Abstract
The innate immune response is a double-edged sword in systemic inflammation and sepsis. Uncontrolled or inappropriate activation can damage and be lethal to the host. Several studies have investigated inhibition of downstream mediators, including tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β). Emerging evidence indicates that upstream inhibition is a better therapeutic approach for attenuating damaging immune activation. Therefore, we investigated inhibition of two central innate immune pathways, those of complement and CD14/Toll-like receptor 4 (TLR4)/myeloid differentiation protein 2 (MD-2), in a porcine in vitro model of Escherichia coli-induced inflammation. Porcine whole blood anticoagulated with lepuridin, which did not interfere with the complement system, was incubated with E. coli lipopolysaccharide (LPS) or whole bacteria. Inhibitors of complement and CD14 and thus the LPS CD14/TLR4/MD-2 receptor complex were tested to investigate the effect on the inflammatory response. A broad range of inflammatory readouts were used to monitor the effect. Anti-CD14 was found to saturate the CD14 molecule on granulocytes and completely inhibited LPS-induced proinflammatory cytokines in a dose-dependent manner. Anti-CD14 significantly reduced the levels of the E. coli-induced proinflammatory cytokines TNF-α and IL-1β, but not IL-8, in a dose-dependent manner. No effect on bacterial clearance was seen. Vaccinia complement control protein and smallpox inhibitor of complement enzymes, two Orthopoxvirus-encoded complement inhibitors, completely inhibited complement activation. Furthermore, these agents almost completely inhibited the expression of wCD11R3, which is associated with CD18 as a β2 integrin, on porcine granulocytes and decreased IL-8 levels significantly in a dose-dependent manner. As expected, complement inhibition reduced bacterial clearance. We conclude that inhibition of complement and CD14 attenuates E. coli-induced inflammation and might be used as a therapeutic regimen in gram-negative sepsis along with appropriate treatment with antibiotics.
Innate immunity plays a key role in protecting a host against invading microorganisms. Several immune cells, like macrophages/monocytes and neutrophils, and cascade systems, like the complement system, are involved. Mediators, like cytokines and chemokines, are important messengers and effector molecules. Receptors, like the Toll-like receptors (TLR) (22), are involved in the inflammatory response and cross talk through direct recognition of pathogen-associated molecular patterns (1).
TLR4 is the receptor for lipopolysaccharide (LPS), an outer membrane constituent of gram-negative bacteria (14, 38). TLR4 is abundant on cells like peripheral blood leukocytes, macrophages, and monocytes that respond to LPS (6). To be functional, TLR4 is incorporated in complexes with other proteins. Glycosylphosphatidylinositol-anchored (not transmembrane) CD14 (6, 42) binds the complex consisting of LPS and the soluble LPS-binding protein (32, 47). Non-membrane-bound myeloid differentiation protein 2 (MD-2), which is physically associated with the extracellular part of TLR4, is necessary for intracellular transduction of the signal after LPS binding (29, 34). The intracellular cascade following activation by LPS ultimately leads to activation of a variety of transcription factors, including nuclear factor κB (4, 11). Such transcription factors activate cytokine and chemokine genes, such as the genes encoding tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8 (6, 11).
The complement system is a cascade system in blood (44). It consists of three known initiating pathways, the classical pathway, the lectin pathway, and the alternative pathway, as well as a common final pathway. The final product of the cascade is the terminal complement complex (TCC), either the membrane-inserted C5b-9 complex (membrane attack complex) or the soluble sC5b-9 complex (27). The important biological properties of this complex include lysis and thus killing of certain bacteria, particularly Neisseria species like Neisseria meningitidis (35), and effects induced by sublytic attack (26). Split products are produced during complement activation. The anaphylatoxins, particularly C3a and C5a, have important roles in the inflammatory response, binding to the corresponding receptors and having effects on various cells (17, 36). Emerging evidence, however, indicates that there are harmful effects of excessive complement activation, which lead to tissue damage under several inflammatory conditions (2, 45, 46).
This complex innate immune response is developed to protect the host, but it can be deleterious if overwhelmed, as is the case in sepsis (7). Due to this fact, several promising studies have been conducted using animal models in which single mediators of innate immunity in sepsis were inhibited. However, the results of such studies with humans have largely been disappointing (21, 45).
A combined approach involving inhibition of both the complement system and upstream induction of proinflammatory cytokines may be a better therapeutic strategy to control inflammation. Recent studies have shown that there is cross talk between TLRs and complement in response to invading microorganisms. Thus, both complement receptor 3 (CR3) and TLR4 were necessary for sufficient uptake and killing of the gram-negative bacterium Salmonella enterica (43). Complement was also shown to be a regulator of TLR-mediated inflammation in mice (50). Recently, we hypothesized that inhibition of complement and CD14 would be an efficient way to attenuate a broad spectrum of inflammatory mediators induced by activation of the innate immune system (24).
To explore this hypothesis, we investigated the effect of inhibition of complement and CD14 on Escherichia coli-induced inflammation in an in vitro porcine whole-blood model. Data were obtained using a model with lepuridin (recombinant hirudin) as an anticoagulant, which enabled the whole inflammatory network to cross talk, in contrast to the data that can be obtained using other anticoagulants, like EDTA, citrate, and heparin, which interfere with and inhibit both complement activation and a number of other plasma and cell inflammation markers (23).
As the pig is a widely used model animal for human diseases, the results can be useful for subsequent in vivo studies.
MATERIALS AND METHODS
Reagents.
