubility and hepatotoxicity (Modi et al., 2007; Solit et al., 2007; Weigel et al., 2007). Attempts to overcome these problems by formulating 17-AAG in solvents, such as dimethylsulfoxide (DMSO) and cremophor are fraught with potentially serious adverse events (Modi et al., 2007; Solit et al., 2007; Weigel et al., 2007; Rowinsky et al., 1993). Given Hsp90 are also expressed in normal cells (Ferrarini et al., 1992; Workman 2004), administration of these and other non-actively targeted formulations of 17-AAG could also be associated with collateral damage to healthy tissues (Modi et al., 2007; Solit et al., 2007; Weigel et al., 2007). Hence, there is an ongoing need to develop new formulations of 17-AAG with improved water-solubility and therapeutic index.
To address both water-insolubility and toxicity of potent anti-cancer drugs, we developed long-circulating, actively-targeted, sterically stabilized phospholipid nanomicelles (∼16 nm) using U.S. FDA generally-regarded as safe (GRAS) compounds, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene)glycol)-2000] (DSPE-PEG2000) to which vasoactive intestinal peptide (VIP), a ubiquitous 28-amino acid pleiotropic mammalian peptide (Gomariz et al., 2001), was grafted to the tip of PEG molecule as an active targeting moiety (Krishnadas et al., 2003; Koo et al., 2005; Working et al., 1996).
These phospholipid nanomicelles are simple to prepare, form spontaneously above their critical micellar concentration (micromolar range), stable upon dilution in aqueous environment with reproducible size distribution and are lyophilized without cryo- and lyoprotectants for long-term storage (Krishnadas et al., 2003; Koo et al., 2005; Ashok et al., 2004; Arleth et al., 2005). Given numerous cancers, most notably breast cancer, overexpress high affinity VIP (VPAC1) receptors on plasma membrane of tumor cells, surface grafting of SSM with VIP could promote active targeting of these drug-loaded nanocarriers selectively to cancer through local microvascular enhanced permeability and retention (EPR) effect and subsequent binding of VIP to its cognate receptors on cancer cells (Dagar et al., 2001; Dagar et al., 2003; Gespach et al., 1988; Reubi 1996, Rubinstein et al., 2007). This, in turn, will improve the therapeutic index of the anti-cancer drug (Krishnadas et al., 2003; Koo et al., 2005). Accordingly, we found that VIP surface-grafted SSM solubilize high concentrations of paclitaxel and camptothecin, two potent, albeit water-insoluble, anti-cancer drugs, within their hydrophobic core and amplified the anti-cancer effects of both drugs (Krishnadas et al., 2003; Koo et al., 2005). Taken together, these data suggest that VIP surface-grafted SSM could also be used to solubilize and actively target 17-AAG to breast cancer.
Thus, the purpose of this study was to begin to address this issue by determining whether 17-AAG can be formulated in VIP surface-grafted SSM and, if so, whether these nanomicelles are cytotoxic to MCF-7 human breast cancer cells which are known to over express VIP receptors (Gespach et al., 1988).
Materials and Methods
Chemicals
N-[Methoxy (polyethylene glycol-2000)] carbonyl-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE-PEG2000) was obtained from Lipoid GmbH (Ludwigshafen, Germany). N-[Methoxy (polyethylene glycol-3400)] carbonyl-1,2-distearoyl-sn-glycero-3-phosphoethanolamine-succinimidylpropionate (DSPE-PEG3400-SPA) was purchased from Nektar Therapeutics (Huntsville, AL). 17-AAG was procured from A.G. Scientific (San Diego, CA). Vasoactive intestinal peptide was synthesized by Protein Research Laboratory, Research Resources Center, University of Illinois at Chicago. MCF-7 cells (#HTB-22), fetal bovine serum, trypsin-EDTA and Eagle's Minimum Essential Medium (EMEM) with Earle's Balanced Salt System (BSS) were obtained from American Type Culture Collection (Manassas, VA). Bovine insulin, DMSO, HEPES buffer, glycine, Tris-HCl, sulforhodamine B, trichloroacetic acid were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Phosphate buffered saline (PBS) was obtained from Mediatech Cellgro (Herndon, VA). HPLC-grade methanol and acetonitrile were procured from Fisher Scientific (Itasca, IL). All chemicals were of analytical grade and used as received.
