Abstract
Transcriptomic and phenotypic studies showed that pyocins are produced in Pseudomonas aeruginosa PAO1 aerobic and anaerobic biofilms. Pyocin activity was found to be high in slow-growing anaerobic biofilms but transient in aerobic biofilms. Biofilm coculture of strain PAO1 and a pyocin-sensitive isolate showed that pyocin production had a significant impact on bacterial population dynamics, particularly under anaerobic conditions.
Pyocins are narrow-spectrum bacteriocins synthesized by most isolates of Pseudomonas aeruginosa and are presumed to play a role in niche establishment and protection in mixed populations (15). There are three types of pyocins (R, F, and S types); R- and F-type pyocins resemble bacteriophage tails (R-type pyocins are nonflexible and contractile, and F-type pyocins are flexible but noncontractile), while S-type pyocins are protease-sensitive proteins (13).
P. aeruginosa is a ubiquitous environmental bacterium and an opportunistic pathogen of humans (10, 17). Central to its survival in many different habitats is the ability to adapt and respond to different environmental stimulants and also to adopt a biofilm (surface-attached) lifestyle. It has been reported that in chronic respiratory infections in cystic fibrosis (CF) patients P. aeruginosa survives in thick, dehydrated, hypoxic airway mucus (21), and anaerobic or microaerobic conditions are also associated with other P. aeruginosa environmental (soils, bogs, and sediments) and infection-related (burn wound) habitats (7, 14, 18, 22). This has led several investigators to study the effect of anaerobiosis on the transcriptome and proteome of P. aeruginosa PAO1 grown in planktonic culture (1, 8, 16, 22). The results of two transcriptomic studies showed that the genes that encode R2 and F2 pyocins and at least one S-type pyocin were upregulated under anaerobic conditions, but this observation was not examined experimentally (1, 16). In this study, we focused on the biofilm mode of growth and investigated whether pyocins are produced in aerobic and anaerobic conditions and whether their induction can affect the population of a pyocin-sensitive isolate within mixed-culture biofilms.
Transcriptomic profiling shows that pyocin genes are upregulated in confluent anaerobic biofilms.
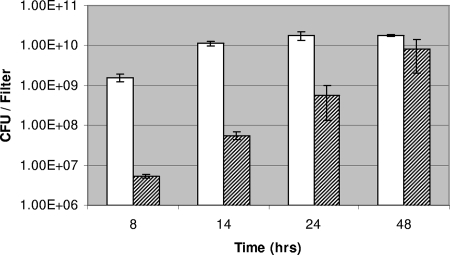
In this study, static biofilms were grown on nitrocellulose filters (diameter, 47 mm; Millipore) placed on reduced-strength LB agar (20%) containing 1% KNO3 to permit anaerobic respiration. Filters were inoculated with 1.0 × 105 CFU of an overnight culture of strain PAO1 and incubated at 37°C under aerobic and anaerobic conditions (anaerobiosis was created using an anaerobic jar containing gas-generating kit BR0038B; Oxoid Ltd.). Preliminary studies showed that P. aeruginosa anaerobic biofilm growth was slower than aerobic biofilm growth; aerobic biofilm populations would typically reach 1.0 × 1010 CFU/filter after 14-h incubation at 37°C, while anaerobic biofilms would take a further 34 h to reach a similar cell density (Fig. 1).
FIG. 1.
Biofilm growth of P. aeruginosa strain PAO1 under aerobic and anaerobic conditions. White columns, biofilms grown under aerobic conditions; shaded columns, biofilms grown under anaerobic conditions. The temperature for both conditions was 37°C. Results shown are averages from two independent experiments.
