Abstract
ISPst9 is an ISL3-like insertion sequence (IS) that was recently described in the naphthalene-degrading organism Pseudomonas stutzeri strain AN10. In this paper we describe a novel strong IS regulation stimulus; transposition of ISPst9 is induced in all P. stutzeri AN10 cells after conjugative interaction with Escherichia coli. Thus, we observed that in all P. stutzeri AN10 cells that received genetic material by conjugation the ISPst9 genomic dose and/or distribution was changed. Furthermore, ISPst9 transposition was also observed when P. stutzeri AN10 cells were put in contact with the plasmidless conjugative strain E. coli S17-1λpir, but not when they were put in contact with E. coli DH5α (a nonconjugative strain). The mechanism of ISPst9 transposition was analyzed, and transposition was shown to proceed by excision from the donor DNA using a conservative mechanism, which generated 3- to 10-bp deletions of the flanking DNA. Our results indicate that ISPst9 transposes, forming double-stranded DNA circular intermediates consisting of the IS and a 5-bp intervening DNA sequence probably derived from the ISPst9 flanking regions. The kinetics of IS circle formation are also described.
Insertion sequences (ISs) are mobile genetic elements that can transpose within the host genome. Most of these movements generate detrimental mutations. Because of this, ISs and their hosts have coevolved so that transposition is downregulated by many different mechanisms (for a review, see reference 28). Nevertheless, the frequency of IS transposition can increase when the cell is under stress in order to increase the chance of beneficial mutations to overcome detrimental conditions. Many physical and chemical stresses, such as starvation, high temperature, the presence of magnetic fields, short-wave irradiation, lack of oxygen availability, and metal ion exposure, have increased transcription of tnpA (transposase-encoding gene) or IS transposition frequencies in different hosts that have been studied (6, 10, 13, 14, 35, 37). Independent of the source of stress used, the IS transposition frequencies obtained in all these studies were relatively low. Furthermore, none of the stimuli used activated transposition in all of the cells influenced by the stimuli.
Many transposition mechanisms have been described, although they can be placed in two groups, replicative transposition (16) and conservative or “cut-and-paste” transposition (36), depending on whether an IS copy is conserved or not conserved in its original localization, respectively. A “cut-and-paste” transposition implies that there is excision of the IS from the original position or donor DNA. This excision can be precise (only the IS is excised) or imprecise (a few extra base pairs are excised together with the IS). It has been shown that imprecise excision can contribute to gene variation (34). Both precise and imprecise “cut-and-paste” transposition events result in changes in the position of the IS in the genome. But the mobile element can also be lost if the IS is not inserted after it is excised from a replicon. An increase in the IS copy number can also be caused by a conservative mechanism if transposition takes place between two sister chromosomes after genome duplication but prior to cell division (2).
ISPst9 is an IS belonging to the ISL3 family recently found in the naphthalene-degrading organism Pseudomonas stutzeri strain AN10 and its 4-chlorosalicylate-degrading derivative AN142 (8). This IS is a 2,472-bp element that is flanked by two perfect 24-bp inverse repeats (IRs), which generates 8-bp AT-rich target duplication upon insertion. ISPst9 was found to transpose in multiple copies, and this IS was responsible for the nahH (catechol 2,3-dioxygenase-encoding gene) insertional inactivation observed in strain AN142 (8). When selective pressure was applied, this IS was excised precisely to generate NahH+ revertants that expressed the required active catechol 2,3-dioxygenase (8).
In this paper we describe a novel stimulus that is able to induce ISPst9 transposition; conjugative interaction seems to activate ISPst9 transposition in all cells that receive the stimulus. The mechanism and kinetics of ISPst9 transposition are also described.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and culture conditions.
P. stutzeri AN10 is a naphthalene-degrading strain that was isolated from West Mediterranean marine sediments (3, 4, 30). Routine strain confirmation analysis was performed by using 16S rRNA gene and the ITS1 sequence as previously described (17). Escherichia coli DH5α (19) was used to maintain plasmids pBluescript SK (Stratagene) and pCR2.1 (Invitrogen) and their derivatives. E. coli S17-1λpir (20) was used as the donor strain for conjugation and for maintenance of plasmids pUT mini-Tn5-Km (9), pDSK519 (24), pGP704 (26), and pKNG101 (23) and their derivatives. E. coli and P. stutzeri strains were grown at 30°C in Luria-Bertani (LB) medium (31) and mineral basal medium (MBM) (1) supplemented with 0.5% (wt/vol) succinate, respectively. When appropriate, ampicillin (Ap) (100 μg/ml), kanamycin (Km) (50 μg/ml), streptomycin (Sm) (50 μg/ml), and trimethoprim (Tp) (50 μg/ml) were added to the media.
Standard DNA manipulation.
Standard DNA procedures were used in this study (31). Genomic DNA was prepared as described previously (11). Plasmid DNA was isolated by alkaline lysis using a QIAprep Spin miniprep kit (Qiagen). Restriction endonuclease digestion (Promega and GE Healthcare) and ligation with T4 DNA ligase (Invitrogen) were performed as recommended by the manufacturers.
Hybridization and gene probes.
Southern blot hybridization was performed as described previously (31). Enhanced chemiluminescence direct labeling (ECL direct nucleic acid labeling and detection system; GE Healthcare) was used for hybridization. Gene probes were prepared by PCR amplification using Taq DNA polymerase (GE Healthcare) and P. stutzeri AN10 or E. coli S17-1λpir genomic DNA as the template. The templates and primers used to obtain the probes were as follows: for the 0.64-kb tnpA4 (ISPst9 transposase-encoding gene) probe, template strain AN10 and primers ISMG3 and ISMG9 (8); for the 0.46-kb tnpA2 (IS5-like transposase-encoding gene) probe, template strain AN10 and primers IS5-1F (5′-ACYTTCGCCGAYGCCGAGTA-3′) and IS5-1R (5′-GCCRTCCTTGTTCTTGGTCG-3′); and for the 1.03-kb RP4-oriT (origin of transfer of plasmid RP4 and flanking regions) probe, template strain S17-1λpir and primers oriT-F (5′-GGCTTGGCCTTGATGTGCCG-3′) and oriT-R (5′-CGTGCTTGGCAATCACGCGC-3′). In all cases, the PCR cycling conditions were as follows: 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min and then 72°C for 10 min.
