Abstract
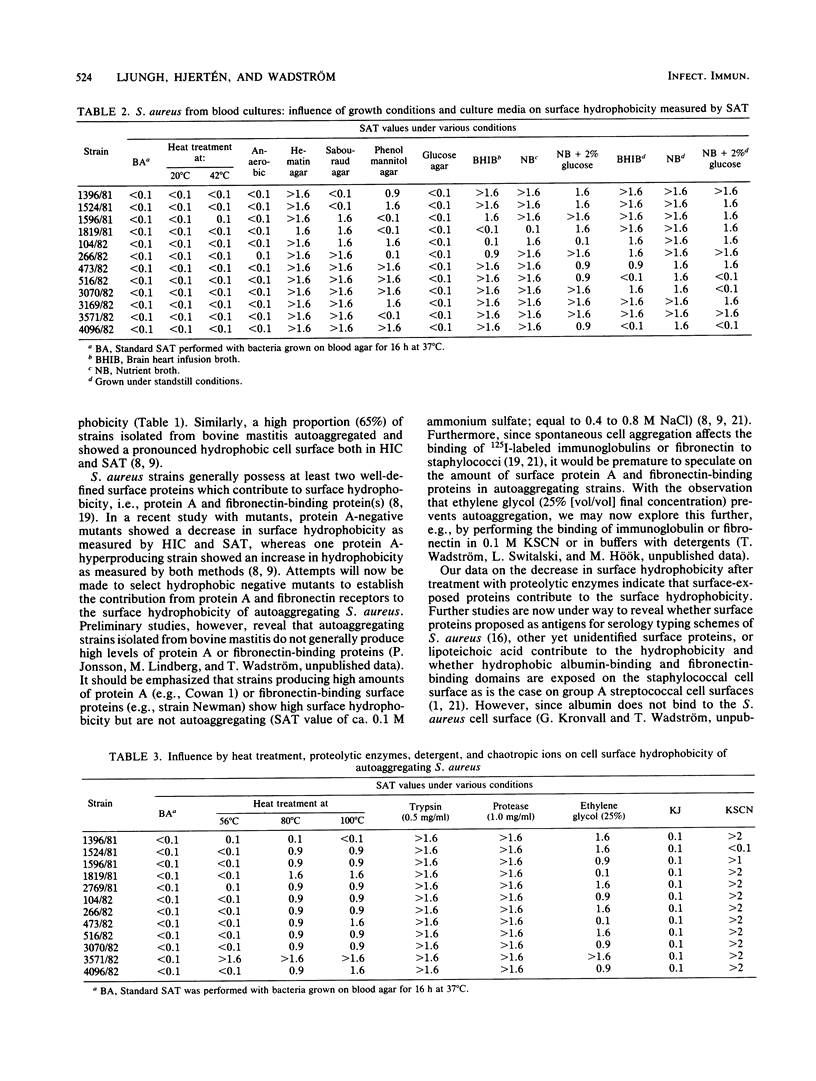
A total of 209 strains of Staphylococcus aureus isolated from infections and 23 strains from nose cultures of healthy laboratory personnel were compared for relative surface hydrophobicity in the salt aggregation test (Lindahl et al., Biochim. Biophys. Acta 677:471-476, 1981). In the standard method, bacterial cell suspensions from blood agar-grown cultures were tested for visible aggregation by "salting out" in serial dilutions of ammonium sulfate (0.1 to 1.6 M [final concentration]). Bacteria were defined as extremely hydrophobic when showing autoaggregation in saline or in 0.002 M sodium phosphate buffer (pH 6.8). Using this definition, we found a large number of strains isolated from various infections to be very hydrophobic: 123 of 135 strains from patients with septicemia (91%), 54 of 60 strains from wound infections (90%), and 12 of 14 strains from urinary tract infections (86%). In contrast, only 9 of 23 strains from nose cultures of healthy carriers (39%) were autoaggregating. A total of 12 autoaggregating strains were grown on various solid and liquid media. Only growth on hematin agar was found to completely suppress surface hydrophobicity as revealed by our salt aggregation test method, and growth in liquid media prevented the expression of hydrophobicity in most strains. Growth at 20 or 42 degrees C or under anaerobic conditions did not affect hydrophobicity. Cells harvested from various phases of growth did not differ significantly in surface hydrophobicity. Heating washed cell suspensions at 56 degrees C did not affect the salt aggregation test values, whereas heating the cell suspensions at 80 and 100 degrees C caused a significant decline in hydrophobicity. The addition of ethylene glycol (25% [vol/vol] final concentration) prevented the autoaggregation of 10 of the 12 strains. Likewise, treating the cell suspensions with proteolytic enzymes decreased the surface hydrophobicity, indicating that surface proteins contribute to high surface hydrophobicity of autoaggregating strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Dankers I. Lectin-like constituents of foods which react with components of serum, saliva, and Streptococcus mutans. Appl Environ Microbiol. 1981 Apr;41(4):880–888. doi: 10.1128/aem.41.4.880-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Ljungh A., Wadström T. Salt aggregation test for measuring cell surface hydrophobicity of urinary Escherichia coli. Eur J Clin Microbiol. 1982 Dec;1(6):388–393. doi: 10.1007/BF02019940. [DOI] [PubMed] [Google Scholar]
- Ludwicka A., Jansen B., Wadström T., Pulverer G. Attachment of staphylococci to various synthetic polymers. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Apr;256(4):479–489. doi: 10.1016/s0174-3031(84)80024-4. [DOI] [PubMed] [Google Scholar]
- Maverakis N. H., Wiley B. B. Capsular antigens of Staphylococcus aureus. I. Further purification of the capsular antigen of a major capsular type of Staphylococcus aureus. Can J Microbiol. 1972 Aug;18(8):1283–1288. doi: 10.1139/m72-198. [DOI] [PubMed] [Google Scholar]
- Morse S. I. Biological attributes of staphylococcal cell walls. Ann N Y Acad Sci. 1965 Jul 23;128(1):191–213. doi: 10.1111/j.1749-6632.1965.tb11639.x. [DOI] [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Rydén C., Rubin K., Ljungh A., Hök M., Wadström T. Binding of fibronectin to Staphylococcus strains. Infect Immun. 1983 Nov;42(2):628–633. doi: 10.1128/iai.42.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylewska S., Hjertén S., Wadström T. Effect of subinhibitory concentrations of antibiotics on the adhesion of Streptococcus pyogenes to pharyngeal epithelial cells. Antimicrob Agents Chemother. 1981 Nov;20(5):563–566. doi: 10.1128/aac.20.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadström T., Schmidt K. H., Kühnemund O., Havlícek J., Köhler W. Comparative studies on surface hydrophobicity of streptococcal strains of groups A, B, C, D and G. J Gen Microbiol. 1984 Mar;130(3):657–664. doi: 10.1099/00221287-130-3-657. [DOI] [PubMed] [Google Scholar]
- Wadström T., Tylewska S. Surface hydrophobicity of Group A streptococci. J Infect Dis. 1982 Oct;146(4):576–578. doi: 10.1093/infdis/146.4.576. [DOI] [PubMed] [Google Scholar]
- Westergren G., Olsson J. Hydrophobicity and adherence of oral streptococci after repeated subculture in vitro. Infect Immun. 1983 Apr;40(1):432–435. doi: 10.1128/iai.40.1.432-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



