Abstract
Brucellosis is an important zoonotic disease of nearly worldwide distribution. Despite the availability of live vaccine strains for bovine (S19, RB51) and small ruminants (Rev-1), these vaccines have several drawbacks, including residual virulence for animals and humans. Safe and efficacious immunization systems are therefore needed to overcome these disadvantages. A vjbR knockout was generated in the S19 vaccine and investigated for its potential use as an improved vaccine candidate. Vaccination with a sustained-release vehicle to enhance vaccination efficacy was evaluated utilizing the live S19 ΔvjbR::Kan in encapsulated alginate microspheres containing a nonimmunogenic eggshell precursor protein of the parasite Fasciola hepatica (vitelline protein B). BALB/c mice were immunized intraperitoneally with either encapsulated or nonencapsulated S19 ΔvjbR::Kan at a dose of 1 × 105 CFU per animal to evaluate immunogenicity, safety, and protective efficacy. Humoral responses postvaccination indicate that the vaccine candidate was able to elicit an anti-Brucella-specific immunoglobulin G response even when the vaccine was administered in an encapsulated format. The safety was revealed by the absence of splenomegaly in mice that were inoculated with the mutant. Finally, a single dose with the encapsulated mutant conferred higher levels of protection compared to the nonencapsulated vaccine. These results suggest that S19 ΔvjbR::Kan is safer than S19, induces protection in mice, and should be considered as a vaccine candidate when administered in a sustained-release manner.
Brucella abortus, a gram-negative, facultative, intracellular bacterium, is a causative agent of brucellosis, a zoonosis of nearly worldwide distribution (5). In animals, brucellosis is a major cause of abortions and infertility (3, 19, 28). In humans, infection can cause a serious debilitating disease manifested as undulant fever, endocarditis, arthritis, and osteomyelitis (23). Due to serious economic losses and public health risk, extensive efforts have been conducted to prevent the disease in animals through vaccination programs (22). Live attenuated vaccines have been developed and successfully used worldwide against bovine brucellosis (26). Currently, no effective vaccines are available for the prevention of human brucellosis.
B. abortus S19 live vaccine has been extensively used to prevent bovine brucellosis (22). The S19 vaccine strain was first isolated from the milk of a Jersey cow in 1923 and, while stored in the laboratory at room temperature, developed an attenuated phenotype (22). Numerous efficacy studies conducted with cattle for this vaccine have demonstrated that 70% of the vaccinated cattle are protected from a wild-type exposure (22). The effectiveness depended on a series of variables, including the age of the vaccinated animal, the prevalence of the disease in vaccinated herds, and the dose and route of the vaccination (22, 26). Although S19 typically exhibits low virulence in cattle, the vaccine can cause abortions when administered to pregnant animals at rates between 1 to 2.5% (26). A less-frequent adverse effect of S19 vaccination is the development of an arthropathy associated with Brucella antigen-containing immune complexes (22, 26).
In many developing nations, immunizations derived from the S19 vaccine have been evaluated in humans. In the former Soviet Union, the administration of live S19 preparations were immunogenic, and protection was achieved and considered to last 1 year but caused a modest but notable incidence of clinical cases, as well as a hypersensitivity reaction (6, 22). As such, S19 is not a safe vaccine candidate for human use.
Previous research in our lab has identified Brucella genes required for virulence and survival via transposon mutagenesis (2, 13). Among these, vjbR (BMEI1116), encoding the luxR-like quorum sensing-related transcriptional regulator is required for virB expression, virulence in mice, and survival in macrophages (8). BALB/c mice immunized with the vjbR mutant were protected against wild-type challenge without exhibiting any local or adverse reactions, making such mutants ideal vaccine candidates for future consideration (4). In the present study, the effects of eliminating the vjbR gene from B. abortus S19 were evaluated in vitro and in BALB/c mice. When vjbR is deleted in S19, two positive effects are observed: diminished inflammation (reduced splenomegaly) and reduced persistence. Taken together, these effects lead to an increased safety of the vaccine strain, since S19 alone elicits splenomegaly, an undesirable side effect of vaccination. The capacity of the B. abortus S19 ΔvjbR::Kan mutant to elicit Brucella-specific immune responses was also evaluated. In an effort to enhance the vaccination efficacy, the knockout was encapsulated into alginate microspheres containing a nonimmunogenic eggshell precursor protein of the parasite Fasciola hepatica (vitelline protein B [VpB]) as previously described (4) that has been used to alter the release properties of the microcapsules with the aim of producing vaccines that are safer while retaining protective efficacy.
MATERIALS AND METHODS
Mice.
One hundred fifty 8- to 10-week-old female BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All experimental procedures and animal care were performed in compliance with institutional animal care regulations.
Bacterial strains.
Bacterial strains used in these experiments include B. abortus S19 (NVSL, Ames, IA), B. abortus S19 ΔvjbR::Kan (engineered for this study), and B. abortus virulent strain 2308 (originally obtained from Billy Deyoe). The bacteria were routinely grown on tryptic soy agar (TSA) at 37°C with 5% (vol/vol) CO2. For B. abortus S19 ΔvjbR::Kan, the medium was supplemented with kanamycin (100 μg/ml). Following 3 days of incubation, the bacteria were harvested from the surface of the plates into phosphate-buffered saline (PBS). The bacteria were pelleted, washed twice by resuspension in MOPS (morpholinepropanesulfonic acid) buffer (10 mM MOPS, 0.85% NaCl [pH 7.4]), and resuspended to a final concentration of 1 × 106 CFU/ml (for encapsulation) or 1 × 105 CFU in 100 μl of PBS (for nonencapsulated bacteria) based upon optical density readings using a Klett meter and a standardized curve. The actual viable counts were confirmed by serial dilution and plating of portions onto TSA plates with or without antibiotic. To inoculate the mice, the bacteria were harvested the same day of challenge. Inoculum doses were plated retrospectively to determine the number of organisms inoculated.