Transformation, conjugation, and cell-cell interaction.
Natural transformation was performed by using the plate transformation procedure (33), with slight modifications. Briefly, an aliquot of a stationary-phase P. stutzeri culture was spotted onto a membrane filter (nitrocellulose; 0.22 μm; Millipore). When the dot containing cells was dry (after approximately 15 min at room temperature), 50 μl of EcoRI-linearized plasmid DNA (300 ng/μl) was added to it. The filter with the DNA-cell mixture was incubated at 30°C for 24 h on the surface of an LB agar plate.
Conjugative plasmids were transferred from E. coli S17-1λpir into P. stutzeri by filter mating. Aliquots of late-exponential-phase cultures (A600, ∼0.8) of donor and recipient strains were spotted together onto a membrane filter (nitrocellulose; 0.22 μm; Millipore) to obtain a cell ratio of 1:1. The mixture was incubated at 30°C for 7 h on the surface of an LB agar plate.
After incubation, cell mixtures from both horizontal gene transfer events (transformation and conjugation) were resuspended in Ringer's solution (Merck). For transconjugant-transformant isolation, cell suspensions and serial dilutions of the suspensions were plated in duplicate onto MBM agar plates containing succinate plus the appropriate antibiotic.
Contact event experiments with P. stutzeri and E. coli (strain DH5α or S17-1λpir lacking a plasmid) were performed like the conjugation experiments, except for the cell ratio used. In order to ensure that there was interaction with P. stutzeri, in the absence of a detectable genetic marker for selection, up to 107 to 108 E. coli cells per cell of P. stutzeri were used. Briefly, 10-fold serial dilutions (10−6 to 10−8) of a late-exponential-phase culture of P. stutzeri (1 to 100 CFU) were spotted together with an aliquot of a late-exponential-phase culture of E. coli (108 CFU) onto membrane filters (nitrocellulose; 0.22 μm; Millipore). After incubation at 30°C for 7 h on the surface of LB agar plates, cell mixtures were resuspended in Ringer's solution and directly plated onto MBM agar plates containing succinate as a unique carbon and energy source for P. stutzeri counting. Serial dilutions of cell mixtures were plated onto LB agar plates for total (P. stutzeri and E. coli) cell counting. For further genomic analysis only P. stutzeri cells from contact events in which the P. stutzeri/E. coli ratio was between 1:107 and 1:108 were used.
ISPst9 transposition analysis.
The location of ISPst9 in the nah cluster of P. stutzeri AN10 and its derivatives was routinely analyzed by PCR amplification with Taq DNA polymerase (GE Healthcare), using primers SAL64 and SAL71 as previously described (8).
In order to evaluate the kinetics of ISPst9 transposition, contact event experiments with E. coli S17-1λpir lacking a plasmid and P. stutzeri were performed as described above but with different incubation times. After contact, cell mixtures were resuspended in 500 μl of Tris-EDTA buffer (50 mM Tris-HCl, 20 mM sodium EDTA; pH 8.0), and genomic and plasmid DNAs were extracted as described above. The quality and concentration of the extracted DNAs were assessed with a NanoDrop ND-1000 UV-visible spectrophotometer (NanoDrop Technologies) used according to the manufacturer's instructions. ISPst9 circle formation was detected by performing PCR with 12 ng/μl (final concentration) of extracted DNA, 0.3 μM outward primer ISMG2 (5′-GCTCACGAAGTCTCAGACC-3′), and 0.3 μM outward primer ISMG4 (5′-TGGTCAGTGCACCTCGTTC-3′). The PCR cycling conditions were as follows: 94°C for 5 min, followed by 25 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min and then 72°C for 10 min. ISPst9 circle formation was considered to occur when a unique 0.95-kb PCR product was observed. After three independent experiments, the PCR products of each set of experiments were resolved on a 1% agarose gel and were stained with ethidium bromide. PCR products were semiquantified using the GeneTools version 3.04.04 analysis program (SynGene). For analysis, the highest measured signal was defined as 100%.
Restriction with mung bean nuclease (Invitrogen) was performed to evaluate the DNA structure (single-stranded DNA or double-stranded DNA) of ISPst9 circle intermediates. Briefly, a 50-μl aliquot containing 1 μg of genomic DNA extracted after a mating event was treated with 10 U of mung bean nuclease for 30 min at 30°C. A control for enzyme functionality was included, using DNA that was thermally denatured (5 min at 100°C and rapid chilling on ice). After treatment, samples were purified by phenol-chloroform-isoamyl alcohol (25:24:1) extraction and ethanol precipitation. Each pellet was resuspended in 30 μl of sterile deionized water for further IS circle amplification by PCR as described above.
Sequencing.
For sequencing, PCR products were purified with a QIAquick PCR purification kit (Qiagen). Sequences were determined a using BigDye Terminator cycle sequencing v3.1 kit (Applied Biosystems) according to the manufacturer's instructions. Sequence analyses were performed using the BioEdit 6.0.5 sequence alignment editor (18).
RESULTS AND DISCUSSION
First evidence of induced ISPst9 transposition.
Since it has been demonstrated previously that ISPst9 can transpose in multiple copies at a reasonably high frequency (up to 10−5) (8), we tried to increase the native ISPst9 transposition frequency to facilitate study of the transposition mechanism. Therefore, harvested P. stutzeri AN10 stationary-phase cells were subjected to different stresses, including high salinity (2 h of incubation at 30°C with up to 27% [wt/vol] NaCl), acidic and basic pHs (2 h of incubation at 30°C at pH 5.6 and 11.6, respectively), an elevated temperature (2 h of incubation at 47°C), and a high level of UV light radiation (45 min of exposure at 20°C to 300 J/m2). In spite of the low level of survival obtained in each case (the survival frequencies were between 10−4 and 10−6), no modification of the ISPst9 pattern was observed for the P. stutzeri AN10 survivors analyzed after genomic DNA digestion, followed by Southern blot hybridization with the tnpA4 (ISPst9 transposase-encoding gene) probe.