We have previously shown using confocal imaging that under aerobic conditions, microcolonies form after 8-h incubation on nitrocellulose filters, and these microcolonies develop into confluent biofilms by 14 h (20). We also found using microarray analysis that gene expression is conserved among 14-, 24-, and 48-h confluent biofilms (<0.62% differential gene expression) (20). Therefore, as confluent growth had also been achieved after 48 h for biofilms incubated under anaerobic conditions, we chose this time point to extract RNA from both aerobic and anaerobic biofilms. Global gene expression was determined using Affymetrix GeneChip microarrays; RNA isolation, cDNA labeling, microarray scanning, and data analysis using GeneSpring software (version 7.1) were performed as previously described (20). Analysis of this microarray data showed 320 and 278 genes to be ≥2.5-fold upregulated or downregulated under anaerobic biofilm conditions, respectively (P < 0.05; Welch Mann-Whitney test) (see Tables ST1 and ST2 in the supplemental material). Among the genes upregulated under anaerobic biofilm conditions were 39 sequential genes (PA0610 to PA0648). This region encodes the R-type (R2) and F-type (F2) pyocins and two pyocin-regulatory genes (prtN and prtR) (Table 1; see also Table ST1 in the supplemental material). In addition, the three S-type pyocins encoded by the genome of strain PAO1 (S5, encoded by PA0985; S2, encoded by PA1150; and an unnamed pyocin, encoded by PA3866) were also upregulated in biofilms grown under anaerobic conditions (Table 1).
TABLE 1.
Pyocin-related genes that are upregulated under anaerobic biofilm conditions
| Gene no.a | Gene name | Fold changeb | Product name | Functional class |
|---|---|---|---|---|
| PA0610 | prtN | 6.07 | Transcriptional regulator PrtN | Transcriptional regulators |
| PA0611 | prtR | 3.34 | Transcriptional regulator PrtR | Transcriptional regulators |
| PA0612 | ptrB | 3.70 | Repressor, PtrB | Transcriptional regulators |
| PA0613 | 4.94 | Hypothetical protein | Hypothetical, unclassified, unknown | |
| PA0614 | 7.74 | Hypothetical protein | Hypothetical, unclassified, or unknown | |
| PA0615 | 4.60 | Hypothetical protein | Hypothetical, unclassified, or unknown | |
| PA0616 | 5.67 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0617 | 5.73 | Probable bacteriophage protein | Related to phage, transposon, or plasmid | |
| PA0618 | 5.54 | Probable bacteriophage protein | Related to phage, transposon, or plasmid | |
| PA0619 | 3.90 | Probable bacteriophage protein | Related to phage, transposon, or plasmid | |
| PA0620 | 4.80 | Probable bacteriophage protein | Related to phage, transposon, or plasmid | |
| PA0621 | 3.98 | Conserved hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0622 | 2.94 | Probable bacteriophage protein | Related to phage, transposon, or plasmid | |
| PA0623 | 3.75 | Probable bacteriophage protein | Related to phage, transposon, or plasmid | |
| PA0624 | 5.49 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0625 | 5.94 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0626 | 5.32 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0627 | 3.15 | Conserved hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0628 | 5.54 | Conserved hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0629 | 4.59 | Conserved hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0630 | 6.12 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0631 | 5.17 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0632 | 5.18 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0633 | 3.09 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0634 | 2.91 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0635 | 3.61 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0636 | 4.17 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0637 | 4.15 | Conserved hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0638 | 5.38 | Probable bacteriophage protein | Related to phage, transposon, or plasmid | |
| PA0639 | 4.08 | Conserved hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0640 | 6.52 | Probable bacteriophage protein | Related to phage, transposon, or plasmid | |
| PA0641 | 6.33 | Probable bacteriophage protein | Related to phage, transposon, or plasmid | |
| PA0642 | 9.15 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0643 | 8.03 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0644 | 7.47 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0645 | 8.18 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0646 | 7.87 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0647 | 6.21 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0648 | 6.09 | Hypothetical protein | Related to phage, transposon, or plasmid | |
| PA0985 | 5.50 | Pyocin S5 | Secreted factors (toxins, enzymes, and alginate) | |
| PA1150 | pys2 | 6.16 | Pyocin S2 | Secreted factors (toxins, enzymes, and alginate) |
| PA3866 | 11.20 | Pyocin | Secreted factors (toxins, enzymes, and alginate) | |
| PA3617 | recA | 3.29 | RecA protein | DNA replication, recombination, modification, and repair |
Genes are identified by open reading frame designation, gene name, and product name (www.pseudomonas.com).
Average increase in gene expression under anaerobic biofilm conditions compared to aerobic biofilm conditions (n-fold).
Experimental data confirm increased bactericidal activity in anaerobic biofilms.