Escherichia coli cultures utilized during the knockout construction were grown on Luria-Bertani (Difco, Becton Dickinson) plates or in broth overnight at 37°C with or without kanamycin (100 mg/liter), carbenicillin (100 mg/liter), or chloramphenicol (50 mg/liter).
Construction of the S19 ΔvjbR::Kan deletion mutant.
The marked S19 ΔvjbR::Kan deletion mutant was constructed in S19 as previously described with some modifications (15). Specifically for this mutant, the sequence downstream of the vjbR gene was amplified from B. abortus 2308 with the primer pair 5′-GTCTTCGAGGATGTACAATTGGC and 5′-CATCTCGTCTGATCAACATGG. The sequence upstream of vjbR was amplified with the primer pair 5′-GAAGCGCCAAAGTATCGC and 5′-CAGTTGGAAAAGGGCTTTTCCAACCG. These two products were ligated to one another via overlapping PCR with an AscI site (New England Biolabs) engineered between the two sequences. This product was then ligated to pEX18Ap, and a kanamycin resistance gene was inserted within the vector at the unique AscI site. This construct was used for the electroporation into S19 as previously described (15). Potential marked deletion mutants were kanamycin resistant and ampicillin sensitive and were verified by PCR and Southern blotting. The S19 ΔvjbR::Kan mutant was then used for this study.
Evaluation of B. abortus S19 ΔvjbR::Kan in J774A.1 macrophages.
Murine macrophage-like J774.A1 (ATCC TIB-67) macrophages were used to assess S19 ΔvjbR::Kan mutant survival compared to that of the parental B. abortus S19. Macrophage survival assays were performed as previously described with some modifications (24). Briefly, macrophages were cultured in Dulbecco's modified Eagle's medium with 10% (vol/vol) fetal bovine serum, 1 mM l-glutamine, and 1 mM nonessential amino acids. Monolayers of macrophages containing 2.5 × 105 cells per well were infected at a multiplicity of infection (MOI) of 1:100 at 37°C with S19 ΔvjbR::Kan, S19, or wild-type 2308. Thirty minutes postinfection, the cells were washed twice with medium without antibiotics and then incubated with 50 μg/ml of gentamicin (Invitrogen) for 30 min to kill any extracellular bacteria. One and 48 h postinfection, the macrophages were lysed using 0.05% (vol/vol) Tween 20 and bacteria collected. Brucella entry and survival were determined by performing serial dilutions and plating onto TSA plates with or without antibiotic for each dilution. All assays were performed in triplicate and repeated at least three times.
Evaluation of B. abortus S19 ΔvjbR::Kan attenuation in mice.
Female BALB/c mice were used to evaluate the survival of the S19 ΔvjbR::Kan mutant. Briefly, 8- to 10-week-old mice were intraperitoneally (i.p.) inoculated with a total of 1 × 106 CFU of either the mutant or parental strain. Groups of five mice were euthanized via carbon dioxide asphyxiation at 1, 3, 5, 7, or 9 weeks postinoculation. At each time point, the spleens were collected, weighed, and homogenized in 1 ml of peptone saline. Serial dilutions were prepared, and 100-μl aliquots of the different dilutions were plated in duplicate onto TSA or TSA with kanamycin. The levels of infection were expressed as the mean ± standard error of the mean (SEM) of individual log CFU/spleen.
Histopathology.
Twelve 8- to 10-week-old female BALB/c mice were distributed into groups of three mice and inoculated with 1 × 105 CFU per mouse of S19, S19 ΔvjbR::Kan, B. abortus 2308, or PBS. At 3 weeks postinoculation, the animals were euthanized by CO2 asphyxiation, and the spleens, lungs, livers, kidneys, and hearts were harvested, fixed in 10% buffered formalin, paraffin embedded, and stained. Histological changes were assessed between the treatment groups.
Preparation of B. abortus antigen-loaded microspheres.
Alginate beads were prepared as previously described with some modifications (1). Briefly, 6 × 106 CFU of live B. abortus S19 ΔvjbR::Kan or B. abortus S19 was resuspended in 1 ml of MOPS buffer (10 mM MOPS, 0.85% NaCl [pH 7.4]) and mixed with 5 ml of alginate solution (1.5% sodium alginate, 10 mM MOPS, 0.85% NaCl [pH 7.3]). VpB was added as a component of the alginate core by the addition of 1 mg of VpB to the bacterium-alginate suspension described above. Spheres were obtained by extruding the suspension through a 200-micron nozzle into a 100 mM calcium chloride solution and stirring for 15 min using the Inotech encapsulator I-50 (Inotech Biosystems International, Rockville, MD). After the bacterium-alginate mixture was extruded into the CaCl2, the capsules were washed twice with MOPS for 5 min and further cross-linked with 0.05% poly-l-lysine (molecular weight, 22,000; Sigma) for 10 min. Following two successive washes, the beads were stirred in a solution of 0.03% (wt/vol) alginate for 5 min to apply a final outer shell and washed twice with MOPS before storage at 4°C. To determine the bacterial viability postencapsulation, 1 ml of capsules was removed from the encapsulator prior to permanent cross-linking with poly-l-lysine. The capsules were allowed to settle and were washed twice with MOPS buffer, and particles were dissolved using 1 ml of depolymerization solution (50 mM Na3-citrate, 0.45% NaCl, 10 mM MOPS [pH 7.2]) with stirring for 10 min. The bacterial number (CFU/ml) in each ml of capsules was determined by plating onto TSA plates.