Preparation and characterization of 17-AAG in phospholipid nanomicelles
Dispersions of 17-AAG solubilized in SSM were prepared by the co-precipitation/reconstitution method as previously employed in our laboratory (Krishnadas et al., 2003; Koo et al., 2005; Ashok et al., 2004). Briefly, varying concentrations of 17-AAG and 5 mM DSPE-PEG2000 were dissolved in methanol. The solvent was then removed using vacuum rotary evaporator to form a dry film. Complete dryness was accomplished by desiccation under vacuum overnight. Thereafter, the film was rehydrated with HEPES buffer (10 mM; pH 7.4) and the resulting dispersion vortexed followed by bath sonication. The dispersion was then flushed with argon, sealed and equilibrated for 3 h at room temperature in complete darkness. Excess unsolubilized 17-AAG was removed by centrifugation at 13,000 g for 5 min to obtain a clear dispersion. Optimal SSM formulations were chosen for further studies based on formation of a homogenous system with maximum solubilization of 17-AAG (see below).
Particle size of aqueous dispersions of 17-AAG in the different formulations of SSM were determined by quasi-elastic light scattering using a NICOMP 380 Submicron Particle Sizer (Particle Sizing Systems Inc, Menlo Park, CA) equipped with a temperature controlled cell holder, a 5 mW helium-neon laser (excitation at 632.8 nm) with detection at a fixed scattering angle of 90° and data were analyzed by volume and intensity-weighted distributions (Krishnadas et al., 2003; Koo et al., 2005; Ashok et al., 2004).
Content of 17-AAG in SSM was determined by RP-HPLC as previously described in our laboratory (Krishnadas et al., 2003; Koo et al., 2005; Ashok et al., 2004). Clear aqueous drug containing micellar dispersions were dissolved in methanol. Each sample preparation was injected (20μl injection volume) in triplicate through a Spectra System AS3500 autosampler (Thermo Separation Products, Waltham, MA) into a Zorbax SB-C18 column (5 μm pore size, 4.6 mm ID, 25 cm length; Agilent Technologies, Santa Clara, CA) equipped with a C18 column guard. The column was eluted with mobile phase composed of acetonitrile and water (70:30 v/v) at 1.0 ml/min flow rate (Spectra System P2000). Detection was by UV absorption measurement at 330 nm (Spectra Focus). Chromatographic peak areas were integrated by using software, Chromquest™ 4.0 (Thermo Separation Products). A standard curve was generated using 17-AAG dissolved in methanol and sample concentrations were determined by regression analysis of the standard curve. The assay was linear over the tested concentration range, and there was no interference of the phospholipids with the assay (Krishnadas et al., 2003; Koo et al., 2005; Ashok et al., 2004).
VIP was conjugated to the distal end of the PEG moiety of DSPE as previously described in our laboratory (Krishnadas et al., 2003; Dagar et al., 2003). Briefly, an activated 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-3400]-succinimidyl propionate (DSPE-PEG3400-SPA) was used to conjugate VIP to DSPE-PEG3400. For the conjugation reaction, VIP and DSPE-PEG3400-SPA in the molar ratio of 1:5 (VIP: DSPE-PEG3400-SPA) were dissolved separately in cold HEPES buffer (10 mM, pH 6.6). DSPE-PEG3400-SPA solution was added in small increments to the VIP solution at 4 °C with gentle stirring. The reaction was allowed to proceed for 2 h at 4 °C and then stopped by adding 1 M glycine solution to the reaction mixture to consume the remaining NHS moieties. Conjugation was ascertained by SDS-PAGE electrophoresis. Thereafter, the DSPE-PEG3400 reaction mixture was added to 17-AAG loaded in SSM to obtain a final concentration of 5 mM phospholipid and 0.3 mM VIP conjugate. The mixture was equilibrated in the dark at 25 °C for 30 min to yield VIP surface-grafted SSM loaded with 17-AAG. Particle size and drug content of this formulation was determined as outlined above.
