Abstract
DNA double-strand breaks are particularly deleterious lesions that can lead to genomic instability and cell death. We investigated the SOS response to double-strand breaks in both Escherichia coli and Bacillus subtilis. In E. coli, double-strand breaks induced by ionizing radiation resulted in SOS induction in virtually every cell. E. coli strains incapable of SOS induction were sensitive to ionizing radiation. In striking contrast, we found that in B. subtilis both ionizing radiation and a site-specific double-strand break causes induction of prophage PBSX and SOS gene expression in only a small subpopulation of cells. These results show that double-strand breaks provoke global SOS induction in E. coli but not in B. subtilis. Remarkably, RecA-GFP focus formation was nearly identical following ionizing radiation challenge in both E. coli and B. subtilis, demonstrating that formation of RecA-GFP foci occurs in response to double-strand breaks but does not require or result in SOS induction in B. subtilis. Furthermore, we found that B. subtilis cells incapable of inducing SOS had near wild-type levels of survival in response to ionizing radiation. Moreover, B. subtilis RecN contributes to maintaining low levels of SOS induction during double-strand break repair. Thus, we found that the contribution of SOS induction to double-strand break repair differs substantially between E. coli and B. subtilis.
Both prokaryotes and eukaryotes respond to DNA damage, in part by altering the global expression profile of hundreds of gene products that function in a variety of cellular pathways (23, 34, 37, 87). In Escherichia coli and Bacillus subtilis the transcriptional response to DNA-damaging agents has been well characterized (11, 23, 31, 37, 67). In both organisms, the highly conserved RecA and LexA proteins control the induction of the protective SOS response following DNA damage (for reviews, see references 83 and 88). RecA positively regulates SOS, while LexA represses this response. RecA binds excess single-stranded DNA (ssDNA) forming a nucleoprotein filament (for a review, see reference 24). The RecA/ssDNA nucleoprotein filament allows for RecA to activate LexA's latent protease activity, resulting in LexA autocleavage and subsequent derepression of the SOS regulon (for a review, see reference 83). In E. coli, the SOS regulon is comprised of 57 genes (for a review, see reference 77). These genes have been identified as part of the SOS regulon because they have a conserved LexA binding site(s) or because their transcription fails to respond to DNA damage in a strain bearing a noncleavable derivative of LexA [lexA(Ind−)] (for reviews, see references 77 and 23).
In B. subtilis, RecA regulates the expression of approximately 600 genes following DNA damage or replication fork arrest (37). The majority of these genes belong to the prophages PBSX and SPβ or are induced in response to the expression of these prophage genes (37). In addition, RecA appears to regulate 26 operons (63 genes) through B. subtilis LexA (11, 37). As in E. coli, the SOS genes are not inducible in a B. subtilis strain bearing the lexA(Ind−) allele and are constitutively expressed in a strain lacking lexA. The B. subtilis SOS regulated genes are also induced following challenge with a variety of DNA damaging agents and following replication fork arrest (11, 36, 37). Interestingly, only eight SOS regulated genes in B. subtilis have orthologs in E. coli that are SOS regulated; three of these are involved in double-strand break repair (11). These observations indicate that although the SOS regulatory mechanism is conserved between these two organisms, the genes under the control of LexA differ significantly.
DNA double-strand breaks are deleterious lesions that are lethal if left unrepaired (for a review, see reference 33). In E. coli, double-strand breaks are almost exclusively repaired through homologous recombination. During homologous recombination, the RecBCD helicase-nuclease enzyme processes double-stranded ends resulting in 3′ ssDNA that is used as a substrate for RecA loading (for a review, see reference 33). RecA polymerization on ssDNA forms a nucleoprotein filament (41, 42; for a review, see reference 77). Once the filament forms, RecA searches for homologous DNA to facilitate base pairing between the broken DNA segment and the intact homolog (40). The prevailing model is that the RecA/ssDNA nucleoprotein filament provides two functions. One function is to catalyze base pairing with the intact sister chromosome. The second function of the filament is to interact with LexA, stimulating LexA autocleavage and subsequent depression of the SOS regulon (43, 44, 63). In E. coli several proteins have been identified as modulating formation of RecA/ssDNA filaments in vitro and in vivo. In particular, the RecFOR proteins assist to nucleate the loading of RecA onto ssDNA at gaps (57). RecBCD can also function to load RecA at the site of a double-strand break. DinI stabilizes RecA/ssDNA filaments, while RecX and RdgC function to limit RecA/ssDNA filament formation (27-29, 48-50, 65, 81, 82). Several of the proteins mentioned above affect the magnitude of SOS induction in vivo. In B. subtilis less is known about the mechanism(s) that regulates the formation of RecA/ssDNA filaments in vivo. B. subtilis does contain homologs or functional analogs of the RecFOR and RecBCD pathways; however, homologs or analogs of DinI, RecX, and RdgC have not been identified in B. subtilis (1, 3, 4, 18, 19). These data suggest that the mechanisms regulating RecA/ssDNA filament formation may be very different between E. coli and B. subtilis.
During exponential growth B. subtilis primarily repairs double-strand breaks through homologous recombination (for a review, see reference 14). E. coli and B. subtilis contain very different homologous recombination pathways (for a review, see reference 33). For example, in B. subtilis gene products involved in homologous recombination comprise seven epistatic groups (for reviews, see references 71 and 72). Protein members from three of these epistatic groups have not been identified in E. coli and four of the epistatic groups function in the processing and loading of RecA onto ssDNA (for reviews, see references 2, 24, 33, 71, and 72). The major differences between the homologous recombination pathways of E. coli and B. subtilis are at the steps of RecA loading and regulating the extent of RecA polymerization on ssDNA.
Because the pathways that regulate RecA binding to ssDNA differ substantially and because the RecA/ssDNA filament is required for SOS induction, we wanted to determine whether SOS induction also differs between E. coli and B. subtilis in response to double-strand breaks. To address this, we measured RecA-green fluorescent protein (GFP) focus formation as an assay for RecA loading in vivo and the transcriptional response in single cells to ionizing radiation in E. coli and B. subtilis. In addition, we measured the transcriptional response of B. subtilis to an enzyme-catalyzed double-strand break induced by the yeast homing endonuclease I-SceI. Based on studies in E. coli, the prevailing view is that double-strand breaks are potent inducers of SOS.
We found here that ionizing radiation readily induced RecA-GFP foci in the majority of E. coli and B. subtilis cells. We found that the percentage of B. subtilis cells showing RecA-GFP foci was similar to the percentage of E. coli cells showing RecA-GFP foci following the induction of double-strand breaks. In contrast to E. coli, we found that SOS induction in B. subtilis was detected in only a small subpopulation of cells following induction of double-strand breaks. These data suggest that the RecA loading response to double-strand breaks is similar between E. coli and B. subtilis; however, the downstream SOS response differs significantly. Furthermore, we measured mRNA levels by using DNA microarrays and found that an I-SceI-catalyzed double-strand break resulted in a slight increase in the expression of LexA repressed genes across a population of cells. These results are in striking contrast to E. coli, which shows SOS induction in most cells following an I-SceI-catalyzed double-strand break (58) or ionizing radiation challenge (the present study). Our comprehensive analysis of B. subtilis in response to double-strand breaks shows that SOS induction is limited and B. subtilis strains incapable of inducing SOS have near wild-type levels of survival to ionizing radiation. Furthermore, we show that wild-type recN+ contributes to maintaining the low levels of spontaneous and DNA damage-inducible SOS that were measured in B. subtilis. We conclude that induction of the SOS response is required for double-strand break repair in E. coli, but SOS is not required for survival, nor even induced in most B. subtilis cells in response to double-strand breaks.
MATERIALS AND METHODS
Bacteriological methods.
Strains used in the present study are described in Table 1. B. subtilis strains were grown in Luria-Bertani (LB) or S750 defined minimal medium at 30°C (12, 38, 79). S750, where indicated, was supplemented with 1% glucose, phenylalanine, and tryptophan at a final concentration of 40 μg/ml. Where indicated, 1% arabinose and 1% succinate were used in place of glucose (12, 38, 79). Antibiotic concentrations were used as described previously (12, 76). Where indicated for ionizing radiation treatment prior to microscopy, 10-ml portions of mid-exponential-phase cultures were placed in 50-ml conical tubes and exposed to a 60Co gamma irradiator for a dose of 100 or 20 Gy, as indicated.
TABLE 1.
