Abstract
The rabbit model of tuberculosis is attractive because of its pathophysiologic resemblance to the disease in humans. Rabbits are naturally resistant to infection but may manifest cavitary lung lesions. We describe here a novel approach that utilizes presensitization and bronchoscopic inoculation to reliably produce cavities in the rabbit model. With a fixed inoculum of bacilli, we were able to reproducibly generate cavities by using Mycobacterium bovis Ravenel, M. bovis AF2122, M. bovis BCG, M. tuberculosis H37Rv, M. tuberculosis CDC1551, and the M. tuberculosis CDC1551 ΔsigC mutant. M. bovis infections generated cavitary CFU counts of 106 to 109 bacilli, while non-M. bovis species and BCG yielded CFU counts that ranged from 104 to 108 bacilli. Extrapulmonary dissemination was almost exclusively noted among rabbits infected with M. bovis Ravenel and AF2122. Though all of the species yielded secondary lesions at intrapulmonary sites, M. bovis infections led to the most apparent gross pathology. Using the M. tuberculosis icl and dosR gene expression patterns as molecular sentinels, we demonstrated that both the cavity wall and cavity lumen are microenvironments associated with hypoxia, upregulation of the bacterial dormancy program, and the use of host lipids for bacterial catabolism. This unique cavitary model provides a reliable animal model to study cavity pathogenesis and extrapulmonary dissemination.
Tuberculosis (TB) continues to be a global public health problem, with approximately one-third of the world's population latently infected (14). Nine million active infections are diagnosed annually, and approximately two million people died of the disease last year (33). Cavitary pulmonary lesions are key means of disease transmission (1, 21). The lesions present an ideal environment for tremendous bacillary growth, with up to 107 to 109 organisms being able to be routinely cultured from a single cavity (4). High titers of bacilli also lead to an increased likelihood of antimicrobial resistance due to spontaneous mutations (11).
For these reasons, animal models of cavity formation and TB disease transmission have been sought. The murine model is limited because mice form cellular granuloma-like lesions which usually lack necrosis, caseation, and cavitation (16). Guinea pigs form necrotic granulomas with caseation but rarely produce cavitary lesions after aerosol Mycobacterium tuberculosis exposure (27). However, rabbits form caseating, necrotic granulomas which, under the correct conditions, may liquefy and cavitate.
Early investigations by Wells and Lurie yielded cavity formation in rabbits presensitized with heat-killed M. bovis and challenged with aerosolized low-dose M. bovis (32). The importance of presensitization was further elucidated by Ratcliffe and Wells in their demonstration that more numerous cavities develop after reinfection with high-dose M. bovis following an initial primary low-dose aerosolized infection (23). Yamamura et al. reliably produced lung cavities in rabbits 30 to 60 days after intrathoracic injection of live or heat-killed mycobacteria when the rabbits were sensitized by repeated subcutaneous injections of heat-killed bacilli (34).
The present report details a novel rabbit model that incorporates several classical approaches to reliably generate cavities in a short time. Alternative means of infection (i.e., aerosol exposure) have been noted in the literature to variably create cavities and require a prolonged formation time (8, 12, 34). The experiments described herein show that cavities can be generated in 6 to 15 weeks via bronchoscopic infection after a standard period of presensitization. This model yielded cavity formation with virulent and attenuated strains of mycobacteria. Gross pathology, histopathology, CFU counts of lung and extrapulmonary lesions, and M. tuberculosis gene expression patterns were explored in selected rabbits.
MATERIALS AND METHODS
Microorganisms.
Cultures for bronchoscopic infection were prepared by thawing frozen stock aliquots of M. bovis Ravenel, M. bovis AF2122, M. bovis BCG, M. tuberculosis H37Rv, M. tuberculosis CDC1551, and the M. tuberculosis CDC1551 ΔsigC mutant. The M. tuberculosis CDC1551 ΔsigC mutant is a gene replacement mutant that showed a modest degree of attenuation in the mouse model (28). Mycobacteria were grown in 7H9 Middlebrook liquid medium supplemented with oleic acid albumin, dextrose, and catalase (Becton Dickinson, Inc., Sparks, MD), 0.5% glycerol, and 0.05% Tween 80. The glycerol-containing medium, as opposed to a pyruvate carbon source, was not found to limit the growth of M. bovis strains.