Cytotoxicity of 17-AAG loaded SSM to MCF-7 cells
Cytotoxicity of 17-AAG loaded in SSM with and without surface-grafted VIP to MCF-7 cells was determined as previously described in our laboratory (Krishnadas et al., 2003; Koo et al., 2005; Rubinstein et al., 2007). Cells were maintained in a humidified atmosphere with 5% CO2 at 37 °C in EMEM with 2 mM L-glutamine and Earle's BSS adjusted to contain 1.5g/L sodium bicarbonate, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate and supplemented with 0.01 mg/ml bovine insulin and 10% fetal bovine serum. Cells (6×104 cells/ml) were plated in a 96-well plate in triplicate. Drugs and controls were serially diluted and added to each well. The concentration of 17-AAG was 0.0025-1.0 μg/ml. Empty SSM and empty VIP surface-grafted SSM at lipid concentration similar to that of the highest concentration of micellar 17-AAG (1μg/ml), DMSO (10%) and HEPES buffer were used as vehicle controls. In addition, 17-AAG (1.0 μg/ml) dissolved in DMSO (10%) was used as positive control. Final concentration of DMSO in each well was 0.5%. Plates were incubated for 72 h in 5% CO2 humidified atmosphere at 37°C. Thereafter, the sulfo rhodamine B cytotoxicity assay was used to determine cell viability spectrophotometrically as previously described in the literature (Vichai et al., 2006) by measuring optical density (O.D.) of acetic acid fixed, sulforhodamine B treated cells at was at 515 nm (SpectraMax Plus384, Molecular Devices, Sunnyvale, CA). Readings obtained for buffer controls were used to define 100% growth. Per cent cell survival was calculated as follows:
Growth curves of per cent survival versus 17-AAG concentration were plotted and GI50 values calculated using nonlinear regression analysis.
Data and statistical analyses
Data are expressed as means±SD. Analysis of variance followed by Tukey's post-hoc test were used for statistical analysis. P<0.05 was considered statistically significant.
Results
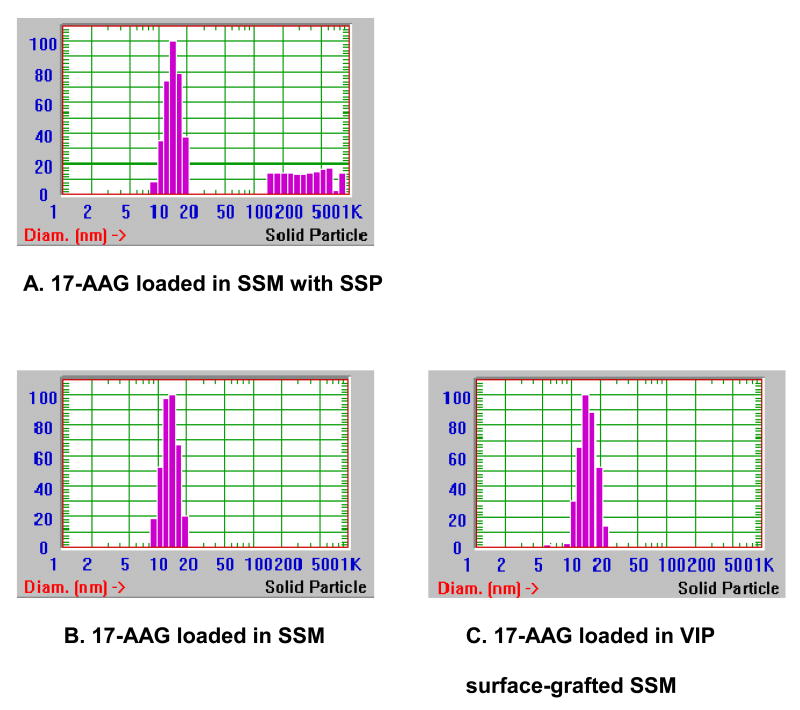
Table 1 depicts formulation characteristics of 17-AAG self-associated with SSM. At 17-AAG concentrations up to 300 μg/ml, only a single homogenous particle species was detected by quasi-elastic light scattering while above it sterically stabilized drug particles (SSP) ranging from 100 to 400 nm in size were observed as well (Figure 1A) (Krishnadas et al., 2003). At 17-AAG concentration of 400 μg/ml, clear precipitation was seen after centrifugation. Thus, lipid:17-AAG molar ratio of 1:0.1 corresponding to 300 μg/ml drug in 5 mM was the optimal solubilization ratio for 17-AAG in SSM with drug content of 93±3% by RP-HPLC analysis (n=3). Particle size (16±1 nm) and drug content (97±2%) of VIP surface-grafted SSM (5 mM) loaded with 17-AAG (300 μg/ml) were similar to those of non-targeted SSM loaded with 17-AAG (Figures 1B&C).