Strains used in this studya
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| B. subtilis | ||
| PY79 | Prototroph, SPβ0 | 89 |
| LAS24 | recA::neo | 75 |
| LAS40 | recA-mgfp-mut2(A206K) (spc) | 78 |
| LAS167 | recN::cat | 70 |
| LAS195 | amyE::PxylI-SceI (cat) | 78 |
| LAS206 | xkdF-yfp (spc) | 15; this study |
| LAS210 | amyE::PxylI-SceI (mls), cgeD::I-SceI recognition site (cat) | 78 |
| LAS239 | amyE::PxylI-SceI (mls), cgeD::I-SceI recognition site (cat), xkdF-yfp (spc) | This study |
| LAS261 | amyE::PxylI-SceI (mls), cgeD::I-SceI recognition site (cat), recA-mgfp(A206K) (spc) | 78 |
| LAS320 | ykoU-ykoV::erm | 85; this study |
| LAS366 | tagC-gfp (spc) | 15; this study |
| LAS367 | amyE::PxylI-SceI (mls) cgeD::I-SceI recognition site (cat), tagC-gfp (spc) | This study |
| LAS600 | trpC2 Δupp | 30 |
| LAS630 | trpC2 Δupp recA::neo | This study |
| LAS631 | trpC2 Δupp dinR3 [lexA(Ind−)] | This study |
| LAS634 | tagC-gfp (spc) recO::cat | This study |
| LAS635 | tagC-gfp (spc) recN::cat | This study |
| E. coli | ||
| MC4100 | F−araD139 Δ(argF-lac)U169 relA1 rpsL150(Strr) flbB5301 deoC1 ptsF25 rbsR | Laboratory stock |
| SS996 | Δattλ::sulApΩgfp-mut2 | 51 |
| BWD06 | MC4100 recA4136-gfp | 64; this study |
| BWD11 | MC4100 ΔrecA::Tc | This study |
| BWD12 | MC4100 lexA3 [lexA(Ind−)] malF::kan | This study |
All B. subtilis strains used in this study are derivatives of 168. All BWD derivatives were constructed from MC4100.
Live cell microscopy.
Microscopy was performed essentially as described previously (78, 79). Cells were grown in defined S750 minimal medium (38) as described in the figure or table legends. Cell membranes were stained with FM4-64 (Molecular Probes). For RecA-GFP images, the exposure time was between 0.5 and 1.0 s. Exposure times for image capture of XkdF-YFP and TagC-GFP were 0.15 and 0.3 s, respectively. Figures were assembled using Photoshop and Illustrator (Adobe).
Microarrays.
DNA microarray procedures were performed essentially as described previously (36, 37). Briefly, PCR arrays representing >99% of the genome printed on Corning GAPS slides were used. Exponentially growing cultures from three independent samples were grown in LB medium (for ionizing radiation) and S750 minimal medium for expression of I-SceI endonuclease. After treatment, cells were collected by centrifugation and processed as described previously (36, 37) to generate a cDNA labeled with Cy5. The Cy5 experimental sample was normalized to a Cy3 signal for a reference cDNA generated as described previously (11, 36, 37). The microarray analysis included spots with >80% of the pixels at one standard deviation above the signal obtained for the background. Significance scores were generated by using the significance analysis of microarrays as described previously, and the cutoff for significance was a 1.5-fold change in gene expression and a Q-value of <5.0 (11, 36, 37).
E. coli growth conditions for microscopy and ionizing radiation treatment.
For microscopy, E. coli strains were grown in M63 minimal medium [15 mM (NH4)2SO4, 0.22 M KH2PO4,0.4 M K2HPO4, 0.2% glucose, 1 mM MgSO4, 5 μM FeSO4] at 30°C until mid-exponential growth (optical density at 600 nm of 0.5). For ionizing radiation treatment, 10 ml of cells were placed in a 50-ml conical tube for exposure to gamma rays from the same 60Co source. After irradiation, cells were placed on agarose pads containing 1% agarose in M63 medium. Cells were stained and visualized as described previously (35). For ionizing radiation killing experiments, exponential-phase cells were placed in 0.85% saline and subjected to the indicated doses of ionizing radiation. Serial dilutions were plated on LB plates, followed by incubation overnight at 37°C prior to scoring for viable cells. Each experiment was repeated at least three times from independent cultures.
RESULTS
Ionizing radiation exposure induces SOS in most E. coli cells.
To establish a basis for comparison to B. subtilis, we pursued experiments in E. coli to determine the level of ionizing radiation sufficient to induce SOS in most E. coli cells. In E. coli, previous work shows that an I-SceI-generated double-strand break induces SOS in the majority of cells (52, 58). It has also been reported that SOS induction in E. coli can be detected throughout a population of cells following ionizing radiation challenge with as little as 1.5 Gy (54).
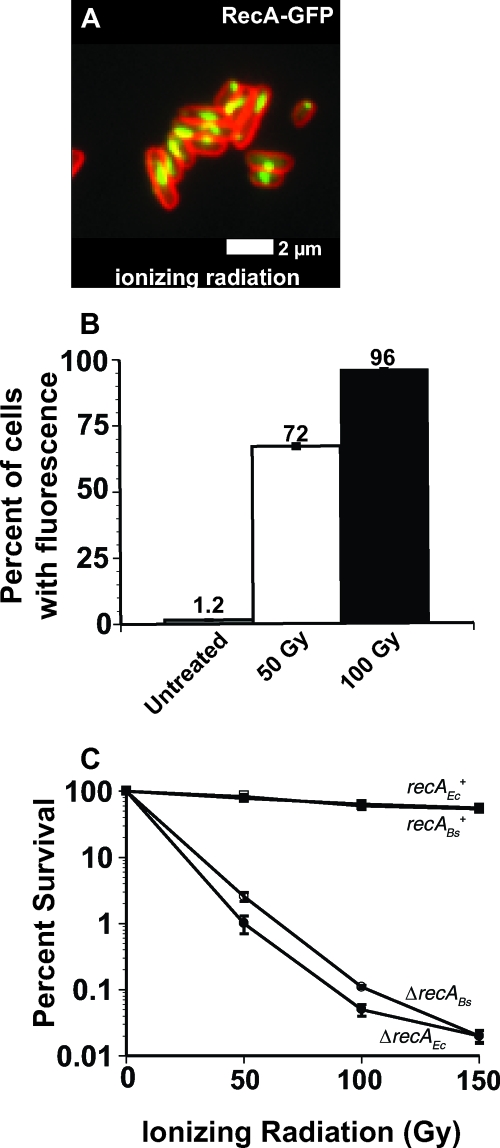
To follow up on these results, we investigated the RecA-GFP focus formation response and the SOS response to ionizing radiation in E. coli. For the untreated control, we found that most cells either had no foci or polar RecA-GFP storage structures (data not shown), confirming previous reports (16, 17, 64-66). In contrast, when we treated cells with 100 Gy of ionizing radiation we observed >99% of cells (n = 978) with RecA-GFP foci with most foci located near mid cell (Fig. 1A). This experiment shows that RecA-GFP focus formation is stimulated in the majority of cells following ionizing radiation challenge (Fig. 1A). We sought to determine what percentage of single E. coli cells had SOS induction after challenge with 50 or 100 Gy of ionizing radiation in order to determine whether the percentage of cells we observed with RecA-GFP foci correlates with the percentage of cells showing SOS induction. To measure the SOS response in single E. coli cells, we used a transcriptional reporter consisting of the gfp gene under the control of the LexA-regulated sulA promoter, as described previously (51). Using this transcriptional reporter strain, we observed SOS induction in 1.2% ± 0.1% (±95% confidence interval, n = 1,021) of cells grown in the absence of exogenous DNA-damaging agents, confirming previously published results (51). When we exposed these cells to 50 Gy of ionizing radiation, which corresponds to approximately 0.2 double-strand breaks per cell (13), we observed SOS induction in 72% ± 0.9% (n = 1,329) of cells (Fig. 1B). When the transcriptional reporter strain was challenged with 100 Gy of ionizing radiation, which corresponds to approximately 0.4 double-strand breaks per cell (13), we observed sulA promoter activity in 96% ± 0.1% (n = 1,420) of cells. Since ionizing radiation induces single-strand breaks and base damage sites in addition to double-strand breaks (for a review, see reference 84), we suggest that the sulA promoter activity is induced in response to other types of ionizing radiation-induced damage. Our results, taken into consideration with the published data for sulA promoter induction following an I-SceI-catalyzed site-specific double-strand break (58), suggest that SOS induction is a normal physiological response to double-strand breaks and other ionizing-radiation-induced DNA damage in E. coli.
FIG. 1.