Animals and infection.
Thirty pathogen-free outbred New Zealand White rabbits (2.5 to 3.5 kg) were obtained from Covance Research Products, Inc. (Denver, PA). Animals were maintained in standard cages under biosafety level 3 conditions. All animals were maintained in accordance with protocols approved by the Institutional Animal Care and Use Committees of Johns Hopkins University. Sensitization was performed by administration of five subcutaneous injections of 107 heat-killed M. bovis cells in incomplete Freund's adjuvant 3 to 4 days apart. An intradermal skin test with 0.1 ml of Old Tuberculin (Synbiotics Corp., Kansas City, MO) was given 25 days after the last sensitization injection. Old Tuberculin, as opposed to purified protein derivative, was chosen secondary to the enhanced ability to detect delayed-type hypersensitivity (DTH) in rabbits. The tuberculin reaction was read 48 to 72 h later to confirm successful acquisition of DTH immunity, measurements of skin fold thickness were taken in two dimensions, and the results were calculated by using the formula for the volume of an oval spheroid. Rabbits were anesthetized with xylazine (5 to 10 mg/kg) and ketamine (15 to 25 mg/kg). Yohimbine (0.1 to 0.2 mg/kg) was used to reverse excessive sedation. A 3.0-mm flexible Pentax FB-8V pediatric bronchoscope (Pentax Medical Company, Montvale, NJ) was wedged into the right basal lobe of the lung. A total of 0.3 ml of a bacillary suspension containing 103 to 104 CFU was delivered via the bronchoscope insertion port. Confirmation of the number of CFU delivered was done via plating of serial dilutions of the inoculated suspension.
Clinical assessment.
After infection, the rabbits were monitored twice weekly for clinical appearance, weight, and rectal temperature.
Necropsy.
Rabbits were observed for a minimum of 50 days and a maximum of 105 days after infection. Criteria for euthanasia included signs of respiratory distress (dyspnea) and/or significant loss of weight (150 to 200 g). Rabbits were euthanized with intravenous Euthasol (Virbac Corporation, Fort Worth, TX). At necropsy, samples were obtained from the lungs and extrapulmonary sites. Cavity specimens that represented the primary lesion included the (i) lumen contents, (ii) wall, and (iii) surrounding inflammatory tissue. Grossly visible secondary lesions of the ipsilateral lung, contralateral lung, and extrapulmonary sites were noted. Extrapulmonary locations included the (i) lymph nodes (mediastinal, thymus, Peyer's patches in the small intestine, and mesentery), (ii) spleen, (iii) liver, (iv) kidney (bilateral), (v) ovary, (vi) adrenal, (vii) bone marrow, and (viii) cecum.
Scoring of gross pathology and cavity histopathology.
At necropsy, grossly visible pulmonary primary lesions in the right lower lobe and secondary lesions in the ipsilateral and contralateral lungs were scored according to their prevalence. Grossly visible extrapulmonary lesions were scored according to both prevalence and location. Paraffin-embedded tissue sections were stained with hematoxylin and eosin and by the Ziehl-Neelsen carbol-fuchsin method (Becton Dickinson and Company, Franklin Lakes, NJ). All slides were examined with a Nikon Microscope Eclipse E800 (Nikon Instruments Inc., Melville, NY).
Determination of bacterial counts.
CFU counts were measured at all of the predetermined pulmonary and extrapulmonary sites of each rabbit infected. Tissue samples from each site were homogenized, and aliquots were plated on selective 7H11 agar supplemented with oleic acid albumin, dextrose, and catalase. CFU counts were enumerated on days 14, 21, and 28.
RNA isolation and transcript analysis.