Lepirudin (Refludan) was purchased from Hoechst Marion Roussel (Frankfurt am Main, Germany). Ultrapure E. coli LPS (5 mg; strain 0111:B4; catalogue no. tlrl-pelps) was purchased from InvivoGen (San Diego, CA). E. coli was purchased from the American Type Culture Collection (Manassas, VA). Human serum albumin (HSA) (200 mg/ml; V) was purchased from Octapharma AG (Lachen, Switzerland). Sterile phosphate-buffered saline (PBS) (catalog no. 14040-083) was purchased from Gibco, Invitrogen Corporation (Paisley, Scotland).
Monoclonal antibodies.
Mouse anti-porcine CD14 monoclonal antibody clone MIL-2 (isotype immunoglobulin G2b [IgG2b]; catalog no. MCA1218ELX [unconjugated] and catalog no. MCA1218 [fluorescein isothiocyanate [FITC] conjugated) was purchased from AbD Serotec (Oxford, United Kingdom). Mouse anti-human IgG2b (clone BH1) was purchased from Diatec Monoclonals AS (Oslo, Norway) and was used as an isotype-matched control with anti-CD14 MIL-2.
Recombinant complement regulatory proteins from Orthopoxvirus.
Vaccinia complement control protein (VCP), which has been described previously (18, 19), was produced using the Pichia pastoris yeast expression system as described previously (10, 28). Smallpox inhibitor of complement enzymes (SPICE), which also has been described previously (30), was produced by cloning and site-directed mutagenesis of VCP (33).
In vitro whole-blood experiments.
Porcine whole blood was collected from 15- to 50-kg pigs (Sus scrofa domesticus; Landrace; outbred stock). The blood was anticoagulated with 50 μg/ml lepirudin as described previously (23), pooled after collection, and immediately placed in 1.8- or 4.5-ml Nunc tubes (catalog no. 375418 and 379146; Nunc, Roskilde, Denmark) for incubation. The blood was used for TCC, cytokine, flow cytometric, and microbiological analyses.
For the complement and cytokine experiments, E. coli or ultrapure E. coli LPS was added directly to the blood, and the samples were incubated at 37°C for 30 min or 2 or 4 h depending on the readout used. Complement activation was stopped by adding EDTA to a final concentration of 20 mM immediately after incubation. The time zero baseline sample was processed immediately. The blood samples were centrifuged for 10 min at 3,000 × g (4°C), and the plasma was collected and stored at −70°C until it was analyzed. In the inhibition experiments, blood was preincubated at 37°C for 5 min with a complement inhibitor (VCP or SPICE) or anti-CD14 and the corresponding controls. E. coli or ultrapure E. coli LPS was then added at the final concentration to whole blood and incubated for 120 min (TNF-α and IL-1β) or 240 min (IL-8).
For the flow cytometry experiments, E. coli was added directly to the blood. The samples were incubated for 10 min at 37°C. Inhibition experiments were performed by preincubating the whole blood for 5 min at 37°C with VCP or anti-CD14 and the corresponding controls. E. coli was then added at the final concentration to whole blood.
For the CFU experiments, blood was preincubated at 37°C for 5 min with a complement inhibitor (SPICE) or anti-CD14 and the corresponding controls. E. coli was then added, and after incubation the blood samples were immediately put on ice before they were processed as described below.
TCC enzyme-linked immunosorbent assay (ELISA).
The soluble C5b-9 TCC was measured using an enzyme immunoassay, as described previously (25). Briefly, monoclonal antibody aE11 that reacted with a neoepitope exposed in C9 after incorporation in the C5b-9 complex was used as a capture antibody, and the final concentration in the wells was 3 μg/ml. A biotinylated anti-C6 monoclonal antibody (Quidel Corporation, San Diego, CA) was used as the detection antibody at a final concentration of 4 μg/ml. Both antibodies cross-react with specific pig epitopes, and the assay can be used to detect porcine TCC (25). The standard used was normal human serum activated with zymosan, which by definition contained 1,000 arbitrary units/ml. Zymosan-activated porcine serum was used as a positive control, and the buffer used as the diluent, PBS, EDTA, and a detergent (Tween), was used as containing a negative control.
Cytokine ELISA.
The immunoassay kits used to detect the porcine cytokines TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-12p70, and gamma interferon (Quantikine) were purchased from R&D Systems (Minneapolis, MN). The immunoassay kit used for human vascular endothelial growth factor, which is known to cross-react with pigs (3), was purchased from R&D Systems (Minneapolis, MN). The immunoassay kits were used according to the instructions of the manufacturer.
Flow cytometry.
After incubation as described above, the whole blood was fixed using a 0.5% paraformaldehyde solution and incubated for 4 min at 37°C. The cells were stained with primary mouse anti-porcine wCD11R3 IgG1 clone 2F4/11 (catalog no. 552291) or an isotype-matched IgG1 control antibody (catalog no. 557273) (both obtained from BD Biosciences Pharmingen, San Jose, CA) for 15 min at room temperature. The cells were washed with PBS and centrifuged at 1,200 × g for 5 min. A phycoerythrin-conjugated rat anti-mouse monoclonal antibody (catalog no. 340270; BD Biosciences, San Jose, CA) was added, and the samples were incubated for 15 min at room temperature in the dark. The red cells were lysed, the samples were centrifuged at 1,200 × g for 5 min, and the pellets were resuspended in PBS.