Ionizing radiation induces sulA promoter activity in E. coli. (A) Representative micrograph of E. coli RecA-GFP after ionizing radiation challenge. RecA-GFP is shown in green, and the cell membrane stained with FM4-64 is shown in red. White bar, 2 μm. (B) E. coli cells bearing the sulApΩgfp-mut2 promoter were used to monitor sulA promoter activity in response to DNA damage in single cells using fluorescence microscopy. The percentages of cells with sulApΩgfp-mut2 fluorescence are shown for untreated cells (n = 1,021) and cells after 50 Gy (n = 1,329) or 100 Gy (n = 1,420) of ionizing radiation. Error bars indicate ± the 95% confidence interval (variance) between at least three independent experiments as follows: untreated, 1.2 ± 0.14; 50 Gy, 72 ± 0.94; and 100 Gy, 96 ± 0.11. (C) Killing curve of wild-type E. coli (strain MC4100) and an isogenic strain with recA deleted. The open symbols represent B. subtilis recA+ (recABs+ [□]) and recA-deficient (ΔrecABs [○]) strains, and the closed symbols are E. coli recA+ (recAEc+ [▪]) and recA-deficient (ΔrecAEc [•]) strains. The error bars represent the standard errors from at least three independent experiments.
We performed an ionizing radiation killing curve from 0 to 150 Gy for wild-type and recA-deficient E. coli strains to compare the percentage of viable cells following ionizing radiation challenge. The wild-type strain survived well, with ∼60% of viable cells recovered following a 100-Gy dose of ionizing radiation, supporting previous observations (52). In contrast, the recA-deficient strain was highly sensitive, with 0.02% of cells surviving a 100-Gy dose (Fig. 1C). We performed a similar killing curve with wild-type B. subtilis cells and an isogenic derivative lacking a functional recA gene for comparison. We found that the survival pattern for E. coli and B. subtilis were very similar for the wild-type and recA-deficient strains (Fig. 1C). Since B. subtilis and E. coli are different organisms and because B. subtilis cells can form chains, we cannot directly compare these killing curves. With that caveat in mind, E. coli and B. subtilis showed similar levels of survival in response to ionizing radiation.
Ionizing radiation induces SOS in a subpopulation of B. subtilis cells.
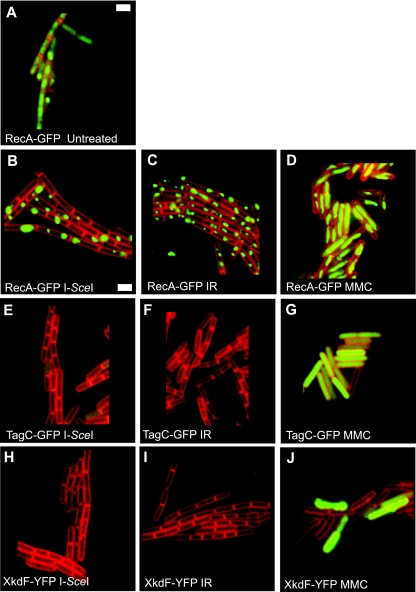
Our results demonstrated that ionizing radiation induces SOS in most E. coli cells. Since E. coli and B. subtilis showed very similar survival curves after ionizing radiation challenge (Fig. 1C), we investigated whether B. subtilis showed similar responses of RecA-GFP foci and SOS induction. RecA-GFP foci were observed in ∼10% (n = 447) of untreated cells. After ionizing radiation challenge, RecA-GFP foci were observed in ∼98% (n = 437) of cells (Fig. 2 and Table 2). The percentages of cells showing RecA-GFP foci following exposure to 100 Gy of ionizing radiation were very similar in E. coli and B. subtilis. To measure DNA damage-inducible gene expression in single B. subtilis cells, we used translational fusions to PBSX phage gene xkdF-yfp and SOS regulated gene tagC-gfp, both of which have been described previously (15). After exposure to 100 Gy of ionizing radiation we only observed PBSX or SOS induction in a subset of cells, 0.4 and 0.3% of cells, respectively (Fig. 2 and Table 2).
FIG. 2.
Visualization of RecA-GFP foci, SOS induction, and PBSX expression after induction of double-strand breaks in B. subtilis. (A to D) Representative micrographs of RecA-GFP foci in B. subtilis. (A) Untreated; (B) I-SceI expression; (C) 100 Gy of ionizing radiation (IR); (D) MMC (1 μg/ml). RecA-GFP foci are shown in green overlaid with the membrane stain FM4-64, which is shown in red. (E to G) Cells with TagC-GFP demonstrating SOS inductions. (E) I-SceI expression; (F) 100 Gy of ionizing radiation (IR), (G) MMC (1 μg/ml). TagC-GFP fluorescent cells are overlaid with the membrane (red). (H to J) Cells with XkdF-YFP representing PBSX expression. (H) I-SceI expression; (I) 100 Gy of ionizing radiation (IR); (J) MMC (1 μg/ml). XkdF-YFP images are overlaid with membrane shown in red. White bar, 2 μm. The numerical scoring of these images is presented in Table 2.
TABLE 2.
Single cell comparison of SOS induction, phage expression (PBSX), and formation of RecA-GFP foci after exposure to DNA damage in B. subtilisa
| Treatment | % of cells with fluorescence or foci (n)
|
||
|---|---|---|---|
| RecA-GFP | XkdF-YFP (PBSX) | TagC-GFP (SOS) | |
| Untreated | 10 (447) | 0.05 (2,006) | 0.06 (3,125)b |
| I-SceI uninduced | 19 (515) | 0.2 (1,050) | 1.9 (1,952) |
| I-SceI repressed | 16 (442) | 0.15 (1,348) | 0.5 (1,808) |
| I-SceI expressed | 75 (424) | 1.4 (789) | 4.6 (829) |
| 100 Gy | 98 (437) | 0.4 (1,851) | 0.3 (1,704) |
| MMC (1 μg/ml) | >99 (1,738) | 19 (776) | 99 (874) |
For the untreated samples and the 100-Gy-treated and MMC-treated strains, PY79 bears the indicated fusion (RecA-GFP, XkdF-YFP, or TagC-GFP) but is otherwise a wild-type strain and does not encode the I-SceI endonuclease or the I-SceI recognition site. These strains were grown in S750 minimal medium supplemented with glucose, tryptophan, and phenylalanine. I-SceI uninduced cells were grown in S750 with arabinose as a carbon source. For repressed conditions the cells were grown in S750 with glucose, and under expressed conditions cells were grown in S750 with arabinose followed by the addition of 0.2% xylose to induce expression of the I-SceI endonuclease. Cells treated with ionizing radiation (100 Gy) were grown in S750 minimal medium supplemented with 1% glucose, tryptophan, and phenylalanine. The data presented in this table are from at least two independent experiments. The number of cells scored (n) is indicated in parentheses for each condition.
Fluorescence was observed for only two cells. The fluorescence intensity was weak compared to the fluorescence intensity after exposure to 1 μg of MMC/ml.
We found it striking that 100 Gy of ionizing radiation was sufficient to elicit RecA-GFP focus formation in ∼98% of B. subtilis cells (n = 437), which is >200-fold higher than the percentage of cells induced for XkdF-YFP and TagC-GFP expression. As we found in E. coli, ∼60% of B. subtilis cells were viable following a 100-Gy dose of ionizing radiation (Fig. 1C). However, we found that less than ∼1% of irradiated cells showed XkdF-YFP or TagC-GFP expression (Fig. 2 and Table 2). We also challenged the SOS and PBSX transcriptional reporter strains with 1 μg of mitomycin C (MMC)/ml as a control to validate the use of these reporter fusions. We found XkdF-YFP (PBSX) expression in ∼19% of cells (n = 776), TagC-GFP (SOS) expression in ∼99% of cells (n = 874), and RecA-GFP focus formation in >99% of cells (n = 1,738) (Table 2). These controls validate that DNA damage does indeed induce expression of XkdF-YFP and TagC-GFP translational reporters (Fig. 2 and Table 2). We conclude that DNA damage caused by ionizing radiation at 100 Gy elicits SOS induction in only a small subpopulation of cells, which is in contrast to our results for an identical ionizing radiation dose to E. coli.
An I-SceI-catalyzed double-strand break induces SOS in a subpopulation of cells.
As mentioned above, ionizing radiation induces several types of DNA damage in addition to double-strand breaks (for a review, see reference 84). Because we only observed increased expression of TagC-GFP or XkdF-YFP by ionizing radiation in a small subpopulation of cells, we determined the expression of TagC-GFP and XkdF-YFP in single cells to a site-specific endonuclease that generates only one double-strand break per chromosome. To achieve this, we used the site-specific endonuclease I-SceI (21, 22, 78). The I-SceI recognition site is located at the cgeD locus in the B. subtilis chromosome at 183° on the chromosomal linkage map (78). This strain also carries the I-SceI endonuclease, with expression controlled by a xylose-inducible promoter located at the amyE locus (78). We have previously shown that after expression of I-SceI, chromosomal breakage is observed in the majority of B. subtilis cells, and RecA-GFP foci are observed in >70% of cells (78). We used this system to compare the percentage of cells with XkdF-YFP and TagC-GFP expression with the percentage of cells showing RecA-GFP foci following a I-SceI-catalyzed double-strand break (Fig. 2 and Table 2).