Immunization of mice.
Twenty 8- to 10-week-old female BALB/c mice were randomly distributed into groups of five mice for i.p. vaccination. Initial preliminary vaccination studies indicated no difference in vaccination efficacy when animals were given either 1 × 105 CFU or 1 × 106 CFU. The treated animals were given a single dose of vaccine containing 1 × 105 CFU of either encapsulated B. abortus S19 ΔvjbR::Kan in alginate with VpB inside the capsule's shell or nonecapsulated B. abortus S19 ΔvjbR::Kan. The control groups received 1 × 105 CFU of either nonencapsulated S19 or empty capsules (no bacteria entrapped) resuspended in 100 μl of MOPS.
Evaluation of Brucella-specific antibody.
To determine the effect of encapsulation in the production of anti-Brucella-specific antibody in sera from inoculated mice, 100 μl of blood was taken from each mouse after 0, 3, and 7 weeks postvaccination as well as 1 week postchallenge. The serum was separated and used for immunoglobulin G1 (IgG1) and IgG2a determination by enzyme-linked immunosorbent assay (ELISA). Heat-killed and sonicated B. abortus whole-cell antigen was used to coat 96-well plates (Nunc-Immuno plates) at a concentration of 25 μg total protein/well. Following overnight incubation at 4°C, the plates were washed, blocked with 0.5 ml of blocking buffer (0.25% [wt/vol] bovine serum albumin), and then incubated with mouse sera diluted 1:100 in the same blocking buffer for 1 h at room temperature. Following the removal of unbound antibody by extensive washing, goat anti-mouse IgG1 or IgG2a horseradish peroxidase conjugate (Serotec) was added at a concentration of 500 ng/ml of antibody and incubation continued for an additional h. Following this incubation, the plates were washed, horseradish peroxidase substrate was added, and then the plates were incubated for 18 min. The reaction was stopped by the addition of 50 μl of 0.5 M NaOH and the absorbance measured at 450 nm. All the assays were performed in triplicate and repeated at least three times.
Efficacy of vaccination.
At selected times postvaccination, the mice (n = 5 per group) were challenged i.p. using 1 × 105 CFU/mouse of B. abortus wild-type 2308. One week postchallenge, the mice were euthanized by CO2 asphyxiation, and their spleens were removed, weighed, and homogenized in 1 ml of peptone saline. Serial dilutions were prepared, and 100-μl portions were plated onto TSA plates. In some instances, 200 to 1,000 μl of spleen homogenate was plated to determine organism recovery. To differentiate between the vaccine candidate and the challenge strain, each dilution was also plated on TSA with kanamycin to identify any residual kanamycin-resistant vaccine strain present. The levels of infection were expressed as means ± SEMs of the individual log10 CFU/spleen. The efficacy of the vaccine in the treated animals compared to the nonvaccinated naïve animals was determined by subtracting the mean CFU/spleen recovered from the mice vaccinated with the nonencapsulated or encapsulated vaccine from the mean CFU/spleen recovered from the naïve nonvaccinated but challenged mice. The efficacy of the encapsulated vaccine compared to that of the nonencapsulated vaccine was determined by subtracting the mean CFU/spleen recovered from the mice vaccinated with the capsules from the mean CFU/spleen recovered from the mice immunized with the nonencapsulated vaccine.
Statistical procedures.
Macrophage infection and survival were expressed as the mean log CFU ± standard deviation for each group and analyzed by analysis of variance (ANOVA) followed by a Tukey's posttest comparing all groups to one another at the same time point. The intensity of infection (bacterial clearance) at each time point was expressed as the mean log CFU ± SEM for each group and analyzed by a two-tailed Student's t test. The efficacy of the vaccination and the differences in spleen weight postchallenge were expressed as the mean log CFU ± SEM for each treatment group and analyzed by ANOVA followed by a Tukey's posttest comparing all groups to one another. IgG production was expressed as the mean absorbance ± SEM. The significance of differences between the groups was determined by ANOVA followed by a Tukey's posttest comparing all groups to one another. P values of <0.05 were considered statistically significant.
RESULTS
B. abortus S19 ΔvjbR::Kan is more attenuated for survival in macrophages and in mice than S19.
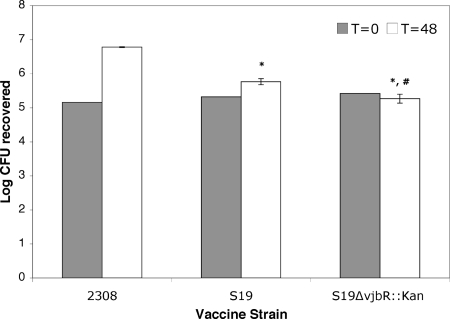
To determine the role of the deletion of the vjbR gene in virulence in S19, J774A.1 macrophages were infected with the marked deletion mutant and compared to the parental strain and to the wild-type B. abortus 2308. Using an MOI of 100, the ability of the bacteria to enter and survive within this cell line was evaluated. At 1 h postinfection (t = 0), there was no difference (P > 0.05) between S19, B. abortus 2308, or S19 ΔvjbR::Kan in the number of bacteria infecting the cell. By 48 h postinfection, there was a 0.96-log difference (P < 0.001) in the number of organisms infecting the cell for S19 versus that for wild-type 2308 or a 1.47-log difference between the number for S19 ΔvjbR::Kan versus that for wild-type 2308 (P < 0.001). When the marked mutant was compared to the parental strain (S19), there was a 0.51-log reduction in the number of bacteria surviving inside the macrophage (P < 0.05) (Fig. 1). These results indicate that S19 ΔvjbR::Kan is attenuated in macrophages.