Table 1. Formulation characteristics of 17-allylamino-17-demethoxy geldanamycin self-associated with sterically stabilized phospholipid nanomicelles*.
| Lipid:17-AAG ratio | 17-AAG concentration (μg/ml) In 5 mM SSM |
Particle size (nm) (n=3) |
SSP |
|---|---|---|---|
| Blank SSM | 0 | 15±1 | No |
| 1:0.035 | 100 | 15±1 | No |
| 1:0.05 | 150 | 15±1 | No |
| 1:0.07 | 200 | 15±1 | No |
| 1:0.085 | 250 | 15±1 | No |
| 1:0.1 | 300 | 14±1 | No |
| 1:0.12 | 350 | 16±1 | Yes |
| 1:0.13 | 375 | 15±1 | Yes |
| 1:0.14 | 400 | 13±2 | Yes |
Data are means±SD
17-AAG, 17-allylamino-17-demethoxy geldanamycin; SSM, sterically stabilized nanomicelles; SSP, sterically stabilized drug particles
Figure 1. Representative particle size distribution of 17-allylamino-17-demethoxy geldanamycin nanoformulations.
17-AAG, 17-allylamino-17-demethoxy geldanamycin; SSM, sterically stabilized nanomicelles; SSP, sterically stabilized drug particles; VIP, vasoactive intestinal peptide
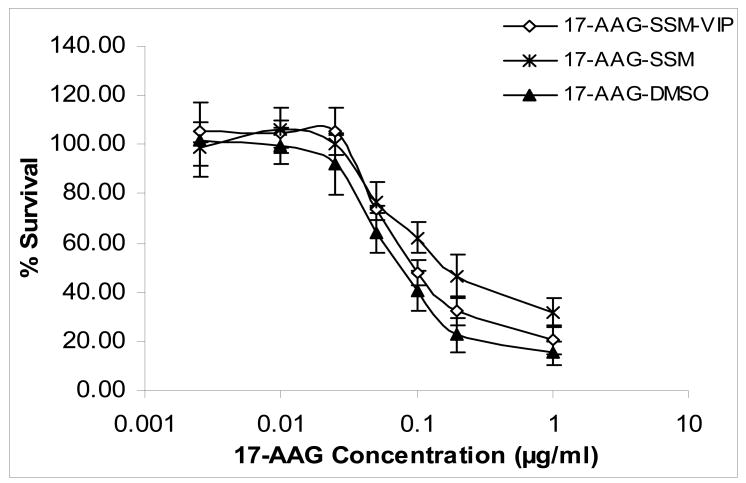
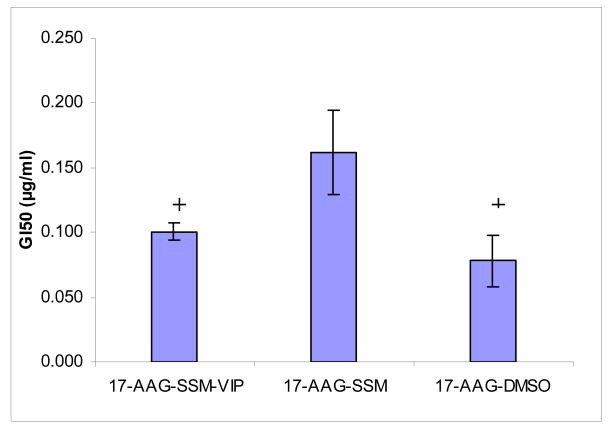
Cytotoxicity and GI50 of various 17-AAG formulations to MCF-7 human breast cancer cells are shown in Figures 2&3. Cytotoxicity of VIP surface-grafted SSM loaded with 17-AAG was significantly higher than that of non-targeted SSM loaded with 17-AAG (Figures 2&3; each, n=3; p<0.05). The cytotoxic effects of VIP surface-grafted SSM loaded with 17-AAG was similar to that of 17-AAG dissolved in DMSO (Figures 2&3; each, n=3; p>0.5). Empty SSM, VIP surface-grafted empty SSM, DMSO and HEPES buffer had no significant effects in MCF-7 cells (n=3; p>0.5; data not shown).