In the CD 14 saturation experiments, different concentrations of nonconjugated anti-porcine CD14 (clone MIL-2) were added (final concentrations, 0.98 to 250 μg/ml) to porcine whole blood and incubated for 10 min at 37°C. An FITC-conjugated anti-CD14 monoclonal antibody (clone MIL-2) was then added at a concentration of 150 μg/ml, which was determined in previous titration experiments, and incubated for 15 min at room temperature. The red cells were lysed, the samples were washed and centrifuged at 1,200 × g for 5 min, and the pellets were resuspended in PBS.
Cell samples were analyzed with a flow cytometer (FACScan; Becton Dickinson, Franklin Lakes, NJ). Unstimulated cells, which were stained with the isotype-matched antibody, were used as an absolute negative control when the flow cytometer was adjusted.
E. coli CFU experiments.
Whole blood incubated as described above was diluted in 10-fold steps in sterile 0.85% NaCl. One hundred microliters of each dilution was plated in parallel on lactose agar plates. The plates were incubated at 36°C overnight. After incubation, the numbers of CFU were determined manually.
Statistical analysis.
Results were analyzed using SPSS for Windows 15.0 (SPSS Inc., Chicago, IL). Pairwise comparisons were performed using a two-sample t test. A P value of <0.05 was considered statistically significant.
Ethics.
The study was approved by The Norwegian Animal Experimental Board, and animals were treated according to Norwegian laboratory animal regulations.
RESULTS
Anti-CD14 monoclonal antibody MIL-2 binds to and saturates CD14 on porcine granulocytes.
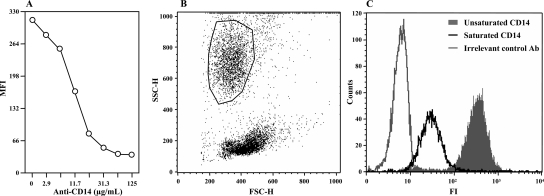
Unconjugated anti-porcine CD14 saturated CD14 on porcine granulocytes in a dose-dependent manner, as determined by flow cytometry (Fig. 1A). Gating of the granulocytes studied is shown in a forward scatter-side scatter plot in Fig. 1B. At an anti-CD14 concentration between 30 and 62.5 μg/ml whole blood the saturation was complete (Fig. 1A). At a concentration as low as 1 μg/ml partial saturation was observed as reduced median fluorescence intensity when FITC-conjugated anti-CD14 was used. The substantial shift of the CD14-saturated granulocyte population compared with the unsaturated population is shown in the flow histogram in Fig. 1C.
FIG. 1.
Anti-CD14 (clone MIL-2) binds to and saturates CD14 on porcine granulocytes. (A) Whole blood from three pigs was incubated for 10 min at 37°C with different doses of unconjugated anti-CD14 (x axis). FITC-conjugated anti-CD14 was then added at a fixed concentration, and the samples were analyzed with a FACScan flow cytometer. The results of one of three experiments are shown. MFI, median fluorescence intensity. (B) Gating of the granulocytes as shown by a forward scatter-side scatter plot. (C) Histogram showing that there was a shift of fluorescence intensity (FI) from unsaturated CD14 (no unconjugated anti-CD14) (filled area) to saturated CD14 (62.5 μg/ml unconjugated anti-CD14). The results for an irrelevant detection control antibody (Ab) are also shown.
Effect of anti-CD14 on LPS-induced cytokine production.
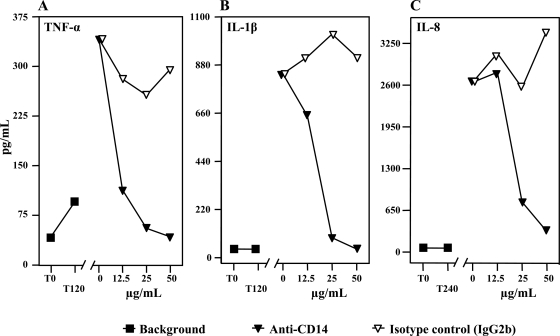
Anti-CD14 reduced TNF-α, IL-1β, and IL-8 production induced by ultrapure E. coli LPS in porcine whole blood in a dose-dependent manner, whereas the isotype-matched control antibody had no effect (Fig. 2). There was complete inhibition of TNF-α (Fig. 2A) and IL-1β (Fig. 2B) with 50 μg anti-CD14/ml whole blood, and there was almost complete of IL-8 (Fig. 2C) with the same concentration. IL-6, IL-10, IL-12p70, vascular endothelial growth factor, and gamma interferon were not produced under the conditions used.
FIG. 2.
Effect of anti-CD14 on LPS-induced cytokine production. Whole blood from four pigs was preincubated with anti-CD14 or an isotype-matched control antibody for 5 min. LPS was then added to a final concentration of 100 ng/ml and incubated at 37°C for 2 h (TNF-α and IL-1β) (A and B) or 4 h (IL-8) (C). Cytokines were measured using ELISA. The results of one of four experiments are shown. T0, baseline sample; T120 and T240, negative control samples obtained after 120 and 240 min, respectively.
E. coli-induced TCC formation and effect of complement inhibitors.
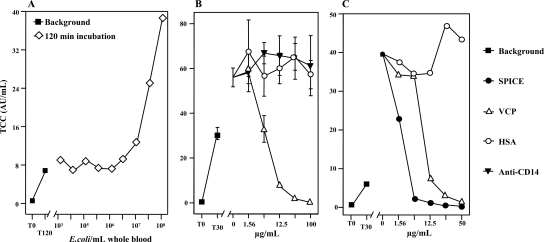
E. coli activated porcine complement in whole blood (as measured using TCC) in a dose-dependent manner. Preparations were incubated for 30, 120, and 240 min, and virtually identical results were obtained (the results for 120 min are shown in Fig. 3A). A measurable increase in the E. coli-induced TCC level in the fluid phase compared with the TCC level induced by incubation alone was induced by 107 to 0.5 × 108 bacteria/ml blood and higher (Fig. 3A).