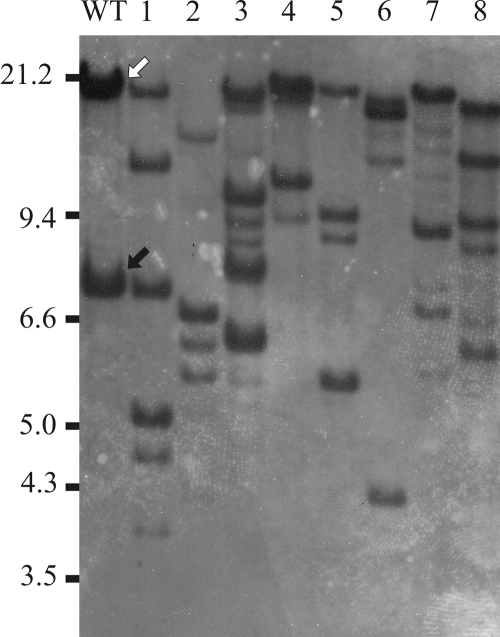
An increase in ISPst9 transposition was detected in an experiment to generate mutants with mutations in the nahAc (naphthalene 1,2-dioxygenase) and nahG (salicylate 1,2-hydroxylase) genes of P. stutzeri AN10. Two plasmids, pLAN04 and pLAN05, containing internal fragments of the nahAc and nahG genes, respectively, both disrupted by a lacZ-Kmr cassette, were constructed to introduce by conjugation and double homologous recombination lacZ-Kmr translational fusions into both genes (M. P. Lanfranconi, unpublished). As both of these plasmids are derivatives of suicide vectors (pLAN04 is a derivative of pKNG101, and pLAN05 is a derivative of pGP704) that are stable only in λpir derivatives of E. coli, they were not able to replicate in P. stutzeri AN10. Both plasmids were transferred from E. coli S17-1λpir into P. stutzeri AN10, and Kmr transconjugants were selected. The resulting Kmr colonies were expected to be transconjugants that had incorporated the ′lacZ-Kmr cassette by homologous recombination between the flanking nah regions in the plasmid and the native nah gene in the recipient cells. Transconjugants in which double homologous recombination had occurred were selected as Km-resistant Sm-sensitive colonies and Km-resistant Ap-sensitive colonies for pLAN04 and pLAN05, respectively. Eight of the transconjugants (four mutants with mutations in nahG and four mutants with mutations in nahAc) were selected, and the fidelity of recombination was checked by Southern blot hybridization. Thus, EcoRI-digested genomic DNAs from transconjugants were hybridized with internal nahG and nahAc probes. The hybridization patterns expected using both internal gene probes were not observed. Further analysis involving hybridization of the same Southern blot with tnpA4 produced two unexpected results (Fig. 1): (i) each of the nahAc and nahG mutants analyzed had a unique tnpA4 hybridization pattern that was totally different from the pattern observed for the wild-type strain, showing that there was an increased number of putative ISPst9 copies; and (ii) the culture of P. stutzeri AN10 used to obtain both mutants had an extra copy of tnpA4 on a 21-kb EcoRI-EcoRI DNA fragment (Fig. 1) compared to the clone in which ISPst9 was found previously, which had a unique copy of tnpA4 in a 7.2-kb fragment (Fig. 1) (8).
FIG. 1.
Southern blot hybridization with the tnpA4 probe of EcoRI-digested genomic DNAs from P. stutzeri AN10 (lane WT) and mutant derivatives with mutations in the nahAc (lanes 1 to 4) and nahG (lanes 5 to 8) genes. Previously described (8) and newly described ISPst9-containing EcoRI-EcoRI bands are indicated by black and white arrows, respectively.
The clone of P. stutzeri strain AN10 used for generation of mutants was recovered in this study from a long-term culture frozen at −80°C, whereas the first analysis described (8) was performed with a culture which had been passed an unknown number of times on LB agar plates with long intervals of plate preservation at 4°C. In order to clarify the difference in the number of tnpA4 copies in the genome of P. stutzeri AN10, up to eight more isolates were obtained from other stored cultures, including the originally deposited AN10 clone from the CCUG culture collection (CCUG 29243) at the University of Göteborg. The identity of these isolates as P. stutzeri strain AN10 was confirmed by 16S rRNA gene and ITS1 sequence analysis (17). Southern blot hybridization of their EcoRI-digested genomic DNAs with the tnpA4 probe revealed the same hybridization pattern in each case: two bands at 7.2 and 21 kb (as shown for the wild type in Fig. 1). PCR amplification using primers SAL64 and SAL71, which hybridize with flanking DNA of the originally described ISPst9 copy (8), was also carried out to demonstrate that this copy remained in its original location. As expected, a 3.32-kb PCR product was obtained. Thus, as all of the results showed unequivocally that in P. stutzeri AN10 there are copies of tnpA4 in two different EcoRI-EcoRI DNA fragments and as both copies were shown to be mobile (Fig. 1), we suggest that they are in two independent mobile elements. In this scenario, we attribute the single copy of ISPst9 found in the strain used in previous work (8) to copy loss that occurred during long-term plate storage. This is not the first time that transposition of mobile elements induced by starvation conditions has been described. Some examples of this behavior are ISH27 of Halobacterium halobium (29), IS5 and IS30 of E. coli (27), and Tn4652 of Pseudomonas putida (21), showing that ISs can be important in bacterial variation under laboratory conditions.
ISPst9 transposition was induced by conjugative interaction.
A series of conjugation experiments with E. coli S17-1λpir carrying pLAN04 or pLAN05 and P. stutzeri AN10 were performed in order to quantify the observed upregulation of ISPst9 transposition. Fifty-four transconjugants in which a double homologous recombination event had occurred were selected (27 nahAc::lacZ-Kmr, Km-resistant, Sm-sensitive transconjugants and 27 nahG::lacZ-Kmr, Km-resistant, Ap-sensitive transconjugants). Before genomic DNA was extracted, at least two passages on LB medium supplemented with kanamycin were performed using single colonies. This strategy was routinely used in order to avoid the presence of low-intensity tnpA4 hybridization bands (as shown in Fig. 1) which might have occurred due to the presence of a mixture of clones with different ISPst9 genomic distributions. As expected, Southern blot hybridization of the EcoRI-digested genomic DNAs of the selected transconjugants with the tnpA4 probe revealed that ISPst9 transposition occurred in all of the AN10 derivatives analyzed; in 44% of both types of mutants (nahAc and nahG) there was an increase in ISPst9 copy number, in around 40% of both types of mutants two copies were maintained, although there were changes in their locations, and in only 15% of both types of mutants one or both copies of ISPst9 were lost (Table 1).