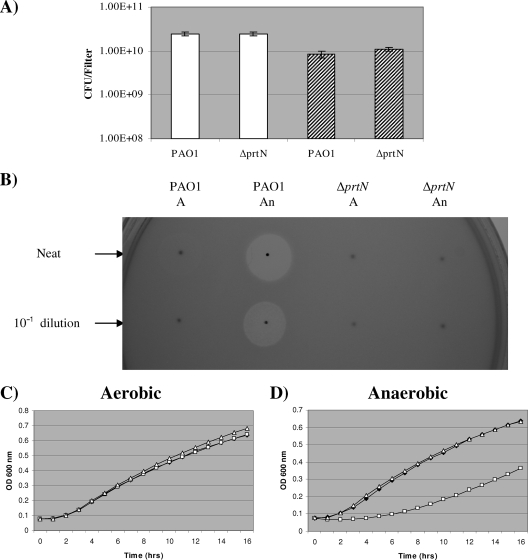
Two-day aerobic and anaerobic biofilms were resuspended in 10 ml of LB broth, and supernatant was extracted and sterilized by membrane filtration. Bactericidal activity was then determined using a variation of the commonly used pyocin-detecting agar overlay assay (9). Briefly, 10 μl of culture supernatant was pipetted onto tryptone soya agar (TSA) plates and left to dry. An overlay of 2.5 ml of soft agar (1% peptone, 0.5% agar) containing 0.1 ml of a 3- to 4-h indicator culture grown in nutrient broth was then poured over the agar surface. Fifteen P. aeruginosa strains were used as indicator strains (2 CF isolates [DWW1 and DWW3; this study] and 13 pyocin indicator strains [In1 to In8 and InA to InE; reference 9]). Supernatant from biofilms grown anaerobically showed significantly more bactericidal activity against the 13 susceptible strains (DWW1, DWW3, In1, In2, In3, In4, In5, In7, In8, InB, InC, InD, and InE) than that from aerobically grown biofilms (results for strain DWW1 are shown in Fig. 2B and Table ST3 in the supplemental material). Further evidence of increased bactericidal activity in anaerobic biofilm supernatant was observed using a semiquantitative planktonic growth inhibition assay. Briefly, 10 μl of an overnight indicator culture (neat initial inoculum) was added to 1 ml of LB broth containing 100-fold diluted biofilm supernatant, and planktonic growth at 37°C was monitored using a FLUOstar Optima microplate reader (BMG Laboratories). The addition of anaerobic biofilm supernatant caused a significant lag in the growth curve, which was indicative of bactericidal activity against the neat initial inoculum of indicator strain DWW1 (Fig. 2D). This lag was not observed using aerobic biofilm supernatant (Fig. 2C). Similar results were obtained using strain In1 as the indicator (data not shown).
FIG. 2.
Determination of pyocin activity in biofilms of strain PAO1. ΔprtN, the prtN mutant. (A) Cell density of 2-day biofilms. White and shaded columns represent biofilms grown under aerobic and anaerobic conditions, respectively. (B) Results obtained using the pyocin overlay assay. Ten microliters of neat supernatant and 10-fold dilutions of supernatant were spotted onto TSA and covered with a soft agar overlay containing an indicator strain (DWW1). A, aerobic biofilm supernatant; An, anaerobic biofilm supernatant. Similar results were obtained when proteinase K (100 μg/ml), which is active against S-type pyocins (6), was incorporated into the overlay agar. Although this observation does not eliminate the possibility that S-type pyocins are active against strain DWW1, it is clear from this result that bactericidal activity can be attributed to R- or F-type pyocins. Results obtained using the planktonic growth inhibition assay, showing the effect of biofilm supernatant (aerobic [C] or anaerobic [D]) on growth of indicator strain DWW1. Filled diamonds, control (no added supernatant); open squares, 10-μl PAO1 biofilm supernatant added; open triangle, 10-μl ΔprtN mutant biofilm supernatant added.
We also observed upregulation of bactericidal activity in biofilms grown under anaerobic conditions when three different full-strength agar media (LB, Pseudomonas isolation agar, and TSA) containing 1% KNO3 were used (see Fig. S1 and S2 in the supplemental material). In addition, we found increased bactericidal activity in planktonic culture grown under anaerobic conditions (see Fig. S3 and Table ST4 in the supplemental material). This confirms the recent observations made by other authors and shows that anaerobic induction of pyocins is not restricted to the biofilm mode of growth (1, 16).