FIG. 3.
E. coli-induced complement activation and effect of complement inhibitors. (A) Whole blood from two pigs was incubated at 37°C with different amounts of E. coli for 2 h. The readout was the amount of the TCC determined using an ELISA. The means of the two experiments are shown. For an explanation of T0 and T120 see the legend to Fig. 2. AU, arbitrary units. (B) Whole blood from two pigs was preincubated for 5 min with different doses (x axis) of VCP, anti-CD14, or HSA as a control. A fixed dose of 108 E. coli bacteria/ml whole blood was then added, and the samples were incubated for 30 min at 37°C. The readout was the amount of the TCC determined using an ELISA. The means and ranges of two separate experiments are shown. (C) Porcine whole blood was preincubated with VCP, SPICE, and a control (HSA) for 5 min. A fixed dose of 108 E. coli bacteria/ml whole blood was then added, and the samples were incubated for 30 min at 37°C. The readout was the amount of TCC determined using an ELISA. The results of one representative experiment are shown.
Based on the experiments whose results are shown in Fig. 3A, a fixed dose of 108 E. coli cells/ml whole blood incubated for 30 min was chosen for the complement inhibition experiments.
VCP reduced soluble TCC formation in a dose-dependent manner (Fig. 3B). There was complete inhibition at a VCP concentration of 100 μg/ml whole blood (Fig. 3B), but lower concentrations of VCP also markedly inhibited TCC formation. Thus, at a 16-fold-lower concentration (6.25 μg/ml) VCP inhibited TCC formation by approximately 40%. Neither anti-CD14 nor HSA had an inhibitory effect on TCC formation (Fig. 3B). VCP and SPICE were then compared in the same experiment, and the results showed that SPICE was more potent than VCP at lower concentrations (Fig. 3C). At a concentration of 6.25 μg/ml, SPICE was approximately five times more potent for inhibiting porcine soluble TCC formation, but at the highest concentration both agents completely inhibited complement activation.
Effect of complement and CD14 inhibition on E. coli-induced wCD11R3 upregulation on porcine granulocytes.
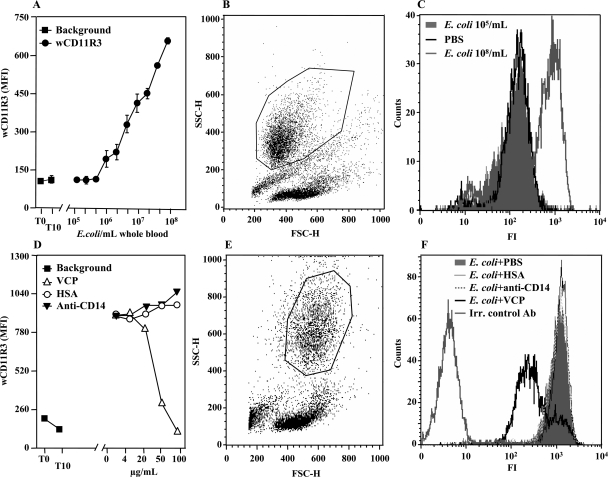
E. coli upregulated expression of the integrin chain wCD11R3 on porcine granulocytes in a dose-dependent manner (Fig. 4A). The upregulation was induced at E. coli concentrations greater than 106 cells/ml whole blood. Blood incubated with PBS did not show any wCD11R3 upregulation (Fig. 4A and C). The wCD11R3 upregulation was very prominent at an E. coli concentration of 108 cells/ml whole blood (Fig. 4A and C). Gating of the granulocytes studied here is shown in a forward scatter-side scatter plot in Fig. 4B. VCP inhibited E. coli-induced wCD11R3 upregulation in a dose-dependent manner (Fig. 4D). The upregulation was virtually abolished at a concentration of 100 μg/ml (Fig. 4D and F). Anti-CD14 and HSA did not have inhibitory effects on the upregulation (Fig. 4D and F). Gating of the granulocytes studied here is shown in a forward scatter-side scatter plot in Fig. 4E.
FIG. 4.
Effect of complement and CD14 inhibition on wCD11R3 expression on granulocytes. (A) Different amounts of E. coli were added to porcine whole blood (n = 2) and incubated for 10 min at 37°C. The samples were analyzed for wCD11R3 expression using a FACScan flow cytometer. The means and ranges of two separate experiments are shown. MFI, median fluorescence intensity. (B) Gating of the granulocytes as shown by a forward scatter-side scatter plot. (C) Histogram showing the shift of the fluorescence intensity (FI) from 105 E. coli bacteria/ml (filled area) or PBS alone to 108 E. coli bacteria/ml. (D) Whole blood from two pigs was preincubated for 5 min with VCP, anti-CD14, or HSA as a control. A fixed dose of 108 E. coli bacteria/ml whole blood was then added and incubated for 10 min at 37°C. The samples were analyzed to determine wCD11R3 expression using a FACScan flow cytometer. The results of one of two virtually identical experiments are shown. (E) Gating of the granulocytes, as shown in a forward scatter-side scatter plot. (F) Histogram showing the shift of fluorescence intensity from 108 E. coli cells/ml with PBS (filled area), with HSA, and with anti-CD14 to VCP. The results for an irrelevant detection control antibody (Irr. control Ab) are also indicated.