FIG. 1.
Survival of the B. abortus S19 ΔvjbR::Kan mutant in J774A.1 macrophages. Wild-type strain 2308, S19, and the B. abortus S19 ΔvjbR::Kan mutant were used to infect J774A.1 macrophages at an MOI of 1:100. After 30 min of incubation followed by 1 h of treatment with gentamicin, infected macrophages were further incubated for 0 or 48 h. Treated cells were lysed, serially diluted, and plated on TSA or TSA-kanamycin plates for CFU determination. The results are represented as the means of three independent experiments ± SEMs. Statistical differences were analyzed by ANOVA followed by Tukey's posttest comparing all groups to one another. *, P < 0.001 compared to the 2308 control; #, P < 0.05 compared to S19.
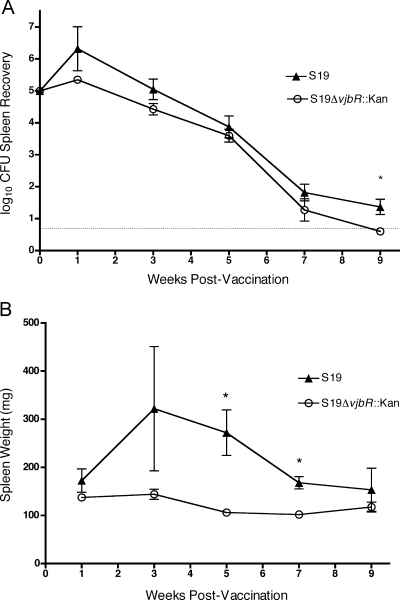
To determine the virulence of S19 ΔvjbR::Kan in vivo, mice were inoculated i.p. with 1 × 106 CFU/mouse of S19 ΔvjbR::Kan or the parental strain S19. Compared to that of S19, the colonization of S19 ΔvjbR::Kan in the spleen did not differ (P > 0.05) at 1, 3, 5, or 7 weeks postinfection, and at only 9 weeks postinfection, bacterial colonization of S19 ΔvjbR::Kan was significantly reduced (P < 0.013) compared to that of S19 (Fig. 2A). Interestingly, inflammation in the spleen was diminished in animals that received S19 ΔvjbR::Kan at the majority of the time points compared to that in the S19-infected animals, as was evident by the lack of splenomegaly (Fig. 2B). Gross morphology was evaluated and again demonstrated the significant reduction in the spleen sizes of mice receiving the S19 ΔvjbR::Kan vaccine compared to those of the S19 controls (Fig. 3). The spleens of the S19 ΔvjbR::Kan-vaccinated mice appeared similar in size to those of the PBS-treated controls, whereas the spleens from the S19-vaccinated mice were comparable to those of the wild-type-treated mice.
FIG. 2.
Clearance of B. abortus S19 ΔvjbR::Kan after infection. BALB/c mice (n = 5/time point) were infected with 1 ×106 CFU/mouse of wild-type 2308 or S19 ΔvjbR::Kan. At 1, 3, 5, 7, and 9 weeks postinfection, mice were euthanized and the spleens were assessed for bacterial colonization (A) and weight (B). Values are the means of the results for individual mice ± standard errors of the means. Differences were determined by two-tailed Student's t test comparing S19 to the mutant (*, P < 0.05). The solid line in panel A represents the limit of detection, which is ≥5 CFU.
FIG. 3.
Spleen morphology in BALB/c mice vaccinated with S19 ΔvjbR::Kan. Mice were inoculated with 1 × 105 CFU of either B. abortus 2308 (A), S19 ΔvjbR::Kan (B), S19 (C), or PBS (D). Animals were euthanized 3 weeks postinoculation, and spleens were weighed, harvested, and fixed for histological analysis.
Evaluation of histological changes in mice inoculated with S19 ΔvjbR::Kan.
Due to the significant differences between the spleen sizes of the animals inoculated with S19 ΔvjbR::Kan and those of the animals inoculated with S19, the mice were inoculated with either S19 ΔvjbR::Kan, S19, B. abortus 2308, or PBS as a control. Three mice per group were euthanized, and the spleens, lungs, livers, kidneys, and hearts were extracted, fixed, and stained to determine the histological changes associated with inflammation. The most dramatic histologic changes were observed in the livers and spleens of all the infected mice. The livers of all the mice inoculated with B. abortus wild-type 2308 and two mice that received S19 had randomly distributed, severe inflammatory foci composed primarily of histiocytes and various numbers of lymphocytes and neutrophils (Fig. 4A and B). Bordering the inflammatory foci were degenerating hepatocytes. Diffusely, the Kupffer cells of these mice were reactive. Hepatic inflammatory foci in either the S19 ΔvjbR::Kan- or PBS-vaccinated mice were uncommon and again characterized by histiocytes, lymphocytes, and rare neutrophils (Fig. 4C and D).
FIG. 4.
Histological analysis of livers and spleens from BALB/c mice inoculated with S19, B. abortus 2308, S19 ΔvjbR::Kan, or PBS. In the livers, random distribution of inflammatory foci in mice vaccinated with either S19 (A) or 2308 (B) was observed (×10 magnification; ×40 magnification in the inset). Normal liver appearance was observed in mice inoculated with either S19 ΔvjbR::Kan (C) or PBS (D). Foci of inflammation are composed primarily of histiocytes. Scale bars in panels A and B, 100 μM; inset scale bars, 25 μM. In the spleens of mice vaccinated with either S19 (E) or 2308 (F), the white pulp is coalescing and enlarged, including marginal zones, and the formation of secondary lymphoid follicles is present (×4 magnification). There was minimal enlargement of the marginal zone in the mice vaccinated with either S19 ΔvjbR::Kan (G) or PBS (H). Scale bars in panels E to H, 200 μM.