TABLE 1.
ISPst9 transposition data obtained with different transconjugants of P. stutzeri AN10
| Plasmid | Event after conjugation | Transconjugants analyzed
|
ISPst9 changesa
|
No. of ISPst9 copies
|
||||
|---|---|---|---|---|---|---|---|---|
| n | ISPst9 affectedb | Increase | Pattern | Loss | Total | Mean | ||
| pLAN04 | Homologous recombination in nahAc gene | 27 | 27 (100) | 12 (44) | 11 (41) | 4 (15) | 55 | 2.04 |
| pLAN05 | Homologous recombination in nahG gene | 27 | 27 (100) | 12 (44) | 10 (37) | 5 (9) | 69 | 2.55 |
| pUT mini-Tn5-Km | Transposition | 28 | 28 (100) | 10 (36) | 5 (18) | 13 (46) | 57 | 2.04 |
| pDSK519 | Plasmid replication | 30 | 28 (93) | 8 (27) | 1 (3) | 19 (63) | 56 | 1.87 |
Increase, presence of more than two copies of ISPst9; Pattern, two copies of ISPst9, but with a different hybridization pattern; Loss, presence of less than two copies. The values are the number (percentages) of transconjugants in each group.
Number (percentage) of transconjugants that had a different ISPst9 genomic distribution than the wild-type strain.
Conjugation experiments with E. coli S17-1λpir carrying pUT mini-Tn5-Km (9) and P. stutzeri AN10 were also performed. The aim of this analysis was to evaluate whether another system of foreign DNA maintenance that disturbed the structure of the chromosome (transposition of mini-Tn5-Km) also upregulated ISPst9 transposition. Twenty-eight transconjugants in which transposition of mini-Tn5-Km occurred were selected as Km-resistant Ap-sensitive clones, and their ISPst9 genomic distribution was checked by hybridization with the tnpA4 probe. As observed with the recombination-dependent DNA acquisition, for all clones that acquired the Kmr determinant by conjugation followed by transposition there were changes in the tnpA4 hybridization pattern (Table 1). The number of clones that showed an increase in the ISPst9 copy number was slightly reduced compared with the values obtained in recombination-dependent DNA acquisition experiments. Interestingly, higher percentages of clones that had lost ISPst9 copies or in which the location was changed were observed (Table 1).
As both foreign DNA chromosomal integration methods resulted in 100% ISPst9 transposition in transconjugants, additional experiments were performed in order to evaluate whether acquisition of self-replicative DNA by conjugation without foreign DNA integration could also induce ISPst9 transposition. Conjugation experiments with E. coli S17-1λpir carrying the broad-host-range plasmid pDSK519 (24) and P. stutzeri AN10 were carried out. Thirty transconjugants harboring the pDSK519 plasmid were selected as Km-resistant clones and were checked to determine whether there were changes in the ISPst9 genomic distribution. For 28 clones (93.3%) that acquired the pDSK519 plasmid by conjugation there were changes in the original tnpA4 hybridization pattern (Table 1 and Fig. 2A), suggesting that the increase in ISPst9 transposition was caused by the addition by conjugation of foreign DNA, independent of the mechanism of maintenance or other events in the host.
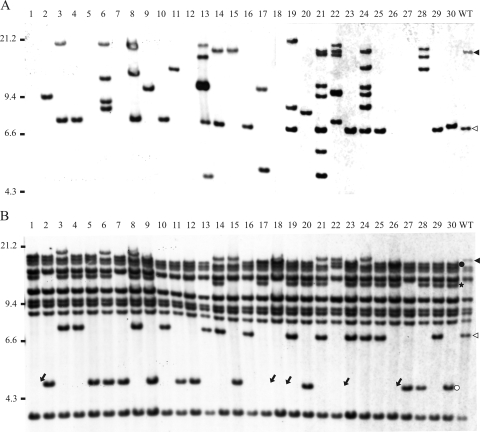
FIG. 2.
Southern blot hybridization with the tnpA4 probe for ISPst9 (A) and the tnpA2 probe (B) of EcoRI-digested genomic DNAs from P. stutzeri AN10 (lane WT) and 30 derivatives that received plasmid pDSK519 by conjugation. Triangles indicate EcoRI DNA fragments that hybridized with the wild-type strain with both probes used. Dots indicate tnpA2-containing EcoRI DNA fragments of transconjugants that lost ISPst9 after conjugation. Relationships between labeled bands are indicated by colors (black and white). The star indicates a tnpA2-containing EcoRI DNA fragment of transconjugants whose presence cannot be directly related to ISPst9 transposition, as explained in the text. Arrows indicate the absence of bands that revealed plausible genome rearrangements produced during ISPst9 transposition, as explained in the text.
Transformation assays were also performed with plasmid pDSK519 and P. stutzeri AN10. These experiments were carried out to clarify whether there were horizontal gene transfer mechanisms other than conjugation that were able to upregulate ISPst9 transposition. Twenty-three transformants were selected as Km-resistant clones. As expected (25), Southern blot hybridization with pDSK519 revealed that the linear plasmid introduced into strain AN10 by natural transformation did not recirculate to reestablish its replicative conformation but was inserted randomly into the genome of all transformants analyzed (results not shown). Although the random insertion altered the genome structure of transformants, none of them showed variation in the tnpA4 hybridization pattern. This suggested that ISPst9 transposition was enhanced only by conjugative DNA acquisition.