Effect of anti-CD14 and SPICE on E. coli CFU.
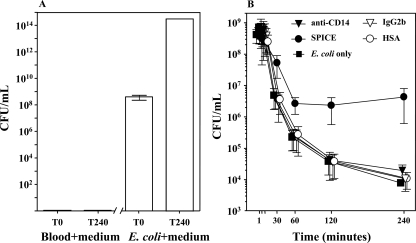
No CFU were detected in whole blood incubated with growth medium for 240 min; however, when E. coli was incubated in growth medium for 240 min, there was a substantial increase in the number of CFU, which reached levels 6 logs higher than the baseline levels (Fig. 5A).
FIG. 5.
Effect of anti-CD14 and SPICE on E. coli CFU. (A) Whole blood from two pigs was incubated with bacterial growth medium, and samples were taken at time zero (T0) and after 240 min (T240) (negative control). E. coli (108 bacteria/ml whole blood) was incubated in bacterial growth medium, and samples were obtained at time zero and after 240 min (positive control). (B) Whole blood from two pigs was preincubated for 5 min with SPICE or anti-CD14, using HSA and an isotype-matched monoclonal antibody (IgG2b), respectively, as the controls. A fixed dose of 108 E. coli bacteria/ml whole blood was then added and incubated for 240 min at 37°C. The data are the means and ranges (n = 2).
Whole blood incubated with E. coli alone for up to 240 min cleared bacteria at concentrations from approximately 108 at time zero to 8 × 103 CFU/ml after 240 min (Fig. 5B). SPICE inhibited the bacterial clearance, in contrast to anti-CD14 and the controls, which had no effect compared to E. coli alone (Fig. 5B).
Effect of anti-CD14 and SPICE on E. coli-induced cytokine production.
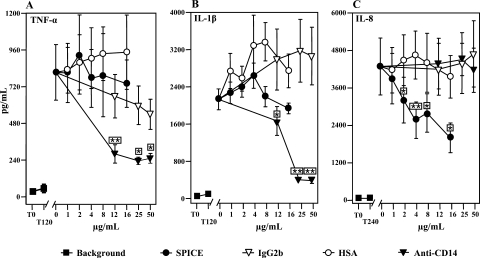
Anti-CD14 inhibited the E. coli-induced proinflammatory cytokines TNF-α (Fig. 6A) and IL-1β (Fig. 6B) significantly and in a dose-dependent manner compared to the isotype-matched control. The level of TNF-α was significantly reduced (P < 0.01) at the lowest concentration used (12.5 μg/ml). The maximal inhibition compared to E. coli alone was 71% inhibition at a concentration of 25 μg/ml. The level of IL-1β was significantly reduced (P < 0.05) at the lowest concentration used (12.5 μg/ml). The maximal inhibition compared to E. coli alone was 81% inhibition at a concentration of 50 μg/ml. In contrast, IL-8 was not inhibited when anti-CD14 was used (Fig. 6C).
FIG. 6.
Effect of SPICE and anti-CD14 on E. coli-induced cytokine production. Whole blood from seven pigs (A and C) and from six pigs (B) was preincubated for 5 min with SPICE or anti-CD14, using HSA and an isotype-matched monoclonal antibody (IgG2b), respectively, as the controls. A fixed dose of 105 E. coli bacteria/ml whole blood was then added and incubated for 2 h (TNF-α and IL-1β) or 4 h (IL-8) at 37°C. The data are the means ± standard errors of the means. A paired-sample t test was used to determine statistical significance (*, P < 0.05; **, P < 0.01). For an explanation of T0, T120, and T240 see the legend to Fig. 2.
SPICE had no effect on TNF-α or IL-1β production (Fig. 6A and B), but it inhibited IL-8 significantly and in a dose-dependent manner compared to HSA (Fig. 6C). Concentrations as low as 2 μg/ml significantly reduced IL-8 production (P < 0.05). The maximal inhibition compared to E. coli alone was 53% inhibition at a concentration of 16 μg/ml.
DISCUSSION
The data described here show for the first time a differentialy effect of inhibiting CD14/TLR4/MD-2 and complement in E. coli-induced inflammation in porcine whole blood. The findings support the hypothesis that these two upstream systems are both pivotal for induction of the inflammatory response. The rationale for inhibiting both the CD14/TLR and complement systems involves blocking not only exogenous microbial ligands but various endogenous ligands as well (24). The main exogenous ligand for the CD14/TLR4/MD-2 complex is LPS, whereas a number of endogenous ligands have recently been described (9, 37). Similarly, complement recognizes not only microbial ligands but also endogenous structures. Interestingly, we recently demonstrated that the inflammatory response induced by meconium, containing no bacteria and having a low LPS content, was completely attenuated by inhibition of complement and CD14 (31). Evidence suggests that “pattern recognition” and “danger signaling” might be important both in the protection of the host against external microbes and in the maintenance of tissue homeostasis, the latter of which contributes to tissue damage during inflammation. Inhibition of complement and CD14 might therefore attenuate the combined exogenous and endogenous activation of the innate immune system in conditions like gram-negative sepsis.