We observed RecA-GFP foci in ∼10% of cells (n = 447) in a control strain that lacks the I-SceI enzyme and cleavage site (Fig. 2A and Table 2). In contrast, expression of I-SceI in a strain, bearing the enzyme and the cleavage site, resulted in RecA-GFP foci in ∼75% of cells (n = 424) (Fig. 2 and Table 2). These results are consistent with published data (78) and suggest RecA-GFP loads onto ssDNA in response to an I-SceI-catalyzed double-strand break (Table 2). In contrast, to the RecA-GFP data, TagC-GFP fluorescence was captured in only ∼4.6% of cells (n = 829) following expression of I-SceI (Table 2). For comparison, we also determined the level of SOS induction in an isogenic strain lacking the I-SceI recognition site and the I-SceI enzyme and found that the spontaneous level of SOS induction was very low, with ∼0.06% of cells (n = 3,125) showing TagC-GFP fluorescence (Table 2 and Fig. 2).
We also used a translational fusion to xkdF-yfp to measure DNA damage-induced PBSX expression at the single cell level following an I-SceI-generated double-strand break. We found that ∼1.4% of cells (n = 789) had XkdF-YFP fluorescence following an I-SceI-induced double-strand break (Table 2). We conclude that an I-SceI-catalyzed double-strand break results in SOS and PBSX gene expression in a small subpopulation of cells. Strikingly, the same condition results in the formation of RecA-GFP foci in the majority of cells (∼75%, n = 424, Table 2). These results suggest that the assembly of RecA-GFP foci does not directly correlate with SOS induction in B. subtilis following double-strand breaks.
Double-strand breaks induce a limited set of LexA-controlled genes in B. subtilis.
In B. subtilis, the transcriptional response to a variety of DNA-damaging agents has been characterized (11, 36, 37, 45-47). We found that both ionizing radiation and a site-specific double-strand break resulted in <5% of cells showing either TagC-GFP or XkdF-YFP expression in single cells (Fig. 2 and Table 2). We thus used microarray analysis to characterize the genome-wide transcriptional response to a site-specific double-strand break to determine whether other LexA regulated genes were also detected.
To explore the genome-wide transcriptional response to a site-specific double-strand break, we used the I-SceI endonuclease system that we have engineered for use in B. subtilis as described above. Expression of I-SceI was induced with the addition of 0.2% xylose, and the transcriptional response was analyzed at 60 min after induction. We were only able to detect a change in gene expression, excluding genes induced by the addition of xylose, at the 60-min time point (Table 3 and data not shown). We chose to examine a time course up to 60 min because we have monitored the appearance of RecA-GFP foci and an SOS reporter (TagC-GFP) and found that the percentage of cells with foci or fluorescence, respectively, peaks near 60 min (data not shown).
TABLE 3.
Expression of SOS regulated genes increases in response to a single site-specific double-strand break in B. subtilis
| Genea | Function or element | Fold change (Q value, %)b
|
|
|---|---|---|---|
| I-SceI + xylose vs ΔI-SceI + xylose | I-SceI + xylose vs I-SceI (no xylose) | ||
| yneB | Site-specific recombinase | 9.7 (1.3) | 7.7 (1.6) |
| yneA | Cell division inhibitor | 5.5 (1.3) | 4.6 (1.6) |
| msmE | Multiple sugar binding protein | 5.1 (1.3) | 2.9 (1.6) |
| dinB | Nuclease inhibitor | 4.4 (1.3) | 3.4 (1.6) |
| i_aprX_ymaC | Alkaline serine protease | 3.7 (1.3) | 2.6 (1.6) |
| yhaZ | Alkylation repair | 3.6 (1.3) | 2.8 (1.6) |
| lexA | Transcriptional repressor of SOS | 2.6 (1.3) | 2.4 (1.6) |
| recA | Homologous recombination | 2.2 (1.3) | ND |
| xkdF | PBSX | 3.3 (1.3) | ND |
| xkdM | PBSX | 3.2 (1.3) | ND |
| xkdV | PBSX | 3.0 (1.3) | ND |
| xkdE | PBSX | 2.9 (1.3) | ND |
| xkdN | PBSX | 2.7 (1.3) | ND |
| xkdG | PBSX | 2.6 (1.3) | ND |
| xhlA | PBSX | 2.5 (1.3) | ND |
| xkdJ | PBSX | 2.5 (1.3) | ND |
| xkdP | PBSX | 2.5 (1.3) | ND |
| xkdH | PBSX | 2.4 (1.3) | ND |
| i_xylB_yncB | Xylulose kinase | ND | 62 (1.6) |
| ptsG | Glucose transport/phosphorylation | ND | 24.3 (1.6) |
| xynB | Xylan β-1,4-xylosidase | ND | 12 (1.6) |
| xylA | Xylose isomerase | ND | 20.1 (1.6) |
“i” indicates “intergenic region.”
The Q value is the rate of false-positive discovery. A Q value of 1% means that 1 out of 100 is likely to be false positive. The list of genes in this table reflects the significantly upregulated genes that were identified. The gene tagC was induced 1.8-fold in the microarray when comparing I-SceI + xylose with ΔI-SceI + xylose (data not shown). Raw microarray data are available at NCBI GEO (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE13694. Gene names are from http://genolist.pasteur.fr/SubtiList/. ND, not determined.
Transcription of LexA controlled genes yhaZ, yneB, yneA, and dinB were induced between three- and ninefold. In B. subtilis, the protein encoded by yneA inhibits cell division and is the functional analog to E. coli SulA (37, 39). The gene yneB is annotated as a site-specific recombinase, and dinB is predicted to encode a nuclease inhibitor (http://genolist.pasteur.fr/SubtiList/). We observed induction of 7 SOS regulated genes out of 63 (11, 36, 37). Included in these seven genes was an increase in transcription of both the lexA and recA genes. In addition to LexA regulated genes, several xkd genes encoded by the defective prophage PBSX (15) were also detected in the microarray, confirming our observations with the XkdF-YFP reporter fusion. We conclude that the small subpopulation of cells induced for SOS are induced enough to allow for detection of seven SOS regulated genes across a population of cells.
Previously, it has been reported that exposure of B. subtilis cells to conditions that inhibit replication fork progression leads to expression of the DnaA regulon (36). Since a double-strand break encountered by a replication fork would likely result in a block of fork progression, we wanted to determine whether DnaA regulated genes were affected. We did not observe activation of DnaA regulated genes following the I-SceI-catalyzed double-strand break (Table 3). The modest induction of the SOS response is the only break-induced transcriptional response detected in cells with an I-SceI-generated double-strand break. Taken together, the microarray analysis confirmed a limited SOS response at the cell population level, and the reporter fusions show high expression in a subpopulation of single cells after induction of an I-SceI-catalyzed double-strand break.
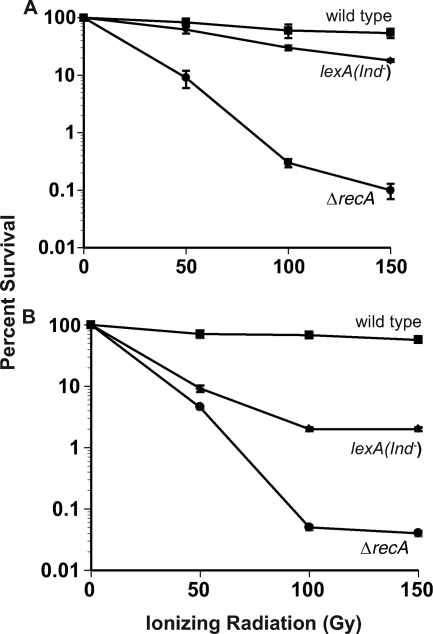
B. subtilis bearing a lexA(Ind−) shows nearly wild-type levels of survival after ionizing radiation challenge.
Because B. subtilis had only modest SOS induction during double-strand break repair, we hypothesized that a B. subtilis strain incapable of inducing SOS might have near wild-type levels of survival in response to ionizing radiation. We examined the ionizing radiation sensitivity of B. subtilis bearing a noncleavable lexA allele [lexA(Ind−)] rendering this strain incapable of SOS induction (23). Indeed, we found that lexA(Ind−) allele conferred ionizing radiation survival to near wild-type levels (Fig. 3A) . In contrast, the recA-deficient strain showed rapid killing after ionizing radiation challenge (Fig. 3A). We found that SOS induction provides a minimal contribution to double-strand break repair in B. subtilis over the ionizing radiation dosage range examined.
FIG. 3.