Pyocins are known to be activated as part of the response to DNA damage. Briefly, an activated RecA protein cleaves PrtR (the negative regulator), and this permits expression of the gene that encodes the positive regulator (prtN). PrtN activates the expression of R-, F-, and S-type pyocin genes (12). To provide evidence that P. aeruginosa pyocins were responsible for this bactericidal activity, a chromosomal deletion mutant of prtN was constructed (see materials and methods in the supplemental material). We found that a deletion in prtN had no effect on biofilm cell density after 2 days of aerobic or anaerobic incubation at 37°C (Fig. 2A). We did find, however, that the prtN mutant produced little bactericidal activity when both the agar overlay (using 15 indicator strains) and planktonic growth inhibition (using indicator strains DWW1 and In1) assays were used (Fig. 2B, C, and D and Table ST3 in the supplemental material; also data not shown). These results demonstrate that pyocins are responsible for the increased bactericidal activity observed under anaerobic conditions. They also show that PrtN is integral to this induction, and therefore, any additional regulatory events are likely to occur further up the hierarchy of pyocin regulation than PrtN.
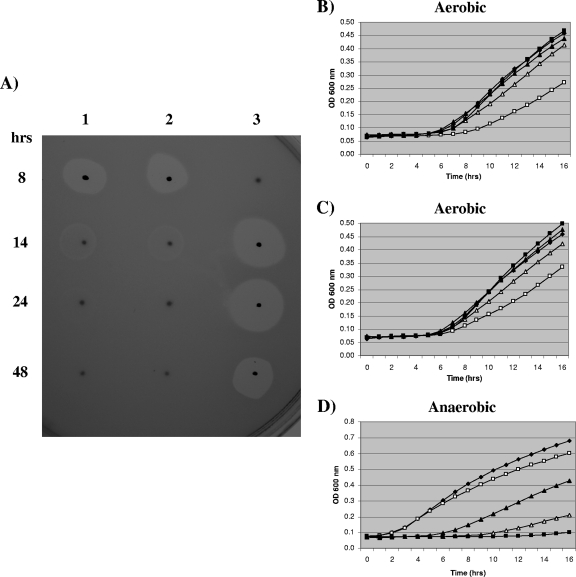
Bactericidal activity is high in slowly developing anaerobic biofilms, while pyocin production in aerobic biofilms is transient.
On analysis of our previous aerobic transcriptomic data set, we found that expression of all PAO1 pyocin genes (R2, F2, and three S types) was higher in biofilms at 8 h than at the confluent time points (14, 24, and 48 h) (see Fig. S4A, B, and C in the supplemental material). We confirmed these results using the pyocin overlay assay. As expected, of the biofilms from the four aerobic time points, supernatant taken from the 8 h aerobic biofilms had the most bactericidal activity against the 13 susceptible P. aeruginosa indicator strains (DWW1 results are shown in Fig. 3A, In1 results are shown in Fig. S5A in the supplemental material, and results for all strains are shown in Table ST5 in the supplemental material). A faint zone of inhibition was also apparent for the 14-h aerobic biofilm supernatant (Fig. 3A). This aerobic pyocin production profile was found for biofilms grown on 20% LB agar alone or with 1% KNO3 (Fig. 3A, lanes 1 and 2, respectively; see Table ST5 in the supplemental material).
FIG. 3.
Bactericidal activities found at different biofilm time points (8, 14, 24, and 48 h). (A) Results obtained using the pyocin overlay assay. Ten microliters of neat supernatant from aerobic or anaerobic PAO1 biofilms was spotted onto TSA and covered with a soft agar overlay containing indicator strain DWW1. Lane 1, aerobic biofilms grown on 20% LB agar; lane 2, aerobic biofilms grown on 20% LB agar containing 1% KNO3; lane 3, anaerobic biofilms grown on 20% LB agar containing 1% KNO3. (B, C, and D) Results obtained using the planktonic growth inhibition assay. The results are averages for two independent biofilms harvested at each time point. Filled diamonds represent the control (no added supernatant), and results are also shown for cultures with biofilm supernatant added (10 μl) 8 h (open squares), 14 h (open triangles), 24 h (filled squares), and 48 h (filled triangles). Panels B and C show data for supernatants from aerobic PAO1 biofilms grown on 20% LB agar and on 20% LB agar containing 1% KNO3, respectively. Panel D shows data for supernatant from the anaerobic PAO1 biofilms used. An overnight culture of strain DWW1 was used as the indicator inoculum (for panels B and C, 10 μl of 100-fold diluted indicator culture was used, and for panel D, 10 μl of neat indicator culture was used).
