Abstract
Bacillus anthracis lethal toxin (LT) was characterized in plasma from infected African Green monkeys, rabbits, and guinea pigs. In all cases, during the terminal phase of infection only the protease-activated 63-kDa form of protective antigen (PA63) and the residual 20-kDa fragment (PA20) were detected in the plasma. No uncut PA with a molecular mass of 83 kDa was detected in plasma from toxemic animals during the terminal stage of infection. PA63 was largely associated with lethal factor (LF), forming LT. Characterization of LT by Western blotting, capture enzyme-linked immunosorbent assay, and size exclusion chromatography revealed that the antiphagocytic poly-γ-d-glutamic acid (γ-DPGA) capsule released from B. anthracis bacilli was associated with LT in animal blood in variable amounts. While the nature of this in vivo association is not understood, we were able to determine that a portion of these LT/γ-DPGA complexes retained LF protease activity. Our findings suggest that the in vivo LT complexes differ from in vitro-produced LT and that including γ-DPGA when examining the effects of LT on specific immune cells in vitro may reveal novel and important roles for γ-DPGA in anthrax pathogenesis.
Bacillus anthracis, the etiologic agent of anthrax, possesses three primary, plasmid-encoded, virulence factors: lethal toxin (LT) and edema toxin (ET), encoded by the pXO1 plasmid (14, 22, 30, 31), and a poly-γ-d-glutamic acid (γ-DPGA) capsule, encoded by the pXO2 plasmid (10, 33). LT is composed of two proteins: lethal factor (LF; 90.5 kDa) and protective antigen (PA; 63 kDa [referred to as PA63]). Similarly, ET is composed of edema factor (EF; 88.8 kDa) (19) and PA63. PA is secreted by the bacterium as an 82.7-kDa protein referred to as PA83 (35). For PA to bind either LF or EF to form LT or ET, respectively, it must first be activated by proteases to form a 63-kDa moiety (PA63) (23, 28). In a model based on studies of cell culture and purified toxin components, PA83 binds to ubiquitous host cell membrane receptors (5) and is cleaved by a cell-associated furin-type protease (16) to form PA63. PA63 then oligomerizes to form a heptamer. The heptamer assembles into a prepore structure (23), to which one to three LF and/or EF molecules bind (24, 25). The complexes are then internalized by receptor-mediated endocytosis, and, upon acidification of the endosome, the PA prepore undergoes a conformational change, resulting in LF and EF being translocated into the target host cell cytosol, where they exert their toxic effects (11, 19). The requirement for LF and multiple PA molecules was also supported by previous data in which titrations of pure PA combined with fixed amounts of pure LF administered to Fischer 344 rats suggested that a ratio of five PA molecules to one LF molecule was lethal (6). PA can be activated in vitro by trypsin to form PA63 and LT upon addition of LF. Recently, it has been shown that monomeric PA63 binds LF in vitro (2), supporting the concept that LT can assemble in the absence of host cell receptors into complexes consisting of multiple stoichiometries. LF, a Zn2+-dependent metalloprotease, cleaves several members of the mitogen-activated protein kinase kinase family (4, 27, 34). EF is a calcium- and calmodulin-dependent adenylate cyclase that elevates intracellular cyclic AMP, resulting in deregulation of cellular physiology and edema (19).
In contrast to in vitro cell culture findings, PA63 but not PA83 was found in the peripheral blood of B. anthracis-infected animals (26). This implies that the LT might have formed directly in the circulation, as suggested by our previous findings that LT complexes can assemble in plasma without binding to host cell receptors (7). Serum protease(s) rapidly cleaved PA83 to form PA63. This proteolytic activity was heat labile at 56°C, required calcium, and was identified in a variety of animals, including primates, horses, bovines, guinea pigs, rabbits, and chickens (7).
During studies to characterize the in vivo-formed LT, we found that the toxin complex was not of uniform size. Further analysis revealed evidence that a portion of the PA63 complexes, with or without LF activity, had various amounts of γ-DPGA associated with them. The association between LT and γ-DPGA was detected in blood from infected monkeys, guinea pigs, and rabbits. This study stresses the need to examine the difference between LT produced in vitro and LT produced during experimental infection. It remains to be determined whether toxin inhibitors and toxin-neutralizing antibodies previously described as effective against LT in vitro have similar activity against LT produced during anthrax infection.