The viabilities of B. subtilis and E. coli bearing the lexA(Ind−) allele differ after ionizing-radiation-induced DNA damage. (A) Sensitivity to ionizing radiation of wild-type (▪), lexA(Ind−) (⧫), and recA::neo (ΔrecA [•]) B. subtilis strains after exposure to a 0 to 150 Gy. (B) Sensitivity to ionizing radiation of wild-type (▪), lexA(Ind−) (⧫), and ΔrecA::tc (ΔrecA [•]) E. coli strains. Each strain was irradiated in at least quadruplicate. Error bars indicate the standard error between samples. For both B. subtilis and E. coli the lexA(Ind−) strains encode a noncleavable form of LexA bearing a G92D missense mutation in B. subtilis and a G85D missense mutation in E. coli.
We examined the ionizing radiation sensitivity of an E. coli strain harboring the lexA(Ind−) allele. We found that SOS induction is important for resistance to ionizing radiation treatment (Fig. 3B). The lexA(Ind−) strain had a level of survival in response to 50 Gy similar to that of an isogenic strain disrupted for recA. When the dose was increased, the strain lacking recA showed less survival than the lexA(Ind−)-harboring strain (Fig. 3B). Taken together, SOS induction is important for survival in response to ionizing radiation-induced damage in E. coli and much less important in B. subtilis.
RecN limits SOS induction in B. subtilis.
We compared the genes that comprise the SOS regulon in E. coli and B. subtilis (11, 23) to identify candidate genes that are under LexA control in E. coli and are regulated independently of LexA in B. subtilis. We performed this comparison to identify potential gene products that are important for suppressing SOS induction in B. subtilis. Of the SOS regulated genes in E. coli that are regulated independently of LexA in B. subtilis, recN was the most conspicuous. E. coli recN is a highly expressed SOS regulated gene (23). The expression of recN appears to be SOS independent in B. subtilis (11, 37), and RecN provides important roles in homologous recombination (70). Based on previous observations that RecN-GFP foci associate with double-strand breaks prior to RecA-GFP and that RecN-GFP foci form in the absence of end processing (73), we found it plausible that RecN might contribute to potent homologous recombination in B. subtilis, effectively minimizing the importance of SOS induction during double-strand break repair.
To determine whether RecN affects SOS in B. subtilis, we used the TagC-GFP translational reporter in a strain disrupted for recN (recN::cat) (73). If RecN functions to limit SOS, then in the absence of recN both the percentage of cells showing spontaneous SOS induction and the percentage of cells showing DNA damage-inducible SOS induction should be elevated relative to a wild-type recN+ control. Indeed, we found that the percentage of cells showing spontaneous SOS induction was elevated 20-fold (P < 0.001) relative to a wild-type recN+ control strain (Table 4). After challenge with 100 Gy of ionizing radiation, we observed SOS induction in 6.0% (n = 1,248) of recN-deficient cells compared to 0.4% in wild-type B. subtilis cells. Clearly, the damage-inducible level of SOS in the recN::cat genetic background is not as high as what we observed for E. coli; however, the recN::cat background shows a 15-fold (P < 0.001) increase in SOS induction after ionizing radiation challenge. We were concerned that the elevated SOS induction we observe in recN-deficient cells simply reflected a general phenomenon associated with a partial defect in homologous recombination. To address this, we measured both the spontaneous and DNA damage-inducible SOS in a strain disrupted for recO. RecO is involved in homologous recombination and is in a different epistasis group from recN (4, 32). We found that the recO::cat allele conferred only a twofold increase (0.2%, n = 2,618) in the spontaneous level of SOS (Table 4). We observed SOS induction in 0.7% of recO::cat cells after ionizing radiation challenge, which corresponds to a twofold increase relative to our wild-type control (Table 4). Taken together, recN is important for limiting SOS induction in B. subtilis.
TABLE 4.
B. subtilis cells lacking recN have elevated SOS inductiona
| Genotype (strain) | % of cells with TagC-GFP fluorescence (n)
|
|
|---|---|---|
| Untreated | 100 Gy of ionizing radiation | |
| Wild type, tagC-gfp (LAS366) | 0.09 (3,122) | 0.4 (1,393) |
| recO::cat, tagC-gfp (LAS634) | 0.2 (2,618) | 0.7 (1,201) |
| recN::cat, tagC-gfp (LAS635) | 2.0 (2,100) | 6.0 (1,248) |
TagC-GFP fluorescence was scored in single cells with the indicated genotype. The numbers of cells scored (n) are indicated in parentheses, and each number is from at least two independent experiments. For each strain the cells were grown to mid-exponential phase (optical density at 600 nm of 0.4 to 0.8) in S750 defined minimal medium. The data in the table are from at least three independent experiments. Cells challenged with 100 Gy of ionizing radiation were treated at room temperature for 100 min prior to microscopic analysis. For both the untreated and the 100-Gy-treated samples, the increase in the percentage of cells with SOS induction of the recN::cat strain is significant (P < 0.001).
DISCUSSION
We measured the transcriptional response of B. subtilis to an I-SceI-catalyzed double-strand break and ionizing radiation by using genomic microarrays and single-cell fluorescent translational reporters. We found that both ionizing radiation and a site-specific double-strand break results in mild SOS induction at the cell population level and high levels of SOS induction in a small subpopulation of single B. subtilis cells (Fig. 2 and Table 2). We observed an increase in expression of seven LexA regulated genes (Table 3) out of approximately 63 (11, 36, 37) in response to an I-SceI-catalyzed double-strand break. We also observed an increase in the expression of genes encoded by the defective prophage PBSX (Table 3). In the case of PBSX, most of the genes upregulated in the array are regulated by RecA, but a few including xkdA, contain a LexA binding site and are therefore regulated directly by LexA (11). Using the fluorescent translational reporters TagC-GFP and XkdF-YFP, we found that SOS and PBSX genes were induced in only a small subpopulation of cells (Fig. 2. and Table 2).
These results also show that double-strand breaks result in RecA-GFP foci formation in most B. subtilis cells, and yet we only observed SOS induction in a small subpopulation of cells. These results are in contrast to what we observe in E. coli. When DNA damage is created from exogenous sources (including ionizing radiation and UV) in E. coli, SOS induction can be visualized in most cells (51; the present study). It should be noted, however, that E. coli RecA-GFP can respond to DNA damage in cells deficient for exonuclease III, with only a slight increase in SOS induction measured (16). These results suggest that under certain circumstances the formation of RecA-GFP foci in E. coli does not necessarily correlate with SOS induction.
NHEJ and SOS induction.
B. subtilis can repair double-strand breaks through two evolutionally distinct pathways: homologous recombination and nonhomologous end joining (NHEJ). Homologous recombination is an error-free repair pathway that uses an intact sister chromosome as the template for repair (for a review, see reference 33). NHEJ rejoins broken DNA ends using limited or no homology between DNA segments (for a review, see reference 25). Until recently, it was thought that NHEJ repair systems were exclusive to eukaryotic organisms (86). A subset of bacteria, including B. subtilis, contains homologs of the Ku and LigD proteins, which mediate NHEJ in bacteria. These gene products have been shown to provide resistance to ionizing radiation or desiccation in several bacterial species, including B. subtilis (26, 55, 56, 59-62, 86).
Because only a very small subpopulation of B. subtilis cells showed SOS induction after challenge with a site-specific double-strand break and ionizing radiation, we investigated the possibility that NHEJ was involved in the repair of some of the double-strand breaks, effectively limiting SOS induction. We found, by using both microarrays and single-cell translational reporter fusions, that NHEJ-deficient cells induced SOS to the same extent as the wild-type control (data not shown). We also found that an NHEJ-deficient strain showed wild-type levels of survival in response to ionizing radiation during exponential growth (data not shown). These data support the observation that NHEJ is growth phase regulated, contributing to double-strand break repair only during the outgrowth of spores or in stationary phase cells (56, 59, 61, 85). These results also suggest that NHEJ does not function to limit SOS following the induction of double-strand breaks in exponentially growing B. subtilis cells.
Double-strand break processing in E. coli and B. subtilis.
We found that a site-specific double-strand break or DNA damage created by ionizing radiation resulted in SOS induction in a small subpopulation of B. subtilis cells (Fig. 2 and Table 2). We found this striking considering that an I-SceI-catalyzed double-strand break results in ∼87% of cells showing SOS induction in E. coli (58) and that exposure of E. coli to ionizing radiation results in sulA promoter activity in 96% of cells (Fig. 1A). In E. coli, double-strand breaks are processed by the RecBCD helicase-nuclease complex, and this enzyme appears to bind and process double-strand breaks in the absence of replication (68, 69, 74). The RecBCD pathway is the overwhelming pathway used for processing of double-strand breaks to generate a substrate for RecA binding and subsequent homologous recombination (5-10). In the absence of recB, homologous recombinational repair of double-strand breaks is virtually absent, and SOS induction is not observed in cells challenged with a I-SceI site-specific double-strand break (58).