On other hand, all Southern blot membranes containing EcoRI-digested DNAs of the 112 AN10 transconjugants analyzed previously (Table 1) were also hybridized with a probe for tnpA2, the plausible transposase gene in an IS5-like insertion sequence located next to nahW (salicylate 1,2-hydroxylase-encoding gene) (5) and close to ISPst9. The aim of these hybridization experiments was to analyze whether there was a similar effect on the transposition of other IS elements present in P. stutzeri AN10. The wild-type strain exhibited hybridization signals with 10 distinct EcoRI-EcoRI DNA fragments (Fig. 2B), suggesting that at least 10 additional tnpA2-like genes were present. In fact, the existence of three such genes has been reported previously; one of them is next to the naphthalene degradation upper pathway genes (tnpA1) (3), and the other two flank the nahW gene (tnpA2 and tnpA3, located in the same EcoRI-EcoRI DNA fragment) (5). As shown for the transconjugants that received pDSK519 (Fig. 2B), only two tnpA2 hybridization bands of P. stutzeri AN10 were affected. Interestingly, both bands were the same size as the tnpA4 hybridization bands observed for the wild-type strain (Fig. 2A). Moreover, in all cases in which a transconjugant did not contain the 7.2-kb EcoRI-EcoRI ISPst9-containing DNA fragment as determined by hybridization, the corresponding tnpA2-containing band was also not present. In almost all these cases, a new tnpA2-containing band was detected (Fig. 2B, lane 30). This new tnpA2-containing band was at 4.7 kb, suggesting that there had been an ISPst9 excision event. Similar behavior was observed for the larger ISPst9-containing band (Fig. 2A). When this IS copy disappeared from its original location, the corresponding tnpA2-containing band (Fig. 2B) also disappeared, and a new smaller 2.5-kb tnpA2-containing band appeared (Fig. 2B, lane 30). Interestingly, a new 13-kb EcoRI-EcoRI tnpA2-containing band was detected in some of the transconjugants (Fig. 2B, lane 30). As far as we could tell, this new tnpA2-containing band was not directly correlated with any observed ISPst9 hybridization pattern, and it was also found in some transconjugants (Fig. 2B, lanes 3 and 14) that did not show changes in the ISPst9 genomic distribution. In any case, as this new tnpA2-containing band always appeared at the same position, independent of the clone analyzed, we assumed that its appearance was not due to IS5-like random transposition and could have been due to other ISPst9 transposition events that have not been detected by hybridization experiments yet. Finally, some transconjugants (Fig. 2B, lane 1) that had lost the ISPst9 copy on the 7.2-kb EcoRI-EcoRI DNA fragment did not contain the tnpA2-containing 4.7-kb EcoRI-EcoRI DNA fragment mentioned above (Fig. 2B). This could be attributed to genome rearrangements resulting from ISPst9 transposition that led to deletion or a change in position of the flanking DNA fragments. Similarly, genome rearrangements caused by ISPpu12, one of the closest relatives of ISPst9, have been reported previously (38). Thus, we concluded that conjugation only upregulates ISPst9 transposition and does not stimulate IS5-like transposition.
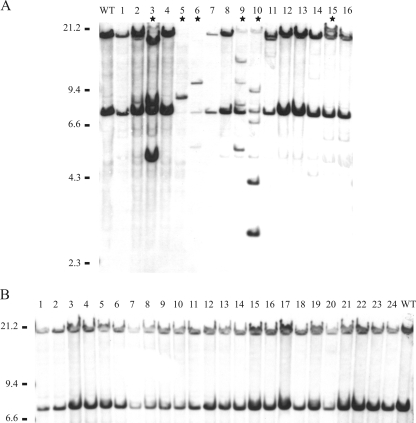
Additional experiments were performed in order to determine whether the upregulation of ISPst9 transposition in P. stutzeri AN10 was due to the conjugative interaction with E. coli or whether the presence of E. coli during the mating event alone was enough to activate ISPst9. Thus, separate contact event experiments with strain AN10 and two E. coli K-12 derivatives, one having the conjugation machinery (strain S17-1λpir) and the other not having the conjugation machinery (strain DH5α), were performed. None of the E. coli strains carried plasmids. Because no genetic exchange was intended to occur between either of the E. coli strains and AN10, there was no marker for selecting AN10 cells which came into contact with E. coli. Therefore, to force interactions between cells of the different species, strain AN10 was serially diluted, and 1 to 100 cells of this strain were spotted together with 108 cells of E. coli. Although there was no absolute guarantee of interaction, up to 6 of the 16 AN10 isolates obtained which had been in contact with E. coli S17-1λpir showed ISPst9 transposition (Fig. 3A). On the other hand, no transposition of this IS was observed in the 24 isolates of P. stutzeri AN10 which had been in contact with E. coli DH5α (Fig. 3B).
FIG. 3.
Southern blot hybridization with the tnpA4 probe of EcoRI-digested genomic DNAs from P. stutzeri AN10 and derivatives isolated after contact with E. coli S17-1λpir (A) or E. coli DH5α (B). Lane WT contained P. stutzeri wild-type strain AN10. Stars indicate the isolates showing ISPst9 transposition.
Simon and coworkers reported (32) that during construction of E. coli S17-1 (the parental strain of E. coli S17-1λpir), in a conjugation experiment the chromosomally integrated RP4 plasmid responsible for the conjugative process could be precisely excised and transferred to the receptor strain at low frequencies. We evaluated this possibility by mating 108 AN10 cells with 108 cells of S17-1λpir. As previously reported (32), we monitored transfer of the Tp resistance determinant (harbored in the chromosomally integrated RP4 plasmid) to strain AN10 by plating preparations in MBM supplemented with Tp. The Tpr acquisition frequencies (10−8) were lower than the acquisition frequencies obtained by Simon and coworkers for E. coli SM10 (10−5), a close relative of S17-1. Furthermore, we analyzed the presence of the Tpr determinant in the 16 AN10 isolates obtained previously after contact with strain S17-1λpir (the isolates shown in Fig. 3A). All of these isolates, including the six isolates that showed ISPst9 transposition (Fig. 3A), were Tp sensitive. We also hybridized the Southern blot membrane shown in Fig. 3A with the RP4-oriT probe and obtained no signal (results not shown), which indicated that plasmid RP4 was not present in genomes of the AN10 derivatives. Thus, although we cannot be completely sure that no genetic material was transferred during the conjugative process, our results suggest that conjugative interaction causes upregulation of ISPst9 transposition. To our knowledge, no similar phenomenon has been described previously. The most similar phenomenon was described by Godoy and Fox (15), who observed a high level of Tn10 loss after conjugational transfer, which they attributed to recombination when this element was inserted by conjugation.
ISPst9 transposed by excision from the donor DNA.