MATERIALS AND METHODS
Bacterial strain preparation, animals, and aerosol exposure.
Studies were conducted with B. anthracis Ames spores prepared as previously described (9, 12). Briefly, the spores were produced in flask cultures of Leighton and Doi medium, harvested by centrifugation, washed in sterile water for injection, purified on a single-step gradient of 60% Hypaque-76 (Nycomed, Inc., Princeton, NJ), and then stored until use at 4°C in 1% phenol. The spores were used to challenge naive guinea pigs, rabbits (29, 36), and monkeys, which were used as controls in other studies unrelated to the study at hand. The guinea pigs, rabbits, and monkeys were all challenged by the aerosol route, and EDTA plasma was collected from moribund animals as described below. In conducting the research described in this report, the investigators adhered to the guidelines promulgated by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (25a). The facilities are fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Blood analysis.
During the terminal stages of infection, blood from monkeys, rabbits, and guinea pigs was collected in Vacutainer tubes containing EDTA (Becton Dickinson, Franklin Lakes, NJ) to inhibit calcium-dependent protease activity on PA (7) and the animals were humanely euthanized. On occasion, blood was collected during necropsy from the hearts of animals that had just died. The animals were first anesthetized with a combination of ketamine, Acepromazine, and xylazine under the guidance of staff veterinarians. Blood cells were removed by centrifugation, and the plasma was filtered through 0.22-μm syringe filters (Millipore, Billerica, MA) and stored at 4°C no longer than 2 days for analysis by Western blotting, fractionation by column chromatography, and LF protease assays. Aliquots were maintained at −70°C for long-term storage.
Western blot analysis of plasma.
Western blotting was performed under native or denaturing polyacrylamide gel electrophoresis (PAGE) conditions as previously described (7) using 4 to 15% or 10 to 15% polyacrylamide gels, respectively (GE Healthcare, Piscataway, NJ). For sodium dodecyl sulfate (SDS)-PAGE, samples were denatured in sample buffer containing SDS and 2-mercaptoethanol and boiled for 5 min. For native PAGE, samples were diluted in nondenaturing buffer and were not heated before application to the gels. The sample components were transblotted onto 0.45-μm nitrocellulose membranes (Bio-Rad, Hercules, CA) and detected with monoclonal antibodies to capsule (FDF-1B9) (3), PA (BA-PA83-2D3 and BA-PA83-18C2) (20), or LF (LFIII-5D2-1-1 and LFIII-10G3-2-1) as previously described (21).
Synthesis and purification of in vitro LT complexes.
Purified recombinant PA83, PA63, and LF proteins (List Biological Laboratories Inc., Campbell, CA) were used for these studies. PA63 and LF were mixed at equimolar concentrations in phosphate-buffered saline (PBS) and incubated for 30 min at room temperature. The resulting LT complex was purified with a Superose 6 size exclusion column (GE Healthcare) and characterized as previously described (26).
Fractionation and analysis of animal plasma.
Filter-sterilized plasma from each infected animal was diluted in PBS and applied to a Superose 6 size exclusion column (GE Healthcare, Piscataway, NJ) at a flow rate of 0.5 ml/min. Capsule from B. anthracis Ames was prepared as described previously (1). Purified capsule, capsule, PA, and LF from fractionated plasma were detected by Western blotting as described above or by methylene blue staining of capsule as previously described (3, 15). Plasma fractions were also subjected to PA capture enzyme-linked immunosorbent assay (ELISA) with a purified anti-PA antibody (immunoglobulin G) produced in goats. LT complexes in plasma were captured with the immobilized goat anti-PA and then assayed for other associated bacterial antigens. Briefly, wells of microdilution plates (Linbro/Titertek) were coated with purified goat anti-PA immunoglobulin G in 0.05 M sodium borate buffer, pH 9.0, and blocked with PBS containing 0.5% gelatin, 0.3% Tween 20, and 1% bovine serum albumin. Horseradish peroxide-conjugated monoclonal antibodies to PA63, to PA20, to LF, and to capsule (FDF-1B9) (3) were used as secondary antibodies. The chromogenic substrate ABTS [2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid)] (Sigma, St. Louis, MO) was used, and absorbance was determined with a Bio-Tek 308 microplate reader (Bio-Rad) at 405 nm. LF protease activity in the LT complexes was determined as previously described (26).