Chi sites (5′-GCTGGTGG-3′) in the E. coli genome function to attenuate double-stranded DNA degradation by RecBCD, switching the mode to that of ssDNA exonuclease activity (80). Chi sites are present in the E. coli genome approximately every 5 kb (53). As with many gram-positive bacteria, B. subtilis AddAB is the functional analog to the E. coli RecBCD enzyme important for double-strand break processing (1-4, 18, 19). The AddAB helicase-nuclease enzyme is not the exclusive enzyme for processing double-strand breaks in B. subtilis. Like RecBCD, AddAB also recognizes a chi site in B. subtilis (5′-AGCGG-3′) (20). This shorter sequence is present approximately every 750 bp in the B. subtilis genome, which is considerably more frequent than the distribution of chi sequences in the E. coli genome (20). Like the chi sequence in E. coli, the B. subtilis version is also co-oriented with DNA replication (20). We have shown in E. coli that SOS induction is important for double-strand break repair, and in B. subtilis SOS is induced only in a small subpopulation of cells. We speculate that the increased frequency of the AddAB chi site in the B. subtilis genome could contribute to the potent double-strand break repair observed in B. subtilis.
Role of RecN in suppressing SOS induction.
As mentioned above, ∼57 genes comprise the SOS response in E. coli, and ∼63 genes comprise the SOS response in B. subtilis (11, 23, 31, 77). Of these, only eight gene products share analogous functions, and only three gene products, recA, ruvA, and ruvB in B. subtilis are involved in double-strand break repair (11, 23, 31, 77). In E. coli, transcription of six gene products involved in double-strand break repair is under LexA control, the most striking is RecN, which is highly induced early in the SOS response. Because in B. subtilis RecN provides a critical role in double-strand break repair, and expression of recN does not appear to be under LexA control, we investigated a role for RecN in limiting SOS in B. subtilis. We found that B. subtilis strains disrupted for recN demonstrated 20-fold-increased SOS under normal growth conditions (Table 4). We interpret these results to mean that RecN binds to double-strand breaks, initiating repair and possibly limiting end processing to a level sufficient for RecA recruitment and DNA strand invasion, or that the double-strand breaks are not processed properly, thus inducing the SOS response. In the absence of recN, double-strand breaks may become overprocessed, leading to an increase in ssDNA bound RecA, resulting in more cells induced for SOS. We examined the level of SOS induction in a recO-deficient strain to determine whether the effect could be explained simply by a partial defect in homologous recombination. We observed only a twofold increase in SOS in a recO::cat strain relative to the wild-type control (Table 4). We conclude that RecN's role in double-strand break repair contributes to potent repair in the absence of SOS induction.
Acknowledgments
We thank Brenda Minesinger, James Foti, and Nicole Dupes for critical reading of the manuscript and helpful discussions. We thank C. Lee for technical assistance with our microarray analysis and for helpful comments on the manuscript. We thank Steve Sandler (University of Massachusetts, Amherst) for two E. coli strains that were used in this study.
G.C.W. is an American Cancer Society Professor and was funded by NCI grant CA021615 and the Massachusetts Institute of Technology Center for Environmental Health Sciences. A.D.G. was funded by GM041934 from the NIH. L.A.S. was funded, in part, by postdoctoral fellowship CA113124 from NCI. JSPS Postdoctoral Fellowships also supported H.K., in part, for Research Abroad. B.D.W. was supported by a scholarship from the National Sciences and Engineering Research Council of Canada.
Footnotes
Published ahead of print on 5 December 2008.
REFERENCES
- 1.Alonso, J. C., and G. Luder. 1991. Characterization of recF suppressors in Bacillus subtilis. Biochimie 73277-280. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, J. C., G. Luder, and R. H. Tailor. 1991. Characterization of Bacillus subtilis recombinational pathways. J. Bacteriol. 1733977-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso, J. C., and A. C. Stiege. 1991. Molecular analysis of the Bacillus subtilis recF function. Mol. Gen. Genet. 228393-400. [DOI] [PubMed] [Google Scholar]
- 4.Alonso, J. C., A. C. Stiege, and G. Luder. 1993. Genetic recombination in Bacillus subtilis 168: effect of recN, recF, recH, and addAB mutations on DNA repair and recombination. Mol. Gen. Genet. 239129-136. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, D. G., J. J. Churchill, and S. C. Kowalczykowski. 1999. A single mutation, RecB(D1080A), eliminates RecA protein loading but not Chi recognition by RecBCD enzyme. J. Biol. Chem. 27427139-27144. [DOI] [PubMed] [Google Scholar]
- 6.Anderson, D. G., J. J. Churchill, and S. C. Kowalczykowski. 1997. Chi-activated RecBCD enzyme possesses 5′→3′ nucleolytic activity, but RecBC enzyme does not: evidence suggesting that the alteration induced by Chi is not simply ejection of the RecD subunit. Genes Cells 2117-128. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, D. G., and S. C. Kowalczykowski. 1998. Reconstitution of an SOS response pathway: derepression of transcription in response to DNA breaks. Cell 95975-979. [DOI] [PubMed] [Google Scholar]
- 8.Anderson, D. G., and S. C. Kowalczykowski. 1998. SSB protein controls RecBCD enzyme nuclease activity during unwinding: a new role for looped intermediates. J. Mol. Biol. 282275-285. [DOI] [PubMed] [Google Scholar]
- 9.Anderson, D. G., and S. C. Kowalczykowski. 1997. The recombination hot spot chi is a regulatory element that switches the polarity of DNA degradation by the RecBCD enzyme. Genes Dev. 11571-581. [DOI] [PubMed] [Google Scholar]
- 10.Anderson, D. G., and S. C. Kowalczykowski. 1997. The translocating RecBCD enzyme stimulates recombination by directing RecA protein onto ssDNA in a chi-regulated manner. Cell 9077-86. [DOI] [PubMed] [Google Scholar]
- 11.Au, N., E. Kuester-Schoeck, V. Mandava, L. E. Bothwell, S. P. Canny, K. Chachu, S. A. Colavito, S. N. Fuller, E. S. Groban, L. A. Hensley, T. C. O'Brien, A. Shah, J. T. Tierney, L. L. Tomm, T. M. O'Gara, A. I. Goranov, A. D. Grossman, and C. M. Lovett. 2005. Genetic composition of the Bacillus subtilis SOS system. J. Bacteriol. 1877655-7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkmen, M. B., and A. D. Grossman. 2006. Spatial and temporal organization of the Bacillus subtilis replication cycle. Mol. Microbiol. 6257-71. [DOI] [PubMed] [Google Scholar]
- 13.Blaisdell, J. O., and S. S. Wallace. 2001. Abortive base-excision repair of radiation-induced clustered DNA lesions in Escherichia coli. Proc. Natl. Acad. Sci. USA 987426-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowater, R., and A. J. Doherty. 2006. Making ends meet: repairing breaks in bacterial DNA by non-homologous end-joining. PLoS Genet. 2e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britton, R. A., E. Kuster-Schock, T. A. Auchtung, and A. D. Grossman. 2007. SOS induction in a subpopulation of structural maintenance of chromosome (Smc) mutant cells in Bacillus subtilis. J. Bacteriol. 1894359-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centore, R. C., R. Lestini, and S. J. Sandler. 2008. XthA (exonuclease III) regulates loading of RecA onto DNA substrates in log phase Escherichia coli cells. Mol. Microbiol. 6788-101. [DOI] [PubMed] [Google Scholar]
- 17.Centore, R. C., and S. J. Sandler. 2007. UvrD limits the number and intensities of RecA-green fluorescent protein structures in Escherichia coli K-12. J. Bacteriol. 1892915-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chedin, F., S. D. Ehrlich, and S. C. Kowalczykowski. 2000. The Bacillus subtilis AddAB helicase/nuclease is regulated by its cognate Chi sequence in vitro. J. Mol. Biol. 2987-20. [DOI] [PubMed] [Google Scholar]
