Abstract
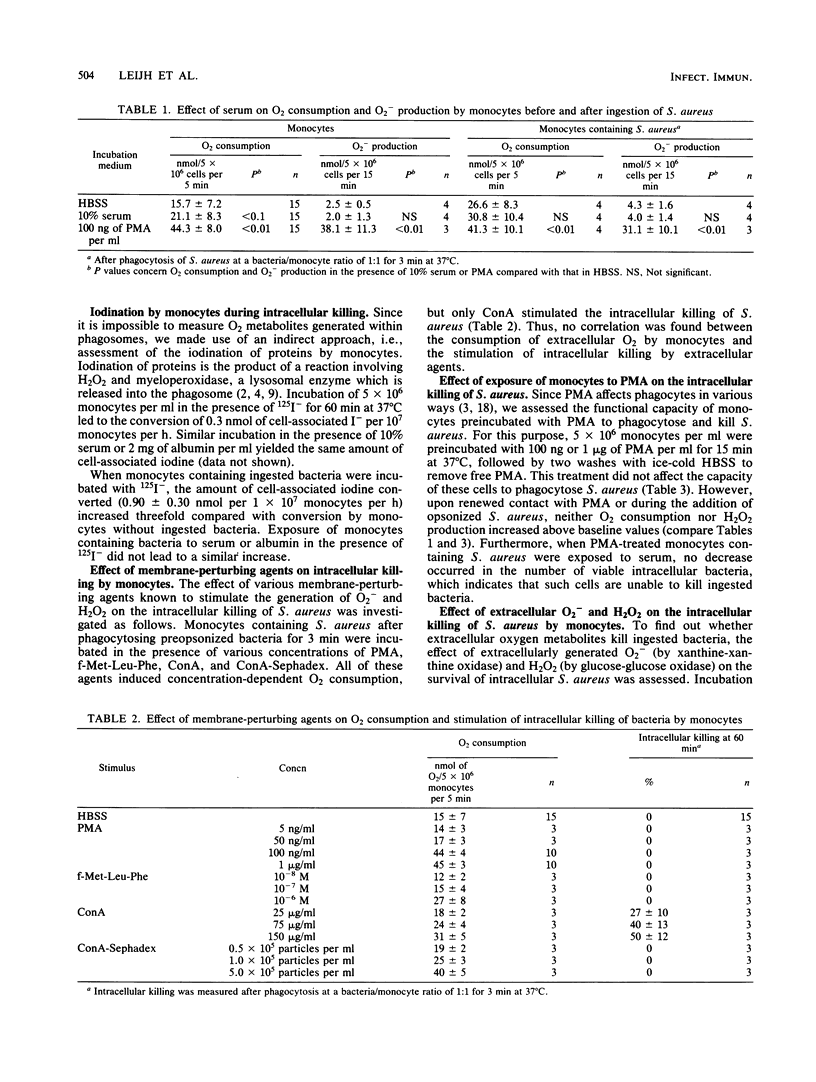
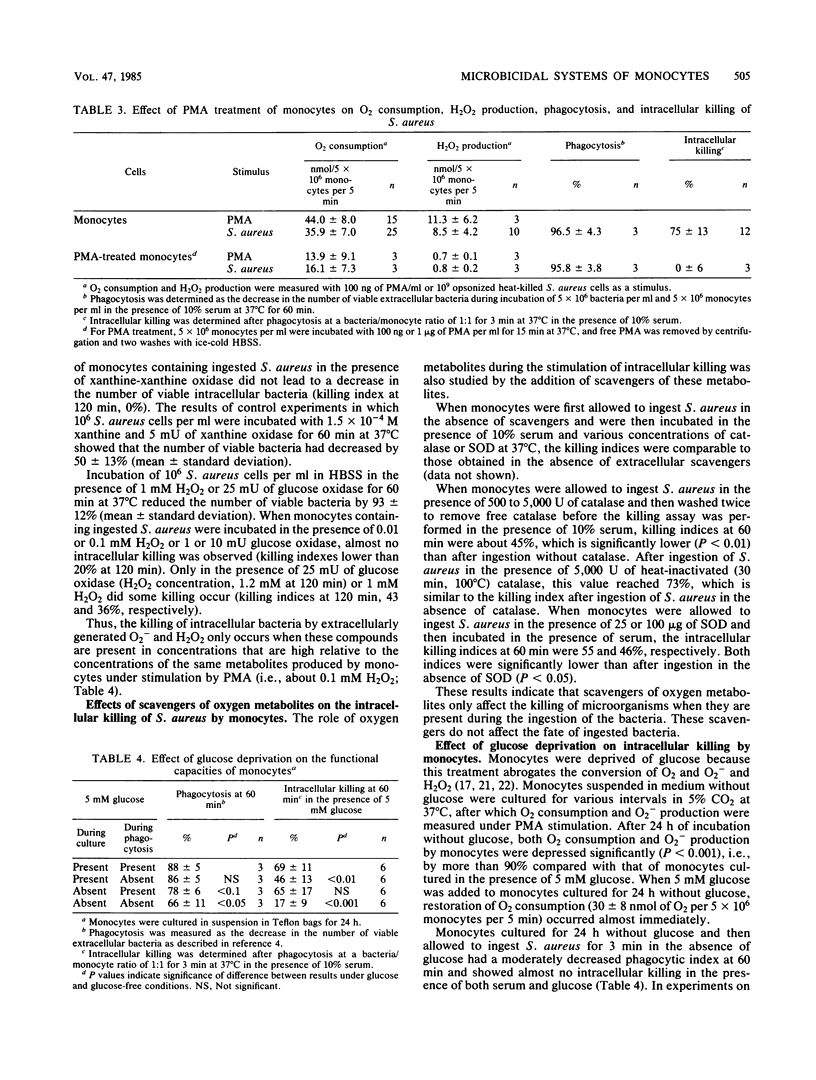
Human monocytes require serum components immunoglobulin G, C3/C3b, and B/Bb to exert optimal microbicidal action against ingested microorganisms. The present study was performed to find out whether these factors act by enhancing oxygen-dependent antimicrobial mechanisms. Serum enhanced oxygen consumption and superoxide production by monocytes before phagocytosis, but did not further increase these processes in monocytes that had recently ingested bacteria. Furthermore, serum did not boost iodination during intracellular killing by monocytes. Phorbol myristate acetate, N-formyl-methyonyl-leucyl-phenylalanine, concanavalin A, and concanavalin A-Sephadex all stimulated the conversion of O2 to H2O2 by monocytes, but only concanavalin A augmented intracellular killing. Reactive oxygen intermediates generated by cell-free enzymes (xanthine oxidase or glucose oxidase) in concentrations comparable to those accumulating extracellularly during incubation of monocytes containing bacteria with phorbol myristate acetate did not promote intracellular killing. The presence of catalase during phagocytosis inhibited killing, but had no effect on killing in the postphagocytic state. Monocytes deprived of glucose for 24 h showed markedly impaired O2 consumption, O2- generation, and bacterial killing; all of these effects were rapidly reversed by restoration of glucose. It is concluded that both an intact respiratory burst and extracellular serum factors are necessary for optimal killing of intracellular Staphylococcus aureus by human monocytes. Serum does not appear to act by enhancing the respiratory burst, but rather to have a separate, synergistic role, the biochemical basis of which is unknown.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Bos A., Wever R., Roos D. Characterization and quantification of the peroxidase in human monocytes. Biochim Biophys Acta. 1978 Jul 7;525(1):37–44. doi: 10.1016/0005-2744(78)90197-3. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Clark R. A., Szot S. The myeloperoxidase-hydrogen peroxide-halide system as effector of neutrophil-mediated tumor cell cytotoxicity. J Immunol. 1981 Apr;126(4):1295–1301. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. A comparison of the superoxide-releasing response in human polymorphonuclear leukocytes and monocytes. J Immunol. 1980 Jul;125(1):359–364. [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1977 Mar;89(3):675–686. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdins J. K., Koech D. K., Karnovsky M. L. Oxidation of glucose by mouse peritoneal macrophages: a comparison of suspensions and monolayers. J Cell Physiol. 1980 Nov;105(2):191–196. doi: 10.1002/jcp.1041050202. [DOI] [PubMed] [Google Scholar]