Figure 2. Cytotoxicity of various 17-allylamino-17-demethoxy geldanamycin formulations to MCF-7 human breast cancer cells*.
*Data are means±SD after 72-h incubation (each data point, n=3 experiments). Significance markers were omitted for clarity (see Results and Figure 3 for explanation).
17-AAG, 17-allylamino-17-demethoxy geldanamycin; SSM; sterically stabilized nanomicelles; VIP, vasoactive intestinal peptide; DMSO, dimethylsulfoxide
Figure 3. GI50 (concentration of drug at which 50% growth inhibition is seen) of various 17-allylamino-17-demethoxy geldanamycin formulations to MCF-7 human breast cancer cells*.
*Data are means±SD after 72-h incubation (each data point, n=3 experiments). +P<0.05 in comparison to 17-AAG-SSM.
17-AAG, 17-allylamino-17-demethoxy geldanamycin; SSM; sterically stabilized nanomicelles; VIP, vasoactive intestinal peptide; DMSO, dimethylsulfoxide
Discussion
The new findings of this study are that 17-AAG was successfully solubilized and monodispersed at therapeutically relevant concentrations (300 μg/ml) in long-acting (PEGylated), biocompatible and biodegradable sterically stabilized phospholipid nanomicelles in the absence and presence of surface-grafted VIP as an active targeting moiety. In addition, cytotoxicity of VIP surface-grafted SSM loaded with 17-AAG to MCF-7 human breast cancer cells was similar to that of the drug dissolved in DMSO and superior to that of non-targeted drug-loaded phospholipid nanomicelles. Taken together, these data indicate that 17-AAG formulated in SSM retains its cytotoxicity to MCF-7 human breast cancer cells and that high affinity overexpressed VIP (VPAC1) receptors on these cells mediate, in part, intracellular uptake of 17-AAG-loaded phospholipid nanomicelles thereby amplifying drug potency.
The results of this study extend previous reports from our laboratory where both water-insoluble paclitaxel and camptothecin, two potent anti-cancer drugs, were successfully formulated in VIP surface-grafted SSM at therapeutically relevant concentrations in the absence of secondary species i.e. sterically stabilized drug particles (Krishnadas et al., 2003; Koo et al., 2005). Accordingly, we propose that VIP surface-grafted SSM loaded with 17-AAG should be further developed as an actively targeted nanomedicine for breast cancer.
We found that unlike VIP surface-grafted SSM loaded with 17-AAG, cytotoxic effects of non-targeted 17-AAG-loaded SSM were smaller than that of 17-AAG dissolved in DMSO. This phenomenon may be related, in part, to greater ability of free 17-AAG molecules to interact with and penetrate the plasma membrane of MCF-7 cells and accumulate intracellularly (Workman 2004; Whitesell et al., 1994; Neckers et al., 2002; Belikoff et al., 2004; DeBoer et al., 1970). By contrast, self-association of 17-AAG with nanomicelles may impede its interactions with plasma membrane over the observation period (72 h) because drug molecules must be first released from the nanocarrier, a time-dependent phenomenon, before interaction with the plasma membrane ensues. This, in turn, may attenuate the cytotoxic effects of nanomicellar 17-AAG.