Severe splenic lesions were similar in animals that received the B. abortus wild-type 2308 and S19 (two out of three mice) (Fig. 4E and F). The lesions were characterized by markedly enlarged and coalescing white pulp marginal zones. These were composed of large numbers of histiocytes with fewer variably degenerate neutrophils and occasional lymphocytes and plasma cells. Often, marginal zones extended both into the red pulp and compressed the follicles as they surrounded central arteries. Up to 60% of the organ-size increase in B. abortus wild-type 2308 and 50% in S19 vaccinates were due to the marginal zone expansion by the described cellular infiltrate. Diffusely, moderate lymphoid hyperplasia with secondary lymphoid follicle formation containing increased mitotic activity and a small number of body macrophages capable of being stained were observed. The changes in the spleens of two of the animals that received the S19 ΔvjbR::Kan mutant were characterized by minimal lymphoid follicle hyperplasia with secondary follicle formation (Fig. 4G). The marginal zones were mildly hyperplastic with a moderate number of histiocytes and plasma cells and fewer lymphocytes and neutrophils. Moderately increased numbers of plasma cells and megakaryocytes were in the red pulp in all treatment groups except in animals that received PBS. No splenic changes were noted in animals that received the PBS (Fig. 4H), one mouse vaccinated with S19, and one mouse that received S19 ΔvjbR::Kan.
Multifocal aggregates of mineralization with accompanying mild fibrosis and small numbers of lymphocytes, histiocytes, and rare neutrophils were observed in the epicardial surfaces of almost all of the mice and were interpreted as unassociated with the inflammatory process (data not shown).
Changes in the lungs were generally unremarkable in all the animals, with the exception of two animals infected with 2308. Changes included minimal edema and a mild-to-moderate perivascular and peribronchiolar cellular infiltrate composed of lymphocytes, histiocytes, and neutrophils. There were no changes observed in any of the kidneys in any group (data not shown).
Evaluation of immune protection provided by S19 ΔvjbR::Kan.
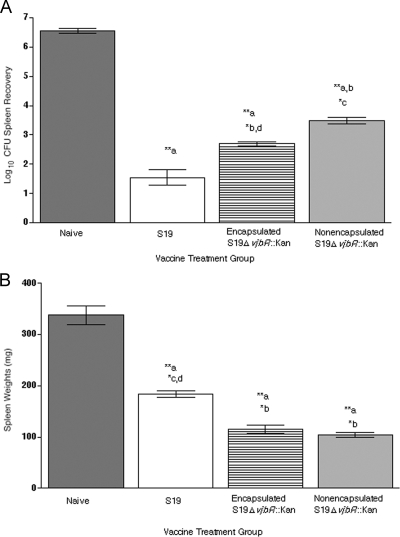
In order to determine the efficacy of the S19 ΔvjbR::Kan mutant as a vaccine, the level of protection provided by equal numbers of either S19 ΔvjbR::Kan or S19 was evaluated against B. abortus wild-type challenge at 20 weeks postvaccination. To try to enhance the efficacy of the S19 ΔvjbR::Kan mutant, the strain was also encapsulated into alginate VpB microcapsules at the same dose. At 21 weeks postvaccination (1 week postchallenge), there was a statistically significant decrease in the splenic bacterial loads from the mice vaccinated with S19 ΔvjbR::Kan relative to those of the naïve mice, with a 3.06-log-unit (P < 0.001) reduction for the nonencapsulated mutant. When administered in a microencapsulated format, the efficacy of the S19 ΔvjbR::Kan vaccine was enhanced by 0.8 log unit compared to that of the nonencapsulated S19 ΔvjbR::Kan vaccine (P < 0.05 encapsulated to nonencapsulated; P < 0.001 naïve to encapsulated) (Fig. 5A). The S19-vaccinated mice exhibited a 5.02-log-unit (P < 0.001) reduction compared to that of the naïve animals.
FIG. 5.
Immunization efficacy and safety of B. abortus S19 ΔvjbR::Kan vaccine formulations. BALB/c mice were immunized i.p. with 1 × 105 of either nonencapsulated or encapsulated S19 ΔvjbR::Kan. Control groups received empty capsules or S19. After 20 weeks, the mice were challenged i.p. with 1 × 105 CFU wild-type 2308. At 1 week postchallenge, the mice were euthanized, their spleens harvested, and the bacterial loads (A) and spleen weights (B) determined. (A) Values are reported as the mean log10 recovery of the 2308 challenge organism recovered from the spleens. Differences in colonization between all the groups were determined by ANOVA followed by a Tukey's posttest (*, P < 0.05; **, P < 0.001). (B) Spleen weights were measured in mg and were compared and analyzed by ANOVA followed by a Tukey's posttest comparing all groups to one another (*, P < 0.01; **, P < 0.001). For the statistical representation on both graphs, “a” symbolizes naïve animals, “b” S19-vaccinated animals, “c” encapsulated S19 ΔvjbR::Kan-vaccinated animals, and “d” nonencapsulated S19 ΔvjbR::Kan-vaccinated animals.
Postchallenge safety was increased in the mice vaccinated with the novel mutant strain, and splenomegaly was significantly reduced in the mice vaccinated with either the encapsulated or the nonencapsulated S19 ΔvjbR::Kan strain compared to the S19-vaccinated mice postchallenge (P < 0.001) (Fig. 5B). Taken together with the efficacy results, these data suggest that the encapsulation of this novel vaccine strain is highly effective, with the improved benefit of diminished splenomegaly and improved safety pre- and postchallenge.