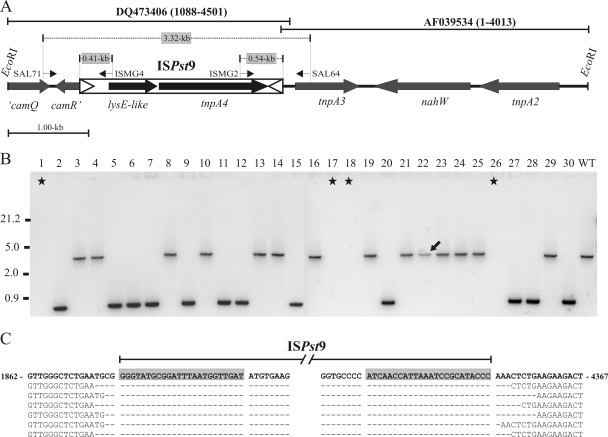
Most of transconjugants analyzed showed a unique and discriminative tnpA4 hybridization pattern, independent of their experimental origin, and seven was the highest number of copies detected (Fig. 2A, lane 21). Despite this, loss of ISPst9 copies and position changes were also observed, as mentioned above (Fig. 2A and Table 1). However, when the total number of putative ISPst9 copies was calculated for all transconjugants used in each experimental approach (Table 1), 2.1 ± 0.2 ISPst9 copies per transconjugant were obtained, suggesting that there was a “cut-and-paste” transposition mechanism (36). PCR amplification using primers SAL64 and SAL71 (Fig. 4A) was performed to determine whether ISPst9 really moved from its original position and originated the movements and losses observed. Wild-type P. stutzeri AN10 and the 30 AN10 transconjugants harboring plasmid pDSK519 shown in Fig. 2 were used. As expected, a single approximately 3.3-kb PCR product was obtained for the wild-type strain and all of the transconjugants in which ISPst9 was on the 7.2-kb EcoRI-EcoRI DNA fragment (Fig. 4B, lane 29). On the other hand, all of the pDSK519-containing transconjugants that had lost ISPst9 at the original position and produced the 4.7-kb tnpA2-containing DNA band (Fig. 2B) produced a unique PCR product that was approximately 0.85 kb long (Fig. 4B, lane 30). This result suggested that there was excision of ISPst9 from the donor DNA, supporting the finding that this IS transposed using a nonreplicative mechanism. Interestingly, no amplification was obtained for four of the five transconjugants (Fig. 4B) that had lost both the tnpA4 and tnpA2 hybridization bands mentioned above (Fig. 2B). This could have been due to plausible deletion of ISPst9-flanking DNA after transposition. The fifth transconjugant (Fig. 2B, lane 22) produced a PCR product whose size was identical to the size of the product observed for the wild-type strain (Fig. 4B). This result supports the hypothesis that there were genome rearrangements during ISPst9 transposition, as mentioned above.
FIG. 4.
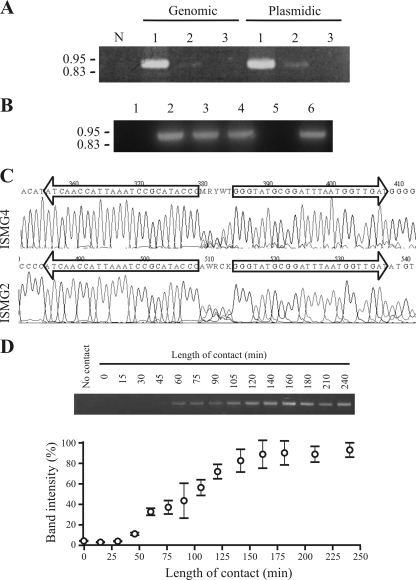
Location of ISPst9 in P. stutzeri AN10. (A) Schematic diagram of the 7.2-kb EcoRI-EcoRI DNA fragment of P. stutzeri AN10 harboring ISPst9. DQ473406 and AF039534 are nucleotide database accession numbers. Coordinates of both sequences are indicated in parentheses. The entire naphthalene degradation lower pathway (nahGTHINLOMKJ) is located in a 5.8-kb EcoRI-EcoRI fragment immediately upstream from the tnpA2 gene (4, 5). Genes code for the following proteins: ′camQ, partial CamQ lactone hydrolase-like protein; camR′, partial CamR transcriptional regulator; lysE-like, LysE-like protein; tnpA4, ISPst9 transposase; tnpA2 and tnpA3, putative IS5-like transposases; nahW, salicylate hydroxylase. The arrows indicate the directions of gene transcription. SAL71, ISMG4, ISMG2, and SAL64 were the primers used; the thin arrows indicate their locations and 5′-3′ orientations. Open triangles indicate IRs of ISPst9. (B) PCR amplification with primers SAL71 and SAL64 of P. stutzeri AN10 (lane WT) and the 30 derivatives that received plasmid pDSK519 by conjugation. Stars and the arrow indicate the isolates suspected to have had genome rearrangements during ISPst9 transposition. (C) Alignment of ISPst9 flanking sequences from P. stutzeri AN10 and six derivatives that received pDSK519 by conjugation and produced a single 0.85-kb SAL71-SAL64 PCR product. Nucleotides 1862 to 4367 of the previously published sequence of strain AN10 (accession number DQ473406) are indicated by bold type. IRs of ISPst9 are shaded.
The first evidence of ISPst9 transposition was observed with P. stutzeri AN142 (8). In this strain, a copy of ISPst9 disrupted the nahH gene. This copy of ISPst9 was flanked by perfect 8-bp direct repeats that were generated at the moment of insertion, and it could be excised precisely in order to reestablish functionality of the nahH gene. This was not observed for the original copy of ISPst9 in P. stutzeri AN10, for which no 8-bp direct repeats were found (8). In order to evaluate whether the excision of ISPst9 occurred together with deletion of a fixed number of nucleotides (the expected 8 bp), six of the 0.85-kb PCR products obtained from transconjugants which had lost the copy of ISPst9 indicated in Fig. 4A were sequenced. Nucleotide sequencing confirmed that ISPst9 was excised nearly perfectly, although the amount of the flanking DNA deleted during transposition ranged from 3 to 10 bp (Fig. 4C). Previous reports indicated that there are more perfect mobile element excision events than imperfect mobile element excision events. This probably occurs because a reestablished phenotype is necessary for detection of IS excision, since regeneration in frame of the inactivated gene is the only way that this can occur. In our experiment there was no phenotypic selection, and thus the deletion of a fixed number of nucleotides (8 bp) was not essential, although perfect excision could also occur, as previously demonstrated (8).