- 19.Chedin, F., N. Handa, M. S. Dillingham, and S. C. Kowalczykowski. 2006. The AddAB helicase/nuclease forms a stable complex with its cognate chi sequence during translocation. J. Biol. Chem. 28118610-18617. [DOI] [PubMed] [Google Scholar]
- 20.Chedin, F., P. Noirot, V. Biaudet, and S. D. Ehrlich. 1998. A five-nucleotide sequence protects DNA from exonucleolytic degradation by AddAB, the RecBCD analogue of Bacillus subtilis. Mol. Microbiol. 291369-1377. [DOI] [PubMed] [Google Scholar]
- 21.Colleaux, L., L. d'Auriol, M. Betermier, G. Cottarel, A. Jacquier, F. Galibert, and B. Dujon. 1986. Universal code equivalent of a yeast mitochondrial intron reading frame is expressed into Escherichia coli as a specific double strand endonuclease. Cell 44521-533. [DOI] [PubMed] [Google Scholar]
- 22.Colleaux, L., L. d'Auriol, F. Galibert, and B. Dujon. 1988. Recognition and cleavage site of the intron-encoded omega transposase. Proc. Natl. Acad. Sci. USA 856022-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Courcelle, J., A. Khodursky, B. Peter, P. O. Brown, and P. C. Hanawalt. 2001. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 15841-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox, M. M. 2007. Regulation of bacterial RecA protein function. Crit. Rev. Biochem. Mol. Biol. 4241-63. [DOI] [PubMed] [Google Scholar]
- 25.Daley, J. M., P. L. Palmbos, D. Wu, and T. E. Wilson. 2005. Nonhomologous end joining in yeast. Annu. Rev. Genet. 39431-451. [DOI] [PubMed] [Google Scholar]
- 26.Della, M., P. L. Palmbos, H. M. Tseng, L. M. Tonkin, J. M. Daley, L. M. Topper, R. S. Pitcher, A. E. Tomkinson, T. E. Wilson, and A. J. Doherty. 2004. Mycobacterial Ku and ligase proteins constitute a two-component NHEJ repair machine. Science 306683-685. [DOI] [PubMed] [Google Scholar]
- 27.Drees, J. C., S. Chitteni-Pattu, D. R. McCaslin, R. B. Inman, and M. M. Cox. 2006. Inhibition of RecA protein function by the RdgC protein from Escherichia coli. J. Biol. Chem. 2814708-4717. [DOI] [PubMed] [Google Scholar]
- 28.Drees, J. C., S. L. Lusetti, S. Chitteni-Pattu, R. B. Inman, and M. M. Cox. 2004. A RecA filament capping mechanism for RecX protein. Mol. Cell 15789-798. [DOI] [PubMed] [Google Scholar]
- 29.Drees, J. C., S. L. Lusetti, and M. M. Cox. 2004. Inhibition of RecA protein by the Escherichia coli RecX protein: modulation by the RecA C terminus and filament functional state. J. Biol. Chem. 27952991-52997. [DOI] [PubMed] [Google Scholar]
- 30.Fabret, C., S. D. Ehrlich, and P. Noirot. 2002. A new mutation delivery system for genome-scale approaches in Bacillus subtilis. Mol. Microbiol. 4625-36. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez De Henestrosa, A. R., T. Ogi, S. Aoyagi, D. Chafin, J. J. Hayes, H. Ohmori, and R. Woodgate. 2000. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol. Microbiol. 351560-1572. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez, S., Y. Kobayashi, N. Ogasawara, and J. C. Alonso. 1999. Analysis of the Bacillus subtilis recO gene: RecO forms part of the RecFLOR function. Mol. Gen. Genet. 261567-573. [DOI] [PubMed] [Google Scholar]
- 33.Friedberg, E. C., G. C. Walker, W. Siede, R. D. Wood, R. A. Schultz, and T. Ellenberger. 2006. DNA repair and mutagenesis, 2nd ed. ASM Press, Washington, DC.
- 34.Fry, R. C., T. J. Begley, and L. D. Samson. 2005. Genome-wide responses to DNA-damaging agents. Annu. Rev. Microbiol. 59357-377. [DOI] [PubMed] [Google Scholar]
- 35.Godoy, V. G., D. F. Jarosz, F. L. Walker, L. A. Simmons, and G. C. Walker. 2006. Y-family DNA polymerases respond to DNA damage-independent inhibition of replication fork progression. EMBO J. 25868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goranov, A. I., L. Katz, A. M. Breier, C. B. Burge, and A. D. Grossman. 2005. A transcriptional response to replication status mediated by the conserved bacterial replication protein DnaA. Proc. Natl. Acad. Sci. USA 10212932-12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goranov, A. I., E. Kuester-Schoeck, J. D. Wang, and A. D. Grossman. 2006. Characterization of the global transcriptional responses to different types of DNA damage and disruption of replication in Bacillus subtilis. J. Bacteriol. 1885595-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardwood, C. R., and S. M. Cutting. 1990. Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom.
- 39.Kawai, Y., S. Moriya, and N. Ogasawara. 2003. Identification of a protein, YneA, responsible for cell division suppression during the SOS response in Bacillus subtilis. Mol. Microbiol. 471113-1122. [DOI] [PubMed] [Google Scholar]
- 40.Kowalczykowski, S. C., and R. A. Krupp. 1995. DNA-strand exchange promoted by RecA protein in the absence of ATP: implications for the mechanism of energy transduction in protein-promoted nucleic acid transactions. Proc. Natl. Acad. Sci. USA 923478-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavery, P. E., and S. C. Kowalczykowski. 1992. Biochemical basis of the constitutive repressor cleavage activity of RecA730 protein. A comparison to RecA441 and RecA803 proteins. J. Biol. Chem. 26720648-20658. [PubMed] [Google Scholar]
- 42.Lavery, P. E., and S. C. Kowalczykowski. 1990. Properties of RecA441 protein-catalyzed DNA strand exchange can be attributed to an enhanced ability to compete with SSB protein. J. Biol. Chem. 2654004-4010. [PubMed] [Google Scholar]
- 43.Little, J. W., S. H. Edmiston, L. Z. Pacelli, and D. W. Mount. 1980. Cleavage of the Escherichia coli LexA protein by the RecA protease. Proc. Natl. Acad. Sci. USA 773225-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Little, J. W., and D. W. Mount. 1982. The SOS regulatory system of Escherichia coli. Cell 2911-22. [DOI] [PubMed] [Google Scholar]
- 45.Love, P. E., M. J. Lyle, and R. E. Yasbin. 1985. DNA-damage-inducible (din) loci are transcriptionally activated in competent Bacillus subtilis. Proc. Natl. Acad. Sci. USA 826201-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Love, P. E., and R. E. Yasbin. 1984. Genetic characterization of the inducible SOS-like system of Bacillus subtilis. J. Bacteriol. 160910-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Love, P. E., and R. E. Yasbin. 1986. Induction of the Bacillus subtilis SOS-like response by Escherichia coli RecA protein. Proc. Natl. Acad. Sci. USA 835204-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lusetti, S. L., J. C. Drees, E. A. Stohl, H. S. Seifert, and M. M. Cox. 2004. The DinI and RecX proteins are competing modulators of RecA function. J. Biol. Chem. 27955073-55079. [DOI] [PubMed] [Google Scholar]
- 49.Lusetti, S. L., M. D. Hobbs, E. A. Stohl, S. Chitteni-Pattu, R. B. Inman, H. S. Seifert, and M. M. Cox. 2006. The RecF protein antagonizes RecX function via direct interaction. Mol. Cell 2141-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lusetti, S. L., O. N. Voloshin, R. B. Inman, R. D. Camerini-Otero, and M. M. Cox. 2004. The DinI protein stabilizes RecA protein filaments. J. Biol. Chem. 27930037-30046. [DOI] [PubMed] [Google Scholar]
- 51.McCool, J. D., E. Long, J. F. Petrosino, H. A. Sandler, S. M. Rosenberg, and S. J. Sandler. 2004. Measurement of SOS expression in individual Escherichia coli K-12 cells using fluorescence microscopy. Mol. Microbiol. 531343-1357. [DOI] [PubMed] [Google Scholar]
- 52.Meddows, T. R., A. P. Savory, J. I. Grove, T. Moore, and R. G. Lloyd. 2005. RecN protein and transcription factor DksA combine to promote faithful recombinational repair of DNA double-strand breaks. Mol. Microbiol. 5797-110. [DOI] [PubMed] [Google Scholar]
- 53.Medigue, C., A. Viari, A. Henaut, and A. Danchin. 1993. Colibri: a functional database for the Escherichia coli genome. Microbiol. Rev. 57623-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min, J., C. W. Lee, S. H. Moon, R. A. LaRossa, and M. B. Gu. 2000. Detection of radiation effects using recombinant bioluminescent Escherichia coli strains. Radiat. Environ. Biophys. 3941-45. [DOI] [PubMed] [Google Scholar]