An anaerobic biofilm pyocin production time course was also determined using the same time points. We found that in addition to the expected activity in supernatant taken from 48 h anaerobic biofilms, supernatant taken from biofilms grown under anaerobic conditions for 14 and 24 h had obvious bactericidal activity against the 13 susceptible P. aeruginosa indicator strains (DWW1 results are shown in Fig. 3A, lane 3; In1 results are shown in Fig. S5A, lane 3, in the supplemental material; and data for all indicator strains are shown in Table ST5 in the supplemental material).
Both the aerobic and anaerobic biofilm results were verified using the planktonic growth inhibition assay (Fig. 3B, C, and D). Bactericidal activity against a neat initial inoculum of indicator strain DWW1 was observed for three anaerobic biofilm samples (14, 24, and 48 h), with the greatest activity observed in the 24-h anaerobic biofilm supernatant (Fig. 3D). Obvious bactericidal activity in 8- and 14-h aerobic biofilms was also found against a 100-fold diluted initial inoculum of indicator strain DWW1 (Fig. 3B and C). This result also shows that bactericidal activities in 14-, 24-, and 48-h anaerobic biofilm supernatants were greater than those observed with aerobic biofilm supernatants (similar results were obtained for activity against In1 [see Fig. S5B, C, and D in the supplemental material]).
Pyocin production can affect bacterial population dynamics within mixed-culture biofilms grown under aerobic and anaerobic conditions.
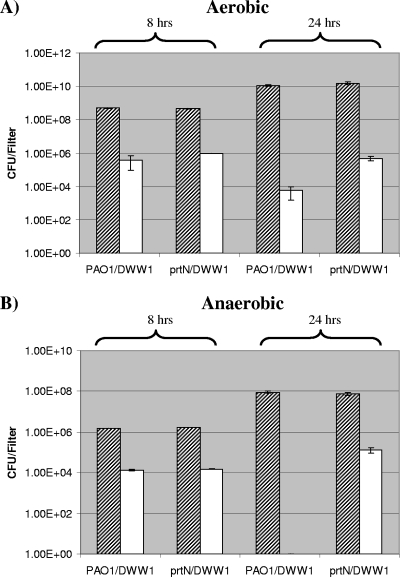
Mixed-culture biofilm competition experiments were performed by culturing a spontaneous rifampin (rifampicin)-resistant (Rifr) mutant obtained from CF patient isolate DWW1 (DWW1 Rifr; see materials and methods in the supplemental material) together with either PAO1 or the prtN mutant. One hundred-microliter mixed cultures containing equal populations (1 × 105 CFU) of both bacterial cultures were spread onto nitrocellulose filters placed on 20% LB agar containing 1% KNO3 and were then incubated under aerobic and anaerobic conditions at 37°C. After 8 and 24 h, biofilms were harvested and viable counts were used to determine both the total biofilm population (using LB agar) and the DWW1 Rifr population (using LB agar containing 100 μg/ml rifampin).
Firstly, when comparing the total biofilm population obtained from the two mixed-culture combinations used (PAO1-DWW1 Rifr and prtN mutant-DWW1 Rifr), no obvious difference was observed at both time points (8 and 24 h) and in both culture conditions (aerobic and anaerobic) used (Fig. 4A and B). However, when the results obtained using LB agar containing rifampin were analyzed, we found contrasting results for mixed-culture biofilms incubated under aerobic and anaerobic conditions. For the aerobic mixed-culture biofilm analysis, we found that after 8- and 24-h incubations, there was an increase in the DWW1 Rifr population (∼2.5-fold and ∼84-fold, respectively) in the prtN mutant-DWW1 Rifr biofilm coculture compared to the PAO1-DWW1 Rifr biofilm coculture (Fig. 4A). The activity after 24 h can be explained by pyocin-producing microcolonies coalescing with pyocin-sensitive microcolonies.
FIG. 4.
Effect of pyocin production on bacterial populations within mixed-culture biofilms incubated under aerobic and anaerobic conditions. Biofilm populations incubated under aerobic conditions (A) and anaerobic conditions (B). Shaded bars, total population (viable counts using LB agar); open bars, population isolated after culture on LB agar containing rifampin. prtN, isogenic prtN mutant of strain PAO1; DWW1 (referred to as DWW1 Rifr in the text) spontaneous rifampin-resistant mutant obtained from CF patient isolate DWW1.