RESULTS
Characterization of the LT complex from infected animals.
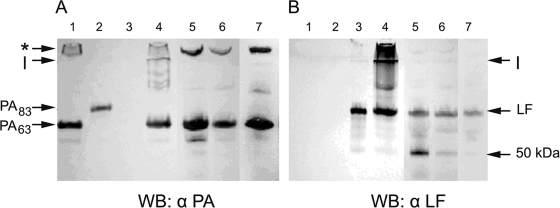
SDS-PAGE followed by Western blot analysis for immunodetection of PA and LF revealed that plasma from infected animals contained PA63 (Fig. 1, lanes 5 to 7) and no detectable PA83 (Fig. 1A). As a control, we demonstrated that in vitro-generated LT disassociated into PA63 (Fig. 1A, lane 4), LF (Fig. 1B, lane 4), and an oligomer resistant to denaturation under SDS-PAGE conditions (Fig. 1). The SDS-resistant oligomer was also present in the animal plasma (Fig. 1A, lanes 5 to 7). LF of the predicted molecular mass was present in all three animal species (Fig. 1B), and an approximately 50-kDa fragment of LF was detected, most notably in the plasma of the monkeys and rabbits (Fig. 1B, lanes 5 and 6).
FIG. 1.
Detection of PA and LF in the plasma of a B. anthracis-infected monkeys, rabbits, and guinea pigs. Shown are results of SDS-PAGE followed by Western blot (WB) analysis of plasma from infected animals. Lane 1, PA63; lane 2, PA83, lane 3, LF; lane 4, in vitro-purified LT complex; lane 5, monkey plasma; lane 6, rabbit plasma; lane 7, guinea pig plasma. (A) Immunoblot stained with anti-PA monoclonal antibodies; (B) immunoblot stained with anti-LF monoclonal antibodies. An SDS-resistant oligomer of PA was detected in both the in vitro and in vivo LT complexes (marked by an asterisk). LF and a 50-kDa fragment of LF were also detected, most notably in plasma from the monkeys and rabbits. I, interface between the stacking and running gels; α, anti.
In order to characterize LT complexes in animal blood, we subjected monkey, rabbit, and guinea pig plasma to size exclusion chromatography. γ-DPGA purified from a B. anthracis culture supernatant (1) had a size of ca. 600 kDa (13) and migrated very slowly into the polyacrylamide gel under SDS-PAGE conditions (Fig. 2A, lane Cap). Analysis of fractionated monkey plasma by SDS-PAGE and staining for γ-DPGA with methylene blue revealed that fractions 6 to 11 contained γ-DPGAs of progressively decreasing molecular mass (Fig. 2A) and of smaller sizes than the purified γ-DPGA (Cap). No staining was observed after fractionation of uninfected normal monkey plasma (data not shown). The large size range of capsule found in vivo contrasts with previous reports of a discrete “low-molecular-weight capsule” (18, 32). When purified γ-DPGA was subjected to native PAGE and transblotted onto nitrocellulose, it did not bind (Fig. 2B, lane Cap); however, in vivo-formed γ-DPGA did bind to nitrocellulose and was detected in fractions 6 to 10 with an anti-γ-DPGA antibody after native PAGE (Fig. 2B). The smearing pattern of γ-DPGA under native PAGE conditions suggested that it consisted of multimers. In addition, the binding of in vivo γ-DPGA to nitrocellulose in contrast with purified γ-DPGA suggested that the in vivo γ-DPGA was associated with other proteins, which allowed it to bind. In order to test this hypothesis, we performed dot blotting using purified capsule. The results revealed that pure capsule binds to nylon membranes but not to nitrocellulose membranes (Fig. 3). We then subjected the fractions to SDS-PAGE and Western blotting to detect PA and LF. Immunodetection revealed PA63 and LF in fractions 6 to 10 (Fig. 2C and D). Fractions 9 and 10 also contained smaller fragments of PA and LF. In order to determine if the PA63 and LF detected in the same fractions as γ-DPGA were associated in vivo, we assayed the fractions by