Encapsulated S19 ΔvjbR::Kan elicited humoral responses that the nonencapsulated vaccine failed to induce.
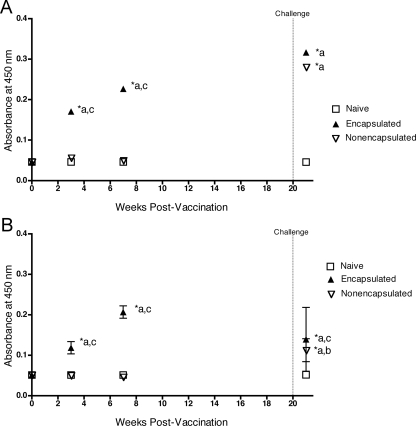
Serum collected at 0, 3, 7, and 21 weeks postvaccination (1 week postchallenge) was assayed for the presence of Brucella-specific IgG1 versus IgG2a antibodies by ELISA. Immunization with encapsulated S19 ΔvjbR::Kan elicited stronger IgG1 and IgG2a responses than the nonencapsulated mutant (P < 0.01) (Fig. 6A and B). During the vaccination stages, both isotypes were induced at similar levels (1:1 ratio), but after challenge, a higher IgG1 (2:1) subtype was seen. In this case, an induction of higher and sustained antibody levels coincides with increased protection from encapsulated S19 ΔvjbR::Kan.
FIG. 6.
IgG1 and IgG2 anti-Brucella antibodies in serum from mice immunized with S19 ΔvjbR::Kan. BALB/c mice were inoculated i.p. with 1 × 105 CFU of either nonencapsulated S19 ΔvjbR::Kan or encapsulated S19 ΔvjbR::Kan. Mice within the control group received empty capsules in lieu of vaccine. At 0, 3, 7, and 21 weeks postvaccination (1 week postchallenge), serum samples were collected for IgG1 (A) and IgG2 (B) determination by ELISA. Results are shown as the means ± SEM of absorbance at 450 nm. For the statistical representation on both graphs, “a” symbolizes naïve animals, “b” encapsulated S19 ΔvjbR::Kan-vaccinated animals, and “c” nonencapsulated S19 ΔvjbR::Kan-vaccinated animals.
DISCUSSION
The development of vaccines to control brucellosis has proven to be a challenge for years. Extensive use of the S19 vaccine has played an enormous role in reducing the disease in cattle, but it became clear that this vaccine in its existing form is of limited use in controlling the disease for humans and in wildlife populations (7). Years of investigation have led to a better understanding of Brucella virulence and the correlates of protective immunity so that vaccines superior to S19 can be developed. The observation that the highest levels of protection are obtained when the host is immunized with live vaccines indicates that persistence and vaccine viability are key aspects required for an efficacious antibrucellosis vaccine (15, 33).
Previous studies in our laboratory have identified genes related to survival and virulence using transposon-based mutagenesis strategies. Among the genes identified, Brucella melitensis vjbR mutants have been evaluated for survival in macrophages and the mouse model to confirm attenuation and immune potential (4). We have previously demonstrated that B. melitensis vjbR mutants are suitable vaccine candidates due to their ability to generate protection in BALB/c mice. Also, by using this mutant, we were able to increase safety by preventing splenomegaly in inoculated mice, a significant improvement over current vaccine strains S19 and Rev-1 that induce splenomegaly in mice (27). In this study, we generated a marked deletion mutant in the S19 vaccine strain with the aim of increasing safety and the possible use of this vaccine in other populations.
The S19 ΔvjbR::Kan mutant was evaluated for survival and attenuation in the macrophage and mouse models. As shown in this study, S19 ΔvjbR::Kan was defective for survival in macrophages and cleared faster than S19 in BALB/c mice. During the initial weeks postinoculation in mice, the mutant and the parental strain did not clear at statistically different rates until 9 weeks postinfection, when S19 ΔvjbR::Kan was undetectable (below the level of detection) in spleens. In contrast, the S19-inoculated mice had 1.36 log units of recoverable bacteria remaining at this time. The increased safety of S19 ΔvjbR::Kan was further revealed by the lack of splenomegaly in inoculated mice. Even at the initial weeks postinoculation (1, 3, and 5 weeks) when the bacterial loads in the spleens were similar to those of the S19-vaccinated mice, the mean spleen weight of the S19 ΔvjbR::Kan-vaccinated mice was decreased compared to that of the S19-vaccinated mice. A histological analysis supported this finding by indicating that the animals that receive the S19 ΔvjbR::Kan mutant did not elicit the degree of inflammatory response observed in the S19-vaccinated animals. Furthermore, inflammatory changes observed in other organs, including the liver, were also significantly diminished with the mutant. This difference in inflammatory response exhibited by the S19 ΔvjbR::Kan-vaccinated animals provides an opportunity to evaluate the use of S19 ΔvjbR::Kan as an improved vaccine candidate.
In vitro studies using antigen-presenting cells have demonstrated that microencapsulated antigens are taken up and processed differently than nonencapsulated materials (12). Similarly, in vivo data has shown that microencapsulation serves to modify the uptake, trafficking, and processing of antigens (29). Additionally, recent reports demonstrate that the persistence of the vaccine strain in the host is needed for the development of suitable and long-term immunity (15). Consistent with this, the S19 vaccine exhibits only modest attenuation, meaning it survives longer in the host but also produces unwanted side effects, such as the severe inflammation reported here. To enhance the immunization efficacy, we investigated the vaccine potential of S19 ΔvjbR::Kan when delivered in a controlled-release vehicle. For this purpose, alginate, a polysaccharide extracted from algae, was used in combination with VpB, derived from the parasite Fasciola hepatica, as the capsular material used to entrap the S19 ΔvjbR::Kan mutant (25, 30, 32). By encapsulating the organism, we attempted to increase its persistence without causing inflammation.