- Leijh P. C., van Zwet T. L., van Furth R. Effect of extracellular serum in the stimulation of intracellular killing of streptococci by human monocytes. Infect Immun. 1980 Nov;30(2):421–426. doi: 10.1128/iai.30.2.421-426.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., Daha M. R., van Furth R. Stimulation of the intracellular killing of Staphylococcus aureus by monocytes: regulation by immunoglobulin G and complement components C3/C3b and B/Bb. J Immunol. 1982 Jul;129(1):332–337. [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Zwet T. L., Daha M. R., van Furth R. Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J Clin Invest. 1979 Apr;63(4):772–784. doi: 10.1172/JCI109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol. 1982 Jul;129(1):351–357. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Interaction of Leishmania with a macrophage cell line. Correlation between intracellular killing and the generation of oxygen intermediates. J Exp Med. 1981 Jun 1;153(6):1690–1695. doi: 10.1084/jem.153.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Pretreatment with phorbol myristate acetate inhibits macrophage activity against intracellular protozoa. J Reticuloendothel Soc. 1982 Jun;31(6):479–487. [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Cohn Z. Role of oxygen-dependent mechanisms in antibody-induced lysis of tumor cells by activated macrophages. J Exp Med. 1980 Jul 1;152(1):198–208. doi: 10.1084/jem.152.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, King G. W., Metz E. N. A comparison of the metabolic response to phagocytosis in human granulocytes and monocytes. J Clin Invest. 1976 May;57(5):1352–1358. doi: 10.1172/JCI108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening R. S., Roos D., Loos J. A. Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med. 1974 Apr;83(4):570–577. [PubMed] [Google Scholar]
- van der Meer J. W., van de Gevel J. S., Elzenga-Claassen I., van Furth R. Suspension cultures of mononuclear phagocytes in the teflon culture bag. Cell Immunol. 1979 Jan;42(1):208–212. doi: 10.1016/0008-8749(79)90236-3. [DOI] [PubMed] [Google Scholar]