antigen capture ELISA. We captured PA with an immobilized goat anti-PA antibody and then used monoclonal antibodies to detect either LF or γ-DPGA associated with PA in each fraction. We also assayed for LF protease in the captured fractions. PA could be detected in fractions 6 to 19. We detected γ-DPGA associated with PA in fractions 6 to 10 using this technique. LF was not detected by ELISA but was detected in fractions 6 to 9 by its protease activity (Fig. 2E) using a modification of the PA capture ELISA. We captured the PA in each fraction with the immobilized goat anti-PA antibody, washed the microtiter plate wells with PBS to remove any nonbound proteins, added LF substrate, and measured LF protease activity. This resulted in an increase in fluorescence in wells containing LF. Very similar results indicating an association of PA, LF, and capsule in some fractions were obtained when fractionated rabbit and guinea pig plasma fractions were assayed by these methods (Fig. 4). The overall distribution of PA, with some plasma fractions containing only PA, in the monkey was more similar to that in the guinea pig than to that in the rabbit. LF was detected by protease activity in plasma fractions from all species. We also determined that PA was associated with γ-DPGA by capturing γ-DPGA with a purified anticapsule antibody followed by detection of PA with anti-PA monoclonal antibodies (not shown). To demonstrate the specificity of the capture of PA, LF, or γ-DPGA from plasma using these monoclonal antibodies, we repeated the antigen capture ELISA using fractionated plasma from a noninfected guinea pig. No PA, LF, or capsule was detected (not shown). The specificities of the monoclonal antibodies used in this study have been previously characterized (3, 7, 20, 21).
FIG. 2.
Detection of PA, LF, and γ-DPGA complexes in fractionated monkey plasma. Infected monkey plasma was fractionated by size exclusion column chromatography, and fractions were analyzed by SDS-PAGE, native PAGE, Western blotting (WB), and PA capture ELISA. γ-DPGAs of decreasing molecular mass were detected in fractions 6 thru 11 (A) by methylene blue staining of components separated by denaturing SDS-PAGE. Immunodetection using anti-γ-DPGA antibody revealed γ-DPGA multimers in fractions 6 through 10 (B) under native PAGE conditions. Note that pure γ-DPGA (B, lane Cap) did not bind to nitrocellulose, suggesting that the γ-DPGA detected in monkey plasma was associated with another protein(s), which allowed γ-DPGA present in the fractions to be detected. Immunodetection of components separated by denaturing SDS-PAGE revealed PA63 and LF in fractions 6 thru 10 (C and D). PA antigen capture ELISA revealed that γ-DPGA was associated with PA (E, fractions 6 through 10) and that PA was distributed over a wide range of fractions (E, fractions 6 through 19), suggesting that multiple oligomeric forms of PA63 were present. LF protease activity was present in fractions 6 through 9 (E). Pure recombinant PA and LF eluted at fraction 16 (not shown). In vitro-produced LT (PA63 plus LF) eluted at fraction 13 (not shown). RFU, relative fluorescence units; Abs, absorbance.
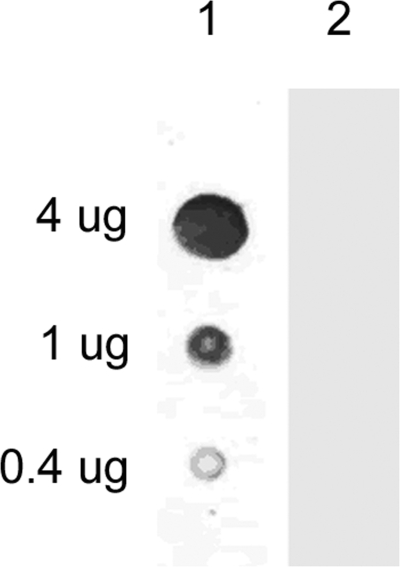
FIG. 3.
Purified capsule binds to nylon but not to nitrocellulose membranes. Indicated amounts of capsule were dotted onto membranes, allowed to air dry, and stained for capsule. Lane 1, nylon membrane stained with methylene blue; lane 2, nitrocellulose membrane stained with methylene blue.
FIG. 4.