ISPst9 transposition forming IS circle intermediates.
IS1411 (22), another member of the ISL3 family, is one of the many ISs that have been shown to transpose via IS circle formation (7). To prove that circle formation also occurred in ISPst9, a PCR was performed using primers ISMG2 and ISMG4, which hybridized at the ends of ISPst9 with an outward orientation (Fig. 4A). Thus, if ISPst9 circle formation occurred, a 0.95-kb PCR product was expected. Genomic and plasmid DNA extraction protocols were used to obtain DNA from contact between P. stutzeri AN10 and E. coli S17-1λpir and from both strains separately as controls. An intense PCR product of the expected size was obtained from the contact event, independent of the DNA extraction protocol used, whereas only a very faint band was obtained when the DNA came from P. stutzeri AN10 alone (Fig. 5A). No amplification was observed with E. coli S17-1λpir (Fig. 5A). ISPst9 circle formation was also analyzed by PCR amplification for contact between strain AN10 and E. coli DH5α. Only a faint band, similar to the band obtained for strain AN10 alone, was observed. Therefore, we suggest that basal ISPst9 circle formation occurs in P. stutzeri AN10 and is enhanced during conjugative interaction. More precisely, as circles were detected after alkaline lysis-based DNA extraction, we also suggest that the IS circles were double-stranded DNA elements. Moreover, IS circle PCR amplification was also obtained after digestion with mung bean nuclease (Fig. 5B). No amplification was observed after thermal DNA denaturation prior to nuclease digestion, confirming the degrading effect of mung bean nuclease on single-stranded DNA.
FIG. 5.
Circle formation by ISPst9. (A) PCR amplification of circle junctions using primers ISMG2 and ISMG4. Lane N, negative control; lane 1, contact between P. stutzeri AN10 and E. coli S17-1λpir; lane 2, strain AN10 alone; lane 3, strain S17-1λpir alone. (B) PCR amplification of circle junctions after mung bean nuclease treatment. Lane 1, negative control; lane 2, positive control; lane 3, addition of mung bean nuclease buffer without enzyme; lane 4, treatment with mung bean nuclease; lane 5, treatment with mung bean nuclease after thermal DNA denaturation; lane 6, thermally denatured DNA without mung bean nuclease treatment. (C) Sequencing electropherograms obtained with primers ISMG2 and ISMG4 for the PCR product shown in panel A, lane 1. Only the circle junction area of chromatograms is shown. IRs of ISPst9 are indicated by arrows. (D) Semiquantification of ISPst9 circle formation in contact experiments with P. stutzeri AN10 and E. coli S17-1λpir for different incubation times. Only one of the three independent gels used for semiquantification is shown. The symbols and error bars indicate the means and standard deviations of three independent experimental determinations.
The amplified 0.95-kb PCR product was sequenced using primers ISMG2 and ISMG4. As expected, the nucleotide sequence confirmed that the two IRs of ISPst9 were contiguous and were separated by an imperfect 5-bp sequence (Fig. 5C). This 5-bp sequence was probably a combination of the two ISPst9 flanking sequences. A similar result was obtained for IS1411, although the 5-bp sequence (5′-AAACC-3′) that separated the IRs was derived from the left IR flanking sequence (22).
In order to evaluate the kinetics of ISPst9 circle formation, contact event experiments with P. stutzeri AN10 and E. coli S17-1λpir were performed using different incubation times (1 to 24 h). PCR amplification with ISMG2 and ISMG4 revealed that maximal ISPst9 circle formation occurred within the first 3 h of contact (Fig. 5D). Three independent experiments were done in order to analyze the kinetics of ISPst9 circle formation during the first 4 h of contact. The results demonstrated that maximum circle formation occurred after 2.5 h, although an increase in ISPst9 circle formation compared to the AN10 basal activity was observed after only 45 min of conjugative interaction (Fig. 5D). It has been shown previously that the transposase of IS911, one of the best-studied IS, is able to generate IS circles after 16 min of induction (12). However, these elevated IS911 circle formation kinetics were obtained by cloning the transposase gene under transcriptional control of the Plac promoter and not under natural conditions used here.
The results obtained for ISPst9 transposition are the first results for self-inducible, real, in vivo kinetics without any artificial transposase transcriptional amplification. This sensitive, easy transposition detection measurement method, together with the fact that conjugative interacting P. stutzeri cells mobilize ISPst9, should allow workers to study in depth the signaling cascade that occurs in the host after the stimulus that upregulates transposition, as well as the consequences of transposition for the host.
Acknowledgments
The support of the Scientific-Technical Service of the University of Balearic Islands during operation of the genetic analyzer for sequencing is acknowledged. B.N. was supported by a contract in the program “Ramon y Cajal” from the M.E.C. (Spanish Ministry of Education and Science). M.P.L. was supported by an F.P.I. doctoral grant from the M.E.C. J.A.C.-O. was supported by a grant from the C.A.I.B. (Government of the Balearic Islands). Funds were obtained from project CTM2005-01783 from the M.E.C. (with FEDER cofunding) and from project PRIB2004-10152 from C.A.I.B.
Footnotes
Published ahead of print on 5 December 2008.
REFERENCES
- 1.Aragno, M., and H. G. Schlegel. 1981. The hydrogen-oxidizing bacteria, p. 865-893. In M. P. Starr, H. Stolp, H. G. Trüper, A. Balows, and H. G. Schlegel (ed.), The prokaryotes. A handbook on habitats, isolation and identification of bacteria. Springer-Verlag, Berlin, Germany.
- 2.Bender, J., J. Kuo, and N. Kleckner. 1991. Genetic evidence against intramolecular rejoining of the donor DNA molecule following IS10 transposition. Genetics 128687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch, R., E. García-Valdés, and E. R. B. Moore. 1999. Genetic characterization and evolutionary implications of a chromosomally encoded naphthalene-degradation upper pathway from Pseudomonas stutzeri AN10. Gene 236149-157. [DOI] [PubMed] [Google Scholar]
- 4.Bosch, R., E. García-Valdés, and E. R. B. Moore. 2000. Complete nucleotide sequence and evolutionary significance of a chromosomally encoded naphthalene-degradation lower pathway from Pseudomonas stutzeri AN10. Gene 24565-74. [DOI] [PubMed] [Google Scholar]
- 5.Bosch, R., E. R. B. Moore, E. García-Valdés, and D. H. Pieper. 1999. NahW, a novel, inducible salicylate hydroxylase involved in mineralization of naphthalene by Pseudomonas stutzeri AN10. J. Bacteriol. 1812315-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brocklehurst, K. R., and A. P. Morby. 2000. Metal-ion tolerance in Escherichia coli: analysis of transcriptional profiles by gene-array technology. Microbiology 1462277-2282. [DOI] [PubMed] [Google Scholar]
- 7.Chandler, M., and J. Mahillon. 2002. Insertion sequence revisited, p. 305-366. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, DC.