For the anaerobic mixed-culture biofilm analysis, we obtained very different results after 8- and 24-h incubations (Fig. 4B). After 8 h, there was no obvious difference in the DWW1 Rifr population obtained from the PAO1-DWW1 Rifr and prtN mutant-DWW1 Rifr biofilm combinations (Fig. 4B). Whereas no DWW1 Rifr colonies were detected for the PAO1-DWW1 Rifr biofilm coculture after 24 h (rifampin mutant detection limit, 20 CFU/filter), a DWW1 Rifr population of 1.25 × 105 CFU/filter was obtained with the prtN-DWW1 Rifr biofilm coculture (Fig. 4B).
These results show that induction of pyocin production in both aerobic and anaerobic biofilms can influence the bacterial composition of mixed-culture biofilms. The 24-h anaerobic incubation results demonstrate the dramatic effect of high-level pyocin induction on the neighboring cells of a pyocin-susceptible strain within the tightly compact confines of a biofilm (Fig. 4B). Therefore, pyocin induction gives an isolate a distinct advantage when the isolate colonizes a niche in the presence of susceptible competitors.
Recent studies using microarray technology have shown that other conditions can affect strain PAO1 pyocin gene transcription. Hydrogen peroxide and ciprofloxacin were found to induce pyocin production, while ceftazidime was found to repress pyocin production (3-5). These data, together with our analysis of pyocin production in biofilm and planktonic culture (under both aerobic and anaerobic conditions), show that pyocin production is under significant environmental and growth-phase regulation. Previous studies using planktonic cultures have demonstrated that R-type pyocin production gives P. aeruginosa strain PA14 a competitive growth advantage when it is cocultured with other susceptible isolates (11). However, this is the first study to (i) observe pyocin production in biofilms, (ii) investigate the effect of oxygen availability on pyocin production, and (iii) observe the role of pyocins on bacterial population dynamics within biofilms.
It is known that biofilms are heterogeneous populations and that within mature aerobic biofilms, oxygen depletion has been demonstrated (23). The authors who determined this found using a drip-flow biofilm reactor that protein synthesis was restricted by oxygen availability to the upper layer (30 μm) of the biofilm. We found previously that confluent membrane filter biofilms grown under aerobic conditions had an average depth of 25 μm (48 h) (20), and while we cannot rule out the possibility that there are regions where oxygen is depleted that trigger localized pyocin production, our results clearly show that pyocin production is not high in confluent membrane filter biofilms incubated under aerobic conditions.
Pyocin production appears to be not just an in vitro phenomenon, as recent studies using serum from CF patients have shown that R-, F-, and S-type pyocins are produced during P. aeruginosa respiratory infection (2, 19). We are currently investigating pyocin production profiles of clinical and environmental isolates of P. aeruginosa.
Supplementary Material
Acknowledgments
Thanks to Tracy Chaplin (Medical Oncology Unit, Barts and The London School of Medicine and Dentistry) for hybridization and scanning of the microarrays. Thanks also to John Govan (University of Edinburgh) and David Wareham (Barts and the London NHS trust) for supplying the pyocin indicator strains used in this analysis.
R.D.W. was funded by a Barts and the London Charity nonclinical fellowship.