The anti-CD14 clone MIL-2 was initially described as a clone that bound to a cell surface molecule which was moderately expressed on granulocytes and strongly expressed on monocytes/macrophages in porcine blood (12). Later, the target of the monoclonal antibody was found to be porcine CD14 (39). The Third Swine CD Workshop determined that anti-CD14 MIL-2 inhibited binding of LPS (39). In the present study, we showed that anti-CD14 MIL-2 bound to and saturated porcine granulocyte CD14 in a dose-dependent manner. Furthermore, anti-CD14 MIL-2 completely inhibited LPS-induced proinflammatory cytokine/chemokine production in porcine whole blood. At a dose of 50 μg anti-CD14/ml whole blood the inhibition was complete. Ultrapure (phenol-extracted) E. coli LPS was used, which is known to activate specifically through the CD14/TLR4/MD-2 complex and not through TLR2, with which crude LPS preparations are known to react through contaminants like lipoproteins (13, 20, 38). Anti-CD14 inhibited E. coli-induced release of TNF-α and IL-1β, but not IL-8, which was significantly reduced by complement inhibition. This was in contrast to the results for LPS-induced IL-8, which anti-CD14 inhibited completely.
Two complement inhibitors were used in this study. VCP is a protein that is secreted by vaccinia virus-infected mammalian cells. It is structurally related to C4b-binding protein and is functionally closely related to CR1. It binds to C3b and C4b in the rodent and human complement cascade and thereby arrests the C3 convertases (15, 19). Several previous studies have demonstrated the beneficial effects of VCP as a complement inhibitor in rodent disease models, such as collagen-induced arthritis (16) and atherosclerosis (40). Recently, we showed that VCP is an efficient inhibitor of complement activation in porcine serum by several known complement activators (41). In the present study we first tested the ability of VCP to inhibit complement in porcine whole blood and found that VCP efficiently and in a dose-dependent manner inhibited E. coli-induced complement activation. SPICE is a protein that is secreted by variola virus-infected human cells. Since smallpox was eradicated in 1977 and live variola virus is not available for study, only molecularly engineered SPICE is available (30). SPICE closely resembles VCP; only 11 of 244 amino acids (approximately 4.5%) are different (30, 33, 49). It was shown previously that SPICE was 100-fold more potent than VCP for inactivation of human C3b (30). A recent study showed that just a few amino acid changes in the VCP molecule may be responsible for this functional difference (48). Interestingly, SPICE was only fivefold more potent than VCP in inhibiting porcine complement, with the reservation that our readout was TCC and not C3b.
Interestingly, the complement inhibitor SPICE reduced bacterial clearance from porcine whole blood. This is not surprising and can be explained by reduced opsonization, phagocytosis, and bacterial killing, in which complement is known to play an essential role (5). In contrast, anti-CD14 had no influence on bacterial clearance, indicating that complement is more important than CD14 for bacterial killing in this setting. Therefore, appropriate antibacterial therapy must be established before complement is inhibited. Since the host defense system is a double-edged sword, a combined strategy involving antimicrobial treatment and attenuation of the host defense, including complement and CD14, might be a rational strategy for treatment of sepsis.
CD11b in complex with CD18 forms the CR3 receptor in humans. CR3 is a β2 integrin and is the principal receptor involved in the phagocytosis of iC3b-coated bacteria. Upregulation of CD11b/CR3 on human granulocytes has previously been shown to be complement dependent (23, 35). Porcine wCD11R3, although slightly smaller than the human α chain of CR3 (155 kDa instead of 165 kDa [8]), is also associated with CD18 and has the same cellular distribution in pigs that CD11b has in humans (8). We found that wCD11R3 on porcine granulocytes was upregulated by E. coli in a dose-dependent manner. Based on these findings, the ability of VCP to inhibit this upregulation was tested. E. coli-induced wCD11R3 upregulation was inhibited by VCP in a dose-dependent manner, and at a concentration of 100 μg VCP/ml whole blood the upregulation was inhibited to background values. These findings indicate that the E. coli-induced upregulation of porcine wCD11R3 is complement dependent, as previously described for CD11b in humans (5).
In summary, inhibition of CD14 and complement was shown to differentially attenuate the E. coli-induced inflammatory response in porcine whole blood. The results obtained with the Orthopoxvirus complement inhibitors VCP and SPICE showed that the wCD11R3 expression induced on granulocytes was completely dependent on complement and that IL-8 production was markedly reduced due to complement inhibition, but this inhibition had no effect on TNF-α or IL-1β. In contrast, the inflammatory cytokines TNF-α and IL-1β were to a large extent CD14 dependent. Furthermore, anti-CD14 inhibited IL-8 induced by LPS, whereas there was no effect on the production induced by whole bacteria. This finding emphasizes the importance of including whole bacteria and not only LPS when the effect of gram-negative bacteria on innate immune responses is studied.
Altogether, these data strengthen the hypothesis that inhibition of complement and CD14/TLR4/MD-2, two main upstream initiators of gram-negative bacterium-induced inflammation, is a rational approach for attenuating inflammation. Further in vivo studies in the pig could provide further support for this hypothesis.
Acknowledgments
This work was supported by National Institutes of Health grant R01 EB003968-01A1, by Helse Sor-Ost grant 2008036, by The Norwegian Research Council, by The Norwegian Council on Cardiovascular Disease, by The Odd Fellow Foundation, by The Research Council of Rikshospitalet, and by The Family Blix Foundation.