- 8.Christie-Oleza, J. A., B. Nogales, C. Martín-Cardona, M. P. Lanfranconi, S. Albertí, J. Lalucat, and R. Bosch. 2008. ISPst9, an ISL3-like insertion sequence from Pseudomonas stutzeri AN10 involved in catabolic gene inactivation. Int. Microbiol. 11101-110. [PubMed] [Google Scholar]
- 9.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 1726568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Re, B., F. Garoia, P. Mesirca, C. Agostini, F. Bersani, and G. Giorgi. 2003. Extremely low frequency magnetic fields affect transposition activity in Escherichia coli. Radiat. Environ. Biophys. 42113-118. [DOI] [PubMed] [Google Scholar]
- 11.Dhaese, P., H. de Greve, H. Decraemer, J. Schell, and M. van Montagu. 1979. Rapid mapping of transposon insertion and deletion mutations in the large Ti-plasmids of Agrobacterium tumefaciens. Nucleic Acids Res. 71837-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duval-Valentin, G., B. Marty-Cointin, and M. Chandler. 2004. Requirement of IS911 replication before integration defines a new bacterial transposition pathway. EMBO J. 233897-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichenbaum, Z., and Z. Livneh. 1998. UV light induces IS10 transposition in Escherichia coli. Genetics 1491173-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanekar, K., A. McBride, O. Dellagostin, S. Thorne, R. Mooney, and J. McFadden. 1999. Stimulation of transposition of the Mycobacterium tuberculosis insertion sequence IS6110 by exposure to a microaerobic environment. Mol. Microbiol. 33982-993. [DOI] [PubMed] [Google Scholar]
- 15.Godoy, V. G., and M. S. Fox. 2000. Transposon stability and a role for conjugational transfer in adaptive mutability. Proc. Natl. Acad. Sci. USA 977393-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grindley, N. D. 2002. The movement of Tn3-like elements: transposition and cointegrate resolution, p. 272-302. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, DC.
- 17.Guasp, C., E. Moore, J. Lalucat, and A. Bennasar. 2000. Utility of internally transcribed 16S-23S rDNA spacer regions for the definition of Pseudomonas stutzeri genomovars and other Pseudomonas species. Int. J. Syst. Evol. Microbiol. 501629-1639. [DOI] [PubMed] [Google Scholar]
- 18.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 4195-98. [Google Scholar]
- 19.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 20.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 1726557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilves, H., R. Horak, and M. Kivisaar. 2001. Involvement of σS in starvation-induced transposition of Pseudomonas putida transposon Tn4652. J. Bacteriol. 1835445-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kallastu, A., R. Horak, and M. Kivisaar. 1998. Identification and characterization of IS1411, a new insertion sequence which causes transcriptional activation of the phenol degradation genes in Pseudomonas putida. J. Bacteriol. 1805306-5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109137-141. [DOI] [PubMed] [Google Scholar]
- 24.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70191-197. [DOI] [PubMed] [Google Scholar]
- 25.Lorenz, M. G., and W. Wackernagel. 1994. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 58563-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1702575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naas, T., M. Blot, W. Fitch, and W. Arber. 1995. Dynamics of IS-related genetic rearrangements in resting Escherichia coli K-12. Mol. Biol. Evol. 12198-207. [DOI] [PubMed] [Google Scholar]
- 28.Nagy, Z., and M. Chandler. 2004. Regulation of transposition in bacteria. Res. Microbiol. 155387-398. [DOI] [PubMed] [Google Scholar]
- 29.Pfeifer, F., and U. Blaseio. 1990. Transposition burst of the ISH27 insertion element family in Halobacterium halobium. Nucleic Acids Res. 186921-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosselló-Mora, R. A., J. Lalucat, and E. García-Valdés. 1994. Comparative biochemical and genetic analysis of naphthalene degradation among Pseudomonas stutzeri strains. Appl. Environ. Microbiol. 60966-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Simon, R., V. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 33.Stewart, G. J., and C. D. Sinigalliano. 1991. Exchange of chromosomal markers by natural transformation between the soil isolate, Pseudomonas stutzeri JM300, and the marine isolate, Pseudomonas stutzeri strain ZoBell. Antonie van Leeuwenhoek 5919-25. [DOI] [PubMed] [Google Scholar]
- 34.Strauch, E., and L. Beutin. 2006. Imprecise excision of insertion element IS5 from the fliC gene contributes to flagellar diversity in Escherichia coli. FEMS Microbiol. Lett. 256195-202. [DOI] [PubMed] [Google Scholar]
- 35.Tachdjian, S., and R. M. Kelly. 2006. Dynamic metabolic adjustments and genome plasticity are implicated in the heat shock response of the extremely thermoacidophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 1884553-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turlan, C., and M. Chandler. 2000. Playing second fiddle: second-strand processing and liberation of transposable elements from donor DNA. Trends Microbiol. 8268-274. [DOI] [PubMed] [Google Scholar]
- 37.Twiss, E., A. M. Coros, N. P. Tavakoli, and K. M. Derbyshire. 2005. Transposition is modulated by a diverse set of host factors in Escherichia coli and is stimulated by nutritional stress. Mol. Microbiol. 571593-1607. [DOI] [PubMed] [Google Scholar]
- 38.Weightman, A. J., A. W. Topping, K. E. Hill, L. L. Lee, K. Sakai, J. H. Slater, and A. W. Thomas. 2002. Transposition of DEH, a broad-host-range transposon flanked by ISPpu12, in Pseudomonas putida is associated with genomic rearrangements and dehalogenase gene silencing. J. Bacteriol. 1846581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]