Protection studies against wild-type challenge with either the S19 or S19 ΔvjbR::Kan strains protected mice significantly, but the efficacy of the vaccine was reduced in the nonencapsulated S19 ΔvjbR::Kan mutant. This indicated that the vjbR gene in S19 is necessary to induce a complete immunity toward Brucella infections. The reduced efficacy was successfully compensated by delivering the mutant vaccine in a sustained, microencapsulated format, corroborating the observation of persistence as a function of vaccine efficacy. The encapsulation of live attenuated organisms is an interesting approach to improve immunization efficacy in potential vaccine candidates. Using alginate-VpB microencapsulation, bacteria are exposed to mild conditions in which bacterial viability is not compromised; this approach permits the development of live vaccines in contrast to previously published encapsulation procedures in which the viability of the bacteria is compromised due to the extreme conditions (17). It is important to mention that the microencapsulation of vaccines has been previously documented as a alternative method to enhance the efficacy of DNA, protein-based, and killed vaccines, but to our knowledge, a microencapsulated live Brucella vaccine has been described only by us (9-11, 14, 16, 18, 20, 21, 31, 34).
The degree of protection (assessed by splenic bacterial burden) conferred by either the encapsulated or nonencapsulated S19 ΔvjbR::Kan mutant was compared to humoral profiles. Immunization with encapsulated S19 ΔvjbR::Kan induced higher IgG1 and IgG2a levels compared to the nonencapsulated S19 ΔvjbR::Kan mutant. The animals that received the encapsulated vaccine mounted a stronger IgG1 response at 1 week postchallenge.
In this study, a vjbR knockout was successfully generated in the S19 vaccine and investigated for its potential use as an improved vaccine candidate. Vaccination with a sustained-release vehicle to enhance the vaccination efficacy was evaluated utilizing live S19 ΔvjbR::Kan to immunize BALB/c mice i.p. with either encapsulated or nonencapsulated S19 ΔvjbR to evaluate immunogenicity, safety, and protective efficacy. Enhanced safety was revealed by the absence of splenomegaly in mice that were inoculated with the mutant. Humoral responses postvaccination indicate that the vaccine candidate was able to elicit an anti-Brucella-specific IgG response even when the vaccine was administered in an encapsulated format. Finally, a single dose with the encapsulated mutant conferred higher levels of protection compared to those of the nonencapsulated vaccine. These results suggest that S19 ΔvjbR is safer than S19, induces protection in mice, and should be considered as a vaccine candidate when administered in a sustained-release manner.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Abraham, S. M., R. F. Vieth, and D. J. Burgess. 1996. Novel technology for the preparation of sterile alginate-poly-l-lysine microcapsules in a bioreactor. Pharm. Dev. Technol. 163-68. [DOI] [PubMed] [Google Scholar]
- 2.Allen, C. A., L. G. Adams, and T. A. Ficht. 1998. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect. Immun. 661008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allsup, T. N. 1969. Abortion in sheep associated with Brucella abortus infection. Vet. Rec. 84104-108. [DOI] [PubMed] [Google Scholar]
- 4.Arenas-Gamboa, A. M., T. A. Ficht, M. M. Kahl-McDonagh, and A. C. Rice-Ficht. 2008. Immunization with a single dose of a microencapsulated Brucella melitensis mutant enhances protection against wild-type challenge. Infect. Immun. 762448-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boschiroli, M. L., V. Foulongne, and D. O'Callaghan. 2001. Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 458-64. [DOI] [PubMed] [Google Scholar]
- 6.Cieslak, T. J., G. W. Christopher, M. G. Kortepeter, J. R. Rowe, J. A. Pavlin, R. C. Culpepper, and E. M. Eitzen, Jr. 2000. Immunization against potential biological warfare agents. Clin. Infect. Dis. 30843-850. [DOI] [PubMed] [Google Scholar]
- 7.Davis, D. S., and P. H. Elzer. 2002. Brucella vaccines in wildlife. Vet. Microbiol. 90533-544. [DOI] [PubMed] [Google Scholar]
- 8.Delrue, R. M., C. Deschamps, S. Leonard, C. Nijskens, I. Danese, J. M. Schaus, S. Bonnot, J. Ferooz, A. Tibor, X. De Bolle, and J. J. Letesson. 2005. A quorum-sensing regulator controls expression of both the type IV secretion system and the flagellar apparatus of Brucella melitensis. Cell. Microbiol. 71151-1161. [DOI] [PubMed] [Google Scholar]
- 9.Estevan, M., C. Gamazo, G. Gonzalez-Gaitano, and J. M. Irache. 2006. Optimization of the entrapment of bacterial cell envelope extracts into microparticles for vaccine delivery. J. Microencapsul. 23169-181. [DOI] [PubMed] [Google Scholar]
- 10.Estevan, M., C. Gamazo, F. Martinez-Galan, and J. M. Irache. 16 October 2008, posting date. Stability of poly(ɛ-caprolactone) microparticles containing Brucella ovis antigens as a vaccine delivery system against brucellosis. AAPS PharmSciTech. doi: 10.1208/s12249-008-9149-2. [DOI] [PMC free article] [PubMed]