We thank Julie Lindstad for excellent laboratory technical assistance and Dorte Christiansen for growing and preparing the bacteria. We thank Adrian Smith, Harry Hjelmseth, and Frank Sundby of The Norwegian Centre for Laboratory Animal and Alternatives, Norwegian School of Veterinary Science, Oslo, Norway, for help with blood sampling and for housing the animals.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. [DOI] [PubMed] [Google Scholar]
- 2.Arumugam, T. V., I. A. Shiels, T. M. Woodruff, D. N. Granger, and S. M. Taylor. 2004. The role of the complement system in ischemia-reperfusion injury. Shock 21401-409. [DOI] [PubMed] [Google Scholar]
- 3.Barboni, B., M. Turriani, G. Galeati, M. Spinaci, M. L. Bacci, M. Forni, and M. Mattioli. 2000. Vascular endothelial growth factor production in growing pig antral follicles. Biol. Reprod. 63858-864. [DOI] [PubMed] [Google Scholar]
- 4.Barton, G. M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 3001524-1525. [DOI] [PubMed] [Google Scholar]
- 5.Brekke, O. L., D. Christiansen, H. Fure, M. Fung, and T. E. Mollnes. 2007. The role of complement C3 opsonization, C5a receptor, and CD14 in E. coli-induced up-regulation of granulocyte and monocyte CD11b/CD18 (CR3), phagocytosis, and oxidative burst in human whole blood. J. Leukoc. Biol. 811404-1413. [DOI] [PubMed] [Google Scholar]
- 6.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 27410689-10692. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420885-891. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez, J., B. Alvarez, F. Alonso, E. Thacker, K. Haverson, K. McCullough, A. Summerfield, and A. Ezquerra. 2001. Workshop studies on monoclonal antibodies in the myeloid panel with CD11 specificity. Vet. Immunol. Immunopathol. 80111-119. [DOI] [PubMed] [Google Scholar]
- 9.Gay, N. J., and M. Gangloff. 2007. Structure and function of Toll receptors and their ligands. Annu. Rev. Biochem. 76141-165. [DOI] [PubMed] [Google Scholar]
- 10.Ghebremariam, Y. T., O. O. Odunuga, K. Janse, and G. J. Kotwal. 2005. Humanized recombinant vaccinia virus complement control protein (hrVCP) with three amino acid changes, H98Y, E102K, and E120K creating an additional putative heparin binding site, is 100-fold more active than rVCP in pageing both classical and alternative complement pathways. Ann. N. Y. Acad. Sci. 1056113-122. [DOI] [PubMed] [Google Scholar]
- 11.Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cell. Signal. 1385-94. [DOI] [PubMed] [Google Scholar]
- 12.Haverson, K., M. Bailey, V. R. Higgins, P. W. Bland, and C. R. Stokes. 1994. Characterization of monoclonal antibodies specific for monocytes, macrophages and granulocytes from porcine peripheral blood and mucosal tissues. J. Immunol. Methods 170233-245. [DOI] [PubMed] [Google Scholar]
- 13.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 165618-622. [DOI] [PubMed] [Google Scholar]
- 14.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1623749-3752. [PubMed] [Google Scholar]
- 15.Jha, P., and G. J. Kotwal. 2003. Vaccinia complement control protein: multi-functional protein and a potential wonder drug. J. Biosci. 28265-271. [DOI] [PubMed] [Google Scholar]
- 16.Jha, P., S. A. Smith, D. E. Justus, and G. J. Kotwal. 2005. Vaccinia virus complement control protein ameliorates collagen-induced arthritic mice. Ann. N. Y. Acad. Sci. 105655-68. [DOI] [PubMed] [Google Scholar]
- 17.Kohl, J. 2001. Anaphylatoxins and infectious and non-infectious inflammatory diseases. Mol. Immunol. 38175-187. [DOI] [PubMed] [Google Scholar]
- 18.Kotwal, G. J., S. N. Isaacs, R. McKenzie, M. M. Frank, and B. Moss. 1990. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250827-830. [DOI] [PubMed] [Google Scholar]
- 19.Kotwal, G. J., and B. Moss. 1988. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature 335176-178. [DOI] [PubMed] [Google Scholar]
- 20.Kurt-Jones, E. A., L. Mandell, C. Whitney, A. Padgett, K. Gosselin, P. E. Newburger, and R. W. Finberg. 2002. Role of Toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood 1001860-1868. [PubMed] [Google Scholar]
- 21.Marshall, J. C. 2003. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat. Rev. Drug Discov. 2391-405. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388394-397. [DOI] [PubMed] [Google Scholar]
- 23.Mollnes, T. E., O. L. Brekke, M. Fung, H. Fure, D. Christiansen, G. Bergseth, V. Videm, K. T. Lappegard, J. Kohl, and J. D. Lambris. 2002. Essential role of the C5a receptor in E. coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 1001869-1877. [PubMed] [Google Scholar]
- 24.Mollnes, T. E., D. Christiansen, O. L. Brekke, and T. Espevik. 2008. Hypothesis: combined inhibition of complement and CD14 as treatment regimen to attenuate the inflammatory response. Adv. Exp. Med. Biol. 632253-263. [PubMed] [Google Scholar]
- 25.Mollnes, T. E., H. Redl, K. Hogasen, A. Bengtsson, P. Garred, L. Speilberg, T. Lea, M. Oppermann, O. Gotze, and G. Schlag. 1993. Complement activation in septic baboons detected by neoepitope-specific assays for C3b/iC3b/C3c, C5a and the terminal C5b-9 complement complex (TCC). Clin. Exp. Immunol. 91295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan, B. P. 1989. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem. J. 2641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan, B. P. 1999. Regulation of the complement membrane attack pathway. Crit. Rev. Immunol. 19173-198. [PubMed] [Google Scholar]