Detection of PA, LF, and γ-DPGA complexes in fractionated rabbit and guinea pig plasma. Infected rabbit and guinea pig plasma was fractionated by size exclusion column chromatography, and fractions were analyzed by PA capture ELISA. In the rabbit (A), PA was associated with γ-DPGA in fractions 6 to 10 and with LF in fractions 6 to 10 and LF protease activity was detected in fractions 7 to 11. In the guinea pig (B), PA was associated with γ-DPGA in fractions 6 to 12 and LF protease activity was detected in fractions 9 to 11. SDS-PAGE and native PAGE showed results similar to those in Fig. 2A through D (not shown). Pure recombinant PA and LF eluted at fraction 16 (not shown). In vitro-produced LT (PA63 plus LF) eluted at fraction 13 (not shown). RFU, relative fluorescence units; Abs, absorbance.
DISCUSSION
Fish and Lincoln began their 1968 article (8) with the following statement: “Little is known about the synthesis of anthrax toxin molecules or the extent of the differences between in vivo and in vitro toxin.” Unfortunately, this statement is still largely true. Our data clearly demonstrated that in vivo-formed toxin exists largely as a complex and that there was no detectable free PA83. All detectable PA had a molecular mass of 63 kDa (Fig. 1) or 20 kDa (not shown), and a major portion of the PA63 appeared to be primarily complexed with LF. We did not assay for EF and so do not know if EF is also present with PA. This supports our previous report (7) showing that native plasma from a wide range of animal species, including monkeys, possesses serum protease activity that cleaves PA83 to form the 63-kDa active moiety (PA63), which binds LF to form a complex (i.e., LT). In that the protease activity requires Ca2+, our use of EDTA in the Vacutainer tubes argues against the cleavage of PA83 by Ca2+-dependent protease activity during the collection of blood. When denatured with SDS, 2-mercaptoethanol, and heat, the in vivo LT complexes yielded PA63 and two bands for LF (Fig. 1). The significance of the smaller-molecular-mass LF (∼50 kDa) is not known, and its relationship to activity and virulence is not understood. However, it is of interest that fractions 9 and 10, containing the LF of ∼50 kDa (Fig. 2D), had low or no detectable protease activity (Fig. 2E), suggesting that this form was inactive. Size exclusion chromatography of in the vitro-synthesized PA63/LF (LT) complex resulted in a major peak, containing detectable LF protease activity, which eluted from the column at fraction 12 (26). In contrast, the in vivo toxin complexes, containing PA63, LF, and detectable LF protease activity, eluted earlier from the sizing column (fractions 6 to 10) (Fig. 2), suggesting that these in vivo LT complexes were much larger than the in vitro-synthesized LT complex. As determined by Western blotting, the in vivo complexes were associated with capsule of various molecular masses (Fig. 2A). Addition of pure capsule to the in vitro-generated PA63/LF complex did not affect the elution profile of in vitro LT (not shown). The finding that in vivo toxin complexes are associated with capsule raises a number of questions with regard to pathogenesis. Is the role of capsule in the virulence of B. anthracis solely the inhibition of phagocytosis, or does capsule also alter the activity of the associated LT complexes? We are currently purifying larger quantities of the different forms of in vivo-formed toxin to address this question. Such studies are complicated by reports of Fish and Lincoln (8) stating that the biological activity of toxin complexes (lethality in Fischer 344 rats and skin edema in guinea pigs) is markedly reduced by freezing the body fluids at −20°C and subsequent thawing, or by storage at 4°C after 6 to 12 h. They found it necessary to perform biological assays within hours after acquiring blood from infected hosts. Adding protease inhibitors would interfere with certain biological assays. Determining which stage of infection an animal is in during the rapidly changing series of terminal events in anthrax infection is also problematic, considering that release of high levels of toxins into the circulation occurs mainly during the final hours before death (17). The data we report here with regard to the absence of PA83 in the blood and the association of capsule with the toxin complex in three rabbits, three guinea pigs, and four monkeys appear consistent. These findings also raise questions about the mechanism of protection afforded by capsule vaccines (1, 15) and antibodies (18). It may be that antibodies to capsule could have unexpected effects on toxin-capsule complexes that contribute to protection. It has been well established that both toxin and capsule are required for virulence, but the present results raise questions about the true relationship between these two virulence factors. Further studies will be required to determine if and how their association in vivo affects their roles in virulence. Do they act separately to produce lethality, in an additive manner, or does the full effect of one (LT) require association with the other (γ-DPGA)?
Acknowledgments
The Defense Advanced Research Projects Agency provided funding for this work.