Alternatively, non-targeted 17-AAG-loaded SSM may be internalized into MCF-7 cells as nanoparticles through various pathways, such as phagocytosis, macropinocytosis, clathrin-mediated endocytosis and caveolae-mediated endocytosis, that express slower kinetics relative to that of free 17-AAG molecules (Working et al., 1996). This hypothesis is supported, in part, by a recent study from our laboratory showing slower intracellular accumulation of non-targeted hydrophobic quantum dots-loaded sterically stabilized nanomicelles in MCF-7 cells relative to that of nanomicelles decorated with VIP (Rubinstein et al., 2007). The results of this study extend these observations by demonstrating that cytotoxicity of VIP surface-grafted SSM loaded with 17-AAG is significantly higher in comparison to non-targeted 17-AAG-loaded nanomicelles in these cells.
Conceivably, the nanosize of VIP surface-grafted SSM loaded with 17-AAG (∼16 nm) coupled with their steric stabilization conferred by PEG2000 moiety and absence of VIP receptors on plasma membrane of endothelial cells could prolong their circulation time because these nanoparticles will not extravasate from intact microvascular beds nor removed by the reticuloendothelial system (Krishnadas et al., 2003; Working et al., 1996 Dagar et al., 2001; Dagar et al., 2003; Reubi 1996). This, in turn, would enable active targeting of VIP surface-grafted SSM loaded with 17-AAG to breast cancer through local enhanced permeability and retention (EPR) effect and VIP binding to its high affinity overexpressed cognate receptors on cancer cells thereby improving the therapeutic index of the drug (Gomariz et al., 2001; Krishnadas et al., 2003; Dagar et al., 2001; Dagar et al., 2003; Gespach et al., 1988; Reubi 1996; Rubinstein et al., 2007). Clearly, additional in vivo studies are warranted to support or refute this hypothesis.
In summary, we found that 17-AAG is solubilized at therapeutically relevant concentrations in actively targeted VIP surface-grafted SSM. Cytotoxicity of these nanomicelles to MCF-7 human breast cancer cells is retained implying high affinity VIP (VPAC1) receptors overexpressed on these cells mediate, in part, their intracellular uptake thereby amplifying drug potency. We propose that 17-AAG loaded in VIP surface-grafted SSM should be further developed as an actively targeted nanomedicine for breast cancer.
Acknowledgments
We thank Ernest Gemeinhart for assistance with cell culture experiments. This study was supported, in part, by NIH grants RO1 AG024026 and R01 CA121797, VA Merit Review and Department of Defense (DOD) grant BCRP DAMD 17-02-1-0415 and DOD contract W81XWH-07-1-0445. Research was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR15482 from the National Center for Research Resources.
List of abbreviations
- 17-AAG
17-allylamino-17-demethoxy geldanamycin
- SSM
sterically stabilized nanomicelles
- SSP
sterically stabilized drug particles
- VIP
vasoactive intestinal peptide
- DMSO
dimethylsulfoxide
- DSPE-PEG2000
poly(ethylene glycol)-2000 -grafted distearoylphosphatidylethanolamine
- DSPE-PEG3400-SPA
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-3400]-succinimidyl propionate
- Hsp90
heat shock protein 90
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arleth L, Ashok B, Önyüksel H, Thiyagarajan P, Jacob J, Hjelm RP. Detailed structure of hairy mixed micelles formed by phosphatidylcholine and PEGylated phospholipids in aqueous media. Langmuir. 2005;21:3279–3290. doi: 10.1021/la047588y. [DOI] [PubMed] [Google Scholar]
- 2.Ashok B, Arleth L, Hjelm RP, Rubinstein I, Önyüksel H. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: effects of PEG chain length and PC incorporation. J Pharm Sci. 2004;93:2476–2487. doi: 10.1002/jps.20150. [DOI] [PubMed] [Google Scholar]