Footnotes
Published ahead of print on 5 December 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alvarez-Ortega, C., and C. S. Harwood. 2007. Responses of Pseudomonas aeruginosa to low oxygen indicate that growth in the cystic fibrosis lung is by aerobic respiration. Mol. Microbiol. 65153-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckmann, C., M. Brittnacher, R. Ernst, N. Mayer-Hamblett, S. I. Miller, and J. L. Burns. 2005. Use of phage display to identify potential Pseudomonas aeruginosa gene products relevant to early cystic fibrosis airway infections. Infect. Immun. 73444-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blázquez, J., J. M. Gomez-Gomez, A. Oliver, C. Juan, V. Kapur, and S. Martin. 2006. PBP3 inhibition elicits adaptive responses in Pseudomonas aeruginosa. Mol. Microbiol. 6284-99. [DOI] [PubMed] [Google Scholar]
- 4.Brazas, M. D., and R. E. Hancock. 2005. Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 493222-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, W., D. A. Small, F. Toghrol, and W. E. Bentley. 2005. Microarray analysis of Pseudomonas aeruginosa reveals induction of pyocin genes in response to hydrogen peroxide. BMC Genomics 6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denayer, S., S. Matthijs, and P. Cornelis. 2007. Pyocin S2 (Sa) kills Pseudomonas aeruginosa strains via the FpvA type I ferripyoverdine receptor. J. Bacteriol. 1897663-7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filiatrault, M. J., K. F. Picardo, H. Ngai, L. Passador, and B. H. Iglewski. 2006. Identification of Pseudomonas aeruginosa genes involved in virulence and anaerobic growth. Infect. Immun. 744237-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filiatrault, M. J., V. E. Wagner, D. Bushnell, C. G. Haidaris, B. H. Iglewski, and L. Passador. 2005. Effect of anaerobiosis and nitrate on gene expression in Pseudomonas aeruginosa. Infect. Immun. 733764-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fyfe, J. A., G. Harris, and J. R. Govan. 1984. Revised pyocin typing method for Pseudomonas aeruginosa. J. Clin. Microbiol. 2047-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardalo, C., and S. C. Edberg. 1997. Pseudomonas aeruginosa: assessment of risk from drinking water. Crit. Rev. Microbiol. 2347-75. [DOI] [PubMed] [Google Scholar]
- 11.Heo, Y. J., I. Y. Chung, K. B. Choi, and Y. H. Cho. 2007. R-type pyocin is required for competitive growth advantage between Pseudomonas aeruginosa strains. J. Microbiol. Biotechnol. 17180-185. [PubMed] [Google Scholar]
- 12.Matsui, H., Y. Sano, H. Ishihara, and T. Shinomiya. 1993. Regulation of pyocin genes in Pseudomonas aeruginosa by positive (prtN) and negative (prtR) regulatory genes. J. Bacteriol. 1751257-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michel-Briand, Y., and C. Baysse. 2002. The pyocins of Pseudomonas aeruginosa. Biochimie 84499-510. [DOI] [PubMed] [Google Scholar]
- 14.Mousa, H. A. 1997. Aerobic, anaerobic and fungal burn wound infections. J. Hosp. Infect. 37317-323. [DOI] [PubMed] [Google Scholar]
- 15.Parret, A. H., and R. De Mot. 2002. Bacteria killing their own kind: novel bacteriocins of Pseudomonas and other gamma-proteobacteria. Trends Microbiol. 10107-112. [DOI] [PubMed] [Google Scholar]
- 16.Platt, M. D., M. J. Schurr, K. Sauer, G. Vazquez, I. Kukavica-Ibrulj, E. Potvin, R. C. Levesque, A. Fedynak, F. S. Brinkman, J. Schurr, S. H. Hwang, G. W. Lau, P. A. Limbach, J. J. Rowe, M. A. Lieberman, N. Barraud, J. Webb, S. Kjelleberg, D. F. Hunt, and D. J. Hassett. 2008. Proteomic, microarray, and signature-tagged mutagenesis analyses of anaerobic Pseudomonas aeruginosa at pH 6.5, likely representing chronic, late-stage cystic fibrosis airway conditions. J. Bacteriol. 1902739-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406959-964. [DOI] [PubMed] [Google Scholar]
- 18.Ueno, C., T. K. Hunt, and H. W. Hopf. 2006. Using physiology to improve surgical wound outcomes. Plast. Reconstr. Surg. 11759S-71S. [DOI] [PubMed] [Google Scholar]
- 19.Upritchard, H. G., S. J. Cordwell, and I. L. Lamont. 2008. Immunoproteomics to examine cystic fibrosis host interactions with extracellular Pseudomonas aeruginosa proteins. Infect. Immun. 764624-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waite, R. D., A. Papakonstantinopoulou, E. Littler, and M. A. Curtis. 2005. Transcriptome analysis of Pseudomonas aeruginosa growth: comparison of gene expression in planktonic cultures and developing and mature biofilms. J. Bacteriol. 1876571-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Doring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 109317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu, M., T. Guina, M. Brittnacher, H. Nguyen, J. Eng, and S. I. Miller. 2005. The Pseudomonas aeruginosa proteome during anaerobic growth. J. Bacteriol. 1878185-8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu, K. D., P. S. Stewart, F. Xia, C. T. Huang, and G. A. McFeters. 1998. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol. 644035-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.