We thank Katheryn Kenyon for her editorial review, Stephen F. Little for providing monoclonal antibodies against PA and LF, and Timothy A. Hoover for critical reading of the manuscript.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 8 December 2008.
REFERENCES
- 1.Chabot, D. J., A. Scorpio, S. A. Tobery, S. F. Little, S. L. Norris, and A. M. Friedlander. 2004. Anthrax capsule vaccine protects against experimental infection. Vaccine 2343-47. [DOI] [PubMed] [Google Scholar]
- 2.Chvyrkova, I., X. C. Zhang, and S. Terzyan. 2007. Lethal factor of anthrax toxin binds monomeric form of protective antigen. Biochem. Biophys. Res. Commun. 360690-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De, B. K., S. L. Bragg, G. N. Sanden, K. E. Wilson, L. A. Diem, C. K. Marston, A. R. Hoffmaster, G. A. Barnett, R. S. Weyant, T. G. Abshire, J. W. Ezzell, and T. Popovic. 2002. A two-component direct fluorescent-antibody assay for rapid identification of Bacillus anthracis. Emerg. Infect. Dis. 81060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280734-737. [DOI] [PubMed] [Google Scholar]
- 5.Escuyer, V., and R. J. Collier. 1991. Anthrax protective antigen interacts with a specific receptor on the surface of CHO-K1 cells. Infect. Immun. 593381-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezzell, J. W., B. E. Ivins, and S. H. Leppla. 1984. Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect. Immun. 45761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezzell, J. W., Jr., and T. G. Abshire. 1992. Serum protease cleavage of Bacillus anthracis protective antigen. J. Gen. Microbiol. 138(Pt. 3)543-549. [DOI] [PubMed] [Google Scholar]
- 8.Fish, D. C., and R. E. Lincoln. 1968. In vivo-produced anthrax toxin. J. Bacteriol. 95919-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz, D. L., N. K. Jaax, W. B. Lawrence, K. J. Davis, M. L. Pitt, J. W. Ezzell, and A. M. Friedlander. 1995. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab Investig. 73691-702. [PubMed] [Google Scholar]
- 10.Green, B. D., L. Battisti, T. M. Koehler, C. B. Thorne, and B. E. Ivins. 1985. Demonstration of a capsule plasmid in Bacillus anthracis. Infect. Immun. 49291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna, P. C., D. Acosta, and R. J. Collier. 1993. On the role of macrophages in anthrax. Proc. Natl. Acad. Sci. USA 9010198-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivins, B. E., S. L. Welkos, G. B. Knudson, and S. F. Little. 1990. Immunization against anthrax with aromatic compound-dependent (Aro-) mutants of Bacillus anthracis and with recombinant strains of Bacillus subtilis that produce anthrax protective antigen. Infect. Immun. 58303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyce, J., J. Cook, D. Chabot, R. Hepler, W. Shoop, Q. Xu, T. Stambaugh, M. Aste-Amezaga, S. Wang, L. Indrawati, M. Bruner, A. Friedlander, P. Keller, and M. Caulfield. 2006. Immunogenicity and protective efficacy of Bacillus anthracis poly-gamma-D-glutamic acid capsule covalently coupled to a protein carrier using a novel triazine-based conjugation strategy. J. Biol. Chem. 2814831-4843. [DOI] [PubMed] [Google Scholar]
- 14.Kaspar, R. L., and D. L. Robertson. 1987. Purification and physical analysis of Bacillus anthracis plasmids pXO1 and pXO2. Biochem. Biophys. Res. Commun. 149362-368. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, K., and Y. Itoh. 2003. Characterization of poly-γ-glutamate hydrolase encoded by a bacteriophage genome: possible role in phage infection of Bacillus subtilis encapsulated with poly-γ-glutamate. Appl. Environ. Microbiol. 692491-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klimpel, K. R., S. S. Molloy, G. Thomas, and S. H. Leppla. 1992. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc. Natl. Acad. Sci. USA 8910277-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobiler, D., S. Weiss, H. Levy, M. Fisher, A. Mechaly, A. Pass, and Z. Altboum. 2006. Protective antigen as a correlative marker for anthrax in animal models. Infect. Immun. 