- 11.Estevan, M., J. M. Irache, M. J. Grillo, J. M. Blasco, and C. Gamazo. 2006. Encapsulation of antigenic extracts of Salmonella enterica serovar: Abortusovis into polymeric systems and efficacy as vaccines in mice. Vet. Microbiol. 118124-132. [DOI] [PubMed] [Google Scholar]
- 12.Eyles, J. E., Z. C. Carpenter, H. O. Alpar, and E. D. Williamson. 2003. Immunological aspects of polymer microsphere vaccine delivery systems. J. Drug Target. 11509-514. [DOI] [PubMed] [Google Scholar]
- 13.Hong, P. C., R. M. Tsolis, and T. A. Ficht. 2000. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 684102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, D. H. 2003. Microencapsulation of vaccine antigens. Methods Mol. Med. 87211-222. [DOI] [PubMed] [Google Scholar]
- 15.Kahl-McDonagh, M. M., and T. A. Ficht. 2006. Evaluation of protection afforded by Brucella abortus and Brucella melitensis unmarked deletion mutants exhibiting different rates of clearance in BALB/c mice. Infect. Immun. 744048-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lameiro, M. H., R. Malpique, A. C. Silva, P. M. Alves, and E. Melo. 2006. Encapsulation of adenoviral vectors into chitosan-bile salt microparticles for mucosal vaccination. J. Biotechnol. 126152-162. [DOI] [PubMed] [Google Scholar]
- 17.Lima, K. M., and J. M. Rodrigues Junior. 1999. Poly-DL-lactide-co-glycolide microspheres as a controlled release antigen delivery system. Braz. J. Med. Biol. Res. 32171-180. [DOI] [PubMed] [Google Scholar]
- 18.Lin, J. H., C. N. Weng, C. W. Liao, K. S. Yeh, and M. J. Pan. 2003. Protective effects of oral microencapsulated Mycoplasma hyopneumoniae vaccine prepared by co-spray drying method. J. Vet. Med. Sci. 6569-74. [DOI] [PubMed] [Google Scholar]
- 19.Mahajan, N. K., R. C. Kulshrestha, and B. Vasudevan. 1986. Brucellosis—cause of abortion in sheep and its public health significance. Int. J. Zoonoses 13174-179. [PubMed] [Google Scholar]
- 20.Malyala, P., J. Chesko, M. Ugozzoli, A. Goodsell, F. Zhou, M. Vajdy, D. T. O'Hagan, and M. Singh. 2008. The potency of the adjuvant, CpG oligos, is enhanced by encapsulation in PLG microparticles. J. Pharm. Sci. 971155-1164. [DOI] [PubMed] [Google Scholar]
- 21.Murillo, M., C. Gamazo, J. M. Irache, and M. M. Goni. 2002. Polyester microparticles as a vaccine delivery system for brucellosis: influence of the polymer on release, phagocytosis and toxicity. J. Drug Target. 10211-219. [DOI] [PubMed] [Google Scholar]
- 22.Nicoletti, P. 1990. Vaccination against Brucella. Adv. Biotechnol. Processes 13147-168. [PubMed] [Google Scholar]
- 23.Pappas, G., N. Akritidis, M. Bosilkovski, and E. Tsianos. 2005. Brucellosis. N. Engl. J. Med. 3522325-2336. [DOI] [PubMed] [Google Scholar]
- 24.Pei, J., and T. A. Ficht. 2004. Brucella abortus rough mutants are cytopathic for macrophages in culture. Infect. Immun. 72440-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice-Ficht, A. C., K. A. Dusek, G. J. Kochevar, and J. H. Waite. 1992. Eggshell precursor proteins of Fasciola hepatica. I. Structure and expression of vitelline protein B. Mol. Biochem. Parasitol. 54129-141. [DOI] [PubMed] [Google Scholar]
- 26.Schurig, G. G., N. Sriranganathan, and M. J. Corbel. 2002. Brucellosis vaccines: past, present and future. Vet. Microbiol. 90479-496. [DOI] [PubMed] [Google Scholar]
- 27.Stevens, M. G., S. C. Olsen, G. W. Pugh, Jr., and D. Brees. 1995. Comparison of immune responses and resistance to brucellosis in mice vaccinated with Brucella abortus 19 or RB51. Infect. Immun. 63264-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuart, F. A., M. J. Corbel, and R. A. Brewer. 1987. Experimental Brucella abortus infection in pigs. Vet. Microbiol. 14365-379. [DOI] [PubMed] [Google Scholar]
- 29.Sun, H., K. G. Pollock, and J. M. Brewer. 2003. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine 21849-855. [DOI] [PubMed] [Google Scholar]
- 30.Waite, J. H., and A. C. Rice-Ficht. 1992. Eggshell precursor proteins of Fasciola hepatica. II. Microheterogeneity in vitelline protein B. Mol. Biochem. Parasitol. 54143-151. [DOI] [PubMed] [Google Scholar]
- 31.Wang, F., X. W. He, L. Jiang, D. Ren, Y. He, D. A. Li, and S. H. Sun. 2006. Enhanced immunogenicity of microencapsulated multiepitope DNA vaccine encoding T and B cell epitopes of foot-and-mouth disease virus in mice. Vaccine 242017-2026. [DOI] [PubMed] [Google Scholar]
- 32.Wee, S., and W. R. Gombotz. 1998. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 31267-285. [DOI] [PubMed] [Google Scholar]
- 33.Zhan, Y., A. Kelso, and C. Cheers. 1995. Differential activation of Brucella-reactive CD4+ T cells by Brucella infection or immunization with antigenic extracts. Infect. Immun. 63969-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zwiorek, K., C. Bourquin, J. Battiany, G. Winter, S. Endres, G. Hartmann, and C. Coester. 2008. Delivery by cationic gelatin nanoparticles strongly increases the immunostimulatory effects of CpG oligonucleotides. Pharm. Res. 25551-562. [DOI] [PubMed] [Google Scholar]