- 28.Murthy, K. H., S. A. Smith, V. K. Ganesh, K. W. Judge, N. Mullin, P. N. Barlow, C. M. Ogata, and G. J. Kotwal. 2001. Crystal structure of a complement control protein that regulates both pathways of complement activation and binds heparan sulfate proteoglycans. Cell 104301-311. [DOI] [PubMed] [Google Scholar]
- 29.Nagai, Y., S. Akashi, M. Nagafuku, M. Ogata, Y. Iwakura, S. Akira, T. Kitamura, A. Kosugi, M. Kimoto, and K. Miyake. 2002. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3667-672. [DOI] [PubMed] [Google Scholar]
- 30.Rosengard, A. M., Y. Liu, Z. Nie, and R. Jimenez. 2002. Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc. Natl. Acad. Sci. USA 998808-8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvesen, B., M. Fung, O. D. Saugstad, and T. E. Mollnes. 2008. Role of complement and CD14 in meconium-induced cytokine formation. Pediatrics 121e496-e505. [DOI] [PubMed] [Google Scholar]
- 32.Schumann, R. R., S. R. Leong, G. W. Flaggs, P. W. Gray, S. D. Wright, J. C. Mathison, P. S. Tobias, and R. J. Ulevitch. 1990. Structure and function of lipopolysaccharide binding protein. Science 2491429-1431. [DOI] [PubMed] [Google Scholar]
- 33.Sfyroera, G., M. Katragadda, D. Morikis, S. N. Isaacs, and J. D. Lambris. 2005. Electrostatic modeling predicts the activities of orthopoxvirus complement control proteins. J. Immunol. 1742143-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 1891777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprong, T., P. Brandtzaeg, M. Fung, A. M. Pharo, E. A. Hoiby, T. E. Michaelsen, A. Aase, J. W. van der Meer, D. M. van, and T. E. Mollnes. 2003. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 1023702-3710. [DOI] [PubMed] [Google Scholar]
- 36.Strainic, M. G., J. Liu, D. Huang, F. An, P. N. Lalli, N. Muqim, V. S. Shapiro, G. R. Dubyak, P. S. Heeger, and M. E. Medof. 2008. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 28425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang, A. H., G. J. Brunn, M. Cascalho, and J. L. Platt. 2007. Pivotal advance: endogenous pathway to SIRS, sepsis, and related conditions. J. Leukoc. Biol. 82282-285. [DOI] [PubMed] [Google Scholar]
- 38.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 1655780-5787. [DOI] [PubMed] [Google Scholar]
- 39.Thacker, E., A. Summerfield, K. McCullough, A. Ezquerra, J. Dominguez, F. Alonso, J. Lunney, J. Sinkora, and K. Haverson. 2001. Summary of workshop findings for porcine myelomonocytic markers. Vet. Immunol. Immunopathol. 8093-109. [DOI] [PubMed] [Google Scholar]
- 40.Thorbjornsdottir, P., R. Kolka, E. Gunnarsson, S. H. Bambir, G. Thorgeirsson, G. J. Kotwal, and G. J. Arason. 2005. Vaccinia virus complement control protein diminishes formation of atherosclerotic lesions: complement is centrally involved in atherosclerotic disease. Ann. N. Y. Acad. Sci. 10561-15. [DOI] [PubMed] [Google Scholar]
- 41.Thorgersen, E. B., Y. T. Ghebremariam, J. M. Thurman, M. Fung, E. W. Nielsen, V. M. Holers, G. J. Kotwal, and T. E. Mollnes. 2007. Candidate inhibitors of porcine complement. Mol. Immunol. 441837-1844. [DOI] [PubMed] [Google Scholar]
- 42.Ulevitch, R. J., and P. S. Tobias. 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13437-457. [DOI] [PubMed] [Google Scholar]
- 43.van Bruggen, R., D. Zweers, A. van Diepen, J. T. van Dissel, D. Roos, A. J. Verhoeven, and T. W. Kuijpers. 2007. Complement receptor 3 and Toll-like receptor 4 act sequentially in uptake and intracellular killing of unopsonized Salmonella enterica serovar Typhimurium by human neutrophils. Infect. Immun. 752655-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walport, M. J. 2001. Complement. First of two parts. N. Engl. J. Med. 3441058-1066. [DOI] [PubMed] [Google Scholar]
- 45.Ward, P. A. 2004. The dark side of C5a in sepsis. Nat. Rev. Immunol. 4133-142. [DOI] [PubMed] [Google Scholar]
- 46.Welch, T. R. 2002. Complement in glomerulonephritis. Nat. Genet. 31333-334. [DOI] [PubMed] [Google Scholar]
- 47.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 2491431-1433. [DOI] [PubMed] [Google Scholar]
- 48.Yadav, V. N., K. Pyaram, J. Mullick, and A. Sahu. 2008. Identification of hot spots in the variola virus complement inhibitor (SPICE) for human complement regulation. J. Virol. 823283-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, L., and D. Morikis. 2006. Immunophysical properties and prediction of activities for vaccinia virus complement control protein and smallpox inhibitor of complement enzymes using molecular dynamics and electrostatics. Biophys. J. 903106-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, X., Y. Kimura, C. Fang, L. Zhou, G. Sfyroera, J. D. Lambris, R. A. Wetsel, T. Miwa, and W. C. Song. 2007. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood 110228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]