- 3.Belikoff J, Whitesell L. Hps 90: an emerging target for breast cancer therapy. Anticancer Drugs. 2004;15:651–662. doi: 10.1097/01.cad.0000136876.11928.be. [DOI] [PubMed] [Google Scholar]
- 4.Dagar S, Sekosan M, Lee BS, Rubinstein I, Önyüksel H. VIP receptors as molecular targets of breast cancer: implications for targeted imaging and drug delivery. J Control Release. 2001;74:129–134. doi: 10.1016/s0168-3659(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 5.Dagar S, Krishnadas A, Rubinstein I, Blend MJ, Önyüksel H. VIP grafted sterically stabilized liposomes for targeted imaging of breast cancer: in vivo studies. J Control Release. 2003;91:123–133. doi: 10.1016/s0168-3659(03)00242-6. [DOI] [PubMed] [Google Scholar]
- 6.DeBoer C, Meulman PA, Wnuk RJ, Peterson DH. Geldanamycin, a new antibiotic. J Antibiot (Tokyo) 1970;23:442–447. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]
- 7.Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992;51:613–619. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- 8.Gespach C, Bawab W, de Cremoux P, Calvo F. Pharmacology, molecular identification and functional characteristics of vasoactive intestinal peptide receptors in human breast cancer cells. Cancer Res. 1988;48:5079–5083. [PubMed] [Google Scholar]
- 9.Gomariz RP, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr Pharm Des. 2001;7:89–111. doi: 10.2174/1381612013398374. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 11.Koo OM, Rubinstein I, Önyüksel H. Camptothecin in sterically stabilized phospholipid micelles: a novel nanomedicine. Nanomedicine. 2005;1:77–84. doi: 10.1016/j.nano.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Krishnadas A, Rubinstein I, Önyüksel H. Sterically stabilized phospholipid mixed micelles: in vitro evaluation as a novel carrier for water-insoluble drugs. Pharm Res. 2003;20:297–302. doi: 10.1023/a:1022243709003. [DOI] [PubMed] [Google Scholar]
- 13.Maloney A, Workman P. HSP90 as a new therapeutic target for cancer therapy: the story unfolds. Expert Opin Biol Ther. 2002;2:3–24. doi: 10.1517/14712598.2.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Modi S, Stopeck AT, Gordon MS, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 15.Neckers L. Heat shock protein 90 is a rational molecular target in breast cancer. Breast Dis. 2002;15:53–60. doi: 10.3233/bd-2002-15106. [DOI] [PubMed] [Google Scholar]
- 16.Reubi JC. In vitro identification of VIP receptors in human tumors: potential clinical implications. Ann NY Acad Sci. 1996;805:753–759. doi: 10.1111/j.1749-6632.1996.tb17553.x. [DOI] [PubMed] [Google Scholar]
- 17.Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- 18.Rubinstein I, Soos I, Önyüksel H. Intracellular delivery of VIP-grafted sterically stabilized phospholipid mixed nanomicelles in human breast cancer cells. Chem Biol Interact. 2008;171:190–194. doi: 10.1016/j.cbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solit DB, Ivy SP, Kopil C, et al. Phase I trial of 17-allylamino-17-demethoxy geldanamycin in patients with advanced cancer. Clin Cancer Res. 2007;13:1775–1782. doi: 10.1158/1078-0432.CCR-06-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 21.Weigel BJ, Blaney SM, Reid JM, et al. A phase I study of 17-allylaminogeldanamycin in relapsed/refractory pediatric patients with solid tumors: a Children's Oncology Group study. Clin Cancer Res. 2007;13:1789–1793. doi: 10.1158/1078-0432.CCR-06-2270. [DOI] [PubMed] [Google Scholar]
- 22.Whitesell L, Mimnaugh E, Costa B, Myers C, Neckers L. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone anamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Working PK, Dayan AD. Pharmacological-toxicological expert report. CAELYX. (Stealth liposomal doxorubicin HCl) Hum Exp Toxicol. 1996;15:751–785. [PubMed] [Google Scholar]
- 24.Workman P. Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett. 2004;206:149–157. doi: 10.1016/j.canlet.2003.08.032. [DOI] [PubMed] [Google Scholar]