745871-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozel, T. R., P. Thorkildson, S. Brandt, W. H. Welch, J. A. Lovchik, D. P. AuCoin, J. Vilai, and C. R. Lyons. 2007. Protective and immunochemical activities of monoclonal antibodies reactive with the Bacillus anthracis polypeptide capsule. Infect. Immun. 75152-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 793162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little, S. F., S. H. Leppla, and E. Cora. 1988. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect. Immun. 561807-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little, S. F., S. H. Leppla, and A. M. Friedlander. 1990. Production and characterization of monoclonal antibodies against the lethal factor component of Bacillus anthracis lethal toxin. Infect. Immun. 581606-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikesell, P., B. E. Ivins, J. D. Ristroph, and T. M. Dreier. 1983. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect. Immun. 39371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milne, J. C., D. Furlong, P. C. Hanna, J. S. Wall, and R. J. Collier. 1994. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J. Biol. Chem. 26920607-20612. [PubMed] [Google Scholar]
- 24.Mogridge, J., K. Cunningham, and R. J. Collier. 2002. Stoichiometry of anthrax toxin complexes. Biochemistry 411079-1082. [DOI] [PubMed] [Google Scholar]
- 25.Mogridge, J., K. Cunningham, D. B. Lacy, M. Mourez, and R. J. Collier. 2002. The lethal and edema factors of anthrax toxin bind only to oligomeric forms of the protective antigen. Proc. Natl. Acad. Sci. USA 997045-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 26.Panchal, R. G., K. M. Halverson, W. Ribot, D. Lane, T. Kenny, T. G. Abshire, J. W. Ezzell, T. A. Hoover, B. Powell, S. Little, J. J. Kasianowicz, and S. Bavari. 2005. Purified Bacillus anthracis lethal toxin complex formed in vitro and during infection exhibits functional and biological activity. J. Biol. Chem. 28010834-10839. [DOI] [PubMed] [Google Scholar]
- 27.Pellizzari, R., C. Guidi-Rontani, G. Vitale, M. Mock, and C. Montecucco. 1999. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNgamma-induced release of NO and TNFalpha. FEBS Lett. 462199-204. [DOI] [PubMed] [Google Scholar]
- 28.Petosa, C., R. J. Collier, K. R. Klimpel, S. H. Leppla, and R. C. Liddington. 1997. Crystal structure of the anthrax toxin protective antigen. Nature 385833-838. [DOI] [PubMed] [Google Scholar]
- 29.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 194768-4773. [DOI] [PubMed] [Google Scholar]
- 30.Robertson, D. L., and S. H. Leppla. 1986. Molecular cloning and expression in Escherichia coli of the lethal factor gene of Bacillus anthracis. Gene 4471-78. [DOI] [PubMed] [Google Scholar]
- 31.Tippetts, M. T., and D. L. Robertson. 1988. Molecular cloning and expression of the Bacillus anthracis edema factor toxin gene: a calmodulin-dependent adenylate cyclase. J. Bacteriol. 1702263-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchida, I., S. Makino, C. Sasakawa, M. Yoshikawa, C. Sugimoto, and N. Terakado. 1993. Identification of a novel gene, dep, associated with depolymerization of the capsular polymer in Bacillus anthracis. Mol. Microbiol. 9487-496. [DOI] [PubMed] [Google Scholar]
- 33.Uchida, I., T. Sekizaki, K. Hashimoto, and N. Terakado. 1985. Association of the encapsulation of Bacillus anthracis with a 60 megadalton plasmid. J. Gen. Microbiol. 131(Pt. 2)363-367. [DOI] [PubMed] [Google Scholar]
- 34.Vitale, G., L. Bernardi, G. Napolitani, M. Mock, and C. Montecucco. 2000. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352(Pt. 3)739-745. [PMC free article] [PubMed] [Google Scholar]
- 35.Welkos, S. L., J. R. Lowe, F. Eden-McCutchan, M. Vodkin, S. H. Leppla, and J. J. Schmidt. 1988. Sequence and analysis of the DNA encoding protective antigen of Bacillus anthracis. Gene 69287-300. [DOI] [PubMed] [Google Scholar]
- 36.Zaucha, G. M., L. M. Pitt, J. Estep, B. E. Ivins, and A. M. Friedlander. 1998. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. Pathol. Lab. Med. 122982-992. [PubMed] [Google Scholar]