- 55.Moeller, R., P. Setlow, G. Horneck, T. Berger, G. Reitz, P. Rettberg, A. J. Doherty, R. Okayasu, and W. L. Nicholson. 2008. Roles of the major, small, acid-soluble spore proteins and spore-specific and universal DNA repair mechanisms in resistance of Bacillus subtilis spores to ionizing radiation from X rays and high-energy charged-particle bombardment. J. Bacteriol. 1901134-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moeller, R., E. Stackebrandt, G. Reitz, T. Berger, P. Rettberg, A. J. Doherty, G. Horneck, and W. L. Nicholson. 2007. Role of DNA repair by nonhomologous-end joining in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV, and ionizing radiation. J. Bacteriol. 1893306-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morimatsu, K., and S. C. Kowalczykowski. 2003. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol. Cell 111337-1347. [DOI] [PubMed] [Google Scholar]
- 58.Pennington, J. M., and S. M. Rosenberg. 2007. Spontaneous DNA breakage in single living Escherichia coli cells. Nat. Genet. 39797-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pitcher, R. S., A. J. Green, A. Brzostek, M. Korycka-Machala, J. Dziadek, and A. J. Doherty. 2007. NHEJ protects mycobacteria in stationary phase against the harmful effects of desiccation. DNA Repair 61271-1276. [DOI] [PubMed] [Google Scholar]
- 60.Pitcher, R. S., L. M. Tonkin, J. M. Daley, P. L. Palmbos, A. J. Green, T. L. Velting, A. Brzostek, M. Korycka-Machala, S. Cresawn, J. Dziadek, G. F. Hatfull, T. E. Wilson, and A. J. Doherty. 2006. Mycobacteriophage exploit NHEJ to facilitate genome circularization. Mol. Cell 23743-748. [DOI] [PubMed] [Google Scholar]
- 61.Pitcher, R. S., L. M. Tonkin, A. J. Green, and A. J. Doherty. 2005. Domain structure of a NHEJ DNA repair ligase from Mycobacterium tuberculosis. J. Mol. Biol. 351531-544. [DOI] [PubMed] [Google Scholar]
- 62.Pitcher, R. S., T. E. Wilson, and A. J. Doherty. 2005. New insights into NHEJ repair processes in prokaryotes. Cell Cycle 4675-678. [DOI] [PubMed] [Google Scholar]
- 63.Rehrauer, W. M., P. E. Lavery, E. L. Palmer, R. N. Singh, and S. C. Kowalczykowski. 1996. Interaction of Escherichia coli RecA protein with LexA repressor. I. LexA repressor cleavage is competitive with binding of a secondary DNA molecule. J. Biol. Chem. 27123865-23873. [PubMed] [Google Scholar]
- 64.Renzette, N., N. Gumlaw, J. T. Nordman, M. Krieger, S. P. Yeh, E. Long, R. Centore, R. Boonsombat, and S. J. Sandler. 2005. Localization of RecA in Escherichia coli K-12 using RecA-GFP. Mol. Microbiol. 571074-1085. [DOI] [PubMed] [Google Scholar]
- 65.Renzette, N., N. Gumlaw, and S. J. Sandler. 2007. DinI and RecX modulate RecA-DNA structures in Escherichia coli K-12. Mol. Microbiol. 63103-115. [DOI] [PubMed] [Google Scholar]
- 66.Renzette, N., and S. J. Sandler. 2008. Requirements for ATP binding and hydrolysis in RecA function in Escherichia coli. Mol. Microbiol. 671347-1359. [DOI] [PubMed] [Google Scholar]
- 67.Robbins-Manke, J. L., Z. Z. Zdraveski, M. Marinus, and J. M. Essigmann. 2005. Analysis of global gene expression and double-strand-break formation in DNA adenine methyltransferase- and mismatch repair-deficient Escherichia coli. J. Bacteriol. 1877027-7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salles, B., and M. Defais. 1984. Signal of induction of RecA protein in Escherichia coli. Mutat. Res. 13153-59. [DOI] [PubMed] [Google Scholar]
- 69.Salles, B., M. Germanier, and M. Defais. 1987. A bacterial strain for detecting agents that produce free radical-mediated DNA strand breaks. Mutat. Res. 183213-217. [DOI] [PubMed] [Google Scholar]
- 70.Sanchez, H., and J. C. Alonso. 2005. Bacillus subtilis RecN binds and protects 3′-single-stranded DNA extensions in the presence of ATP. Nucleic Acids Res. 332343-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanchez, H., B. Carrasco, S. Ayora, and J. C. Alonso. 2007. Dynamics of DNA double-strand break repair in Bacillus subtilis. Caister Academic Press, Norfolk, United Kingdom.
- 72.Sanchez, H., B. Carrasco, S. Ayora, and J. C. Alonso. 2006. Homologous recombination in low dC+dG gram-positive bacteria. Springer, Berlin, Germany.
- 73.Sanchez, H., D. Kidane, M. Castillo Cozar, P. L. Graumann, and J. C. Alonso. 2006. Recruitment of Bacillus subtilis RecN to DNA double-strand breaks in the absence of DNA end processing. J. Bacteriol. 188353-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sassanfar, M., and J. W. Roberts. 1990. Nature of the SOS-inducing signal in Escherichia coli: the involvement of DNA replication. J. Mol. Biol. 21279-96. [DOI] [PubMed] [Google Scholar]
- 75.Sciochetti, S. A., P. J. Piggot, D. J. Sherratt, and G. Blakely. 1999. The ripX locus of Bacillus subtilis encodes a site-specific recombinase involved in proper chromosome partitioning. J. Bacteriol. 1816053-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simmons, L. A., B. W. Davies, A. D. Grossman, and G. C. Walker. 2008. Beta clamp directs localization of mismatch repair in Bacillus subtilis. Mol. Cell 29291-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simmons, L. A., J. J. Foti, S. E. Cohen, and G. C. Walker. 25 July 2008, posting date. Chapter 5.4.3, The SOS regulatory network. In R. Curtiss et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://ecosal.org.
- 78.Simmons, L. A., A. D. Grossman, and G. C. Walker. 2007. Replication is required for the RecA localization response to DNA damage in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 1041360-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith, B. T., A. D. Grossman, and G. C. Walker. 2001. Visualization of mismatch repair in bacterial cells. Mol. Cell 81197-1206. [DOI] [PubMed] [Google Scholar]
- 80.Spies, M., I. Amitani, R. J. Baskin, and S. C. Kowalczykowski. 2007. RecBCD enzyme switches lead motor subunits in response to chi recognition. Cell 131694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stohl, E. A., J. P. Brockman, K. L. Burkle, K. Morimatsu, S. C. Kowalczykowski, and H. S. Seifert. 2003. Escherichia coli RecX inhibits RecA recombinase and coprotease activities in vitro and in vivo. J. Biol. Chem. 2782278-2285. [DOI] [PubMed] [Google Scholar]
- 82.Stohl, E. A., and H. S. Seifert. 2001. The recX gene potentiates homologous recombination in Neisseria gonorrhoeae. Mol. Microbiol. 401301-1310. [DOI] [PubMed] [Google Scholar]
- 83.Sutton, M. D., B. T. Smith, V. G. Godoy, and G. C. Walker. 2000. The SOS response: recent insights into umuDC-dependent mutagenesis and DNA damage tolerance. Annu. Rev. Genet. 34479-497. [DOI] [PubMed] [Google Scholar]
- 84.Wallace, S. S. 1998. Enzymatic processing of radiation-induced free radical damage in DNA. Radiat. Res. 150S60-S79. [PubMed] [Google Scholar]
- 85.Wang, S. T., B. Setlow, E. M. Conlon, J. L. Lyon, D. Imamura, T. Sato, P. Setlow, R. Losick, and P. Eichenberger. 2006. The forespore line of gene expression in Bacillus subtilis. J. Mol. Biol. 35816-37. [DOI] [PubMed] [Google Scholar]
- 86.Weller, G. R., B. Kysela, R. Roy, L. M. Tonkin, E. Scanlan, M. Della, S. K. Devine, J. P. Day, A. Wilkinson, F. d'Adda di Fagagna, K. M. Devine, R. P. Bowater, P. A. Jeggo, S. P. Jackson, and A. J. Doherty. 2002. Identification of a DNA nonhomologous end-joining complex in bacteria. Science 2971686-1689. [DOI] [PubMed] [Google Scholar]
- 87.Workman, C. T., H. C. Mak, S. McCuine, J. B. Tagne, M. Agarwal, O. Ozier, T. J. Begley, L. D. Samson, and T. Ideker. 2006. A systems approach to mapping DNA damage response pathways. Science 3121054-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yasbin, R. E., D. L. Cheo, and K. W. Bayles. 1992. Inducible DNA repair and differentiation in Bacillus subtilis: interactions between global regulons. Mol. Microbiol. 61263-1270. [DOI] [PubMed] [Google Scholar]
- 89.Youngman, P., J. B. Perkins, and R. Losick. 1984. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid 121-9. [DOI] [PubMed] [Google Scholar]