For transcriptional analyses, total RNA was isolated from scrapings of caseating lesions (homogenized inner cavitary contents), fragments of the cavitary wall (homogenized segment of the fibrous cavitary wall) of rabbits infected with M. tuberculosis CDC1551 or M. bovis AF2122, and in vitro-grown log-phase (A600 of 0.8) and stationary-phase (A600 of 2.0) cultures of the two organisms by the Trizol method according to the supplier's instructions (Invitrogen Corporation, Carlsbad, CA). Enrichment for bacterial RNA isolated from tissues of infected animals was carried out with the MICROBEnrich kit (Ambion Inc., Austin, TX) in accordance with the manufacturer's protocol. To quantify the level of expression of each transcript, RNA was treated with RNase-free DNase (Ambion), and 1 μg of RNA was subjected to reverse transcription with the iScript cDNA synthesis kit (Bio-Rad Laboratories). This was followed by real-time quantitative PCR with the SYBR Green Supermix (Bio-Rad Laboratories) and gene-specific primers (dosR-F [5′ GAGCTTGACGTCGTAGGTGA 3′], dosR-R [5′ TCTAGCATGGCCTCGTCAG 3′], icl-F [5′ AGATGCTGGCCTACAACTGC 3′], and icl-R [5′ GACATACGCGCTCATCTGGT 3′]). The n-fold differences in transcript levels were derived by comparing the cycle threshold values of the test samples with those of samples from cultures grown to log phase, following normalization to the M. tuberculosis sigA transcript (amplified with primers sigA-F (5′ CGATGACGACGAGGAGATCGC 3′) and sigA-R (5′ CAGCGCTACCTTGCCGATCTG 3′).
Statistical analysis.
Data are reported as mean values unless otherwise stated. Mean paired values of intrathoracic sites were compared to cavity center values by two-tailed t tests. The level of significance was set at P < 0.10.
RESULTS
Reproducibility of rabbit lung cavity formation following presensitization and bronchoscopic infection.
All rabbits were sensitized with heat-killed M. bovis, and all except one converted their tuberculin skin tests to positive prior to infection. The single nonconverter was a rabbit subsequently infected with the M. tuberculosis CDC1551 ΔsigC mutant (SC2); despite a negative DTH skin reaction, this rabbit successfully generated a cavity.
Rabbits were sacrificed at various times after infection (range = 30 to 105 days). All of the strains of mycobacteria studied reliably produced lung cavities at the site of initial infection in the right basal lobe via bronchoscopy. The largest cavities occurred at the primary site of infection. Despite an approximately threefold range of inoculation titers (range = 5,000 to 18,000 bacilli), cavity formation of similar gross pathology and histopathology was observed. Notably, cavity formation could be elicited in every rabbit infected with the attenuated ΔsigC mutant. Each rabbit formed cavities, with the exceptions of a single rabbit infected with M. bovis AF2122 and one infected with M. tuberculosis CDC1551, both of which formed multiple coalescing granulomas at their site of inoculation. One rabbit infected with M. tuberculosis H37Rv died prematurely, and the dimensions of its cavity were difficult to accurately discern.
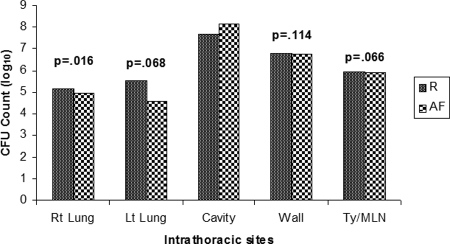
Histopathology revealed that the most numerous acid-fast bacterial burdens were evident in the central portion of the cavity (Fig. 1). With the exception of the cavity wall, which contained portions of the liquefied caseum, the cavity center contained the highest CFU count compared to any other intrathoracic site. This paradigm held true for both M. bovis-infected and non-M. bovis-infected rabbits. A notable exception includes M. bovis BCG-infected rabbits, which had notable bacilli in both the cavitary center and wall. Moreover, the gross pathology revealed the most striking abnormalities within the right lower lobe at the site of the primary lesions (Fig. 2). Cavitary necrosis was most obvious in M. bovis-infected rabbits, but central caseation and liquefaction were evident in all of the lung cavities formed. Phenotypic differences in the central caseation were also noted among M. bovis infections that produced a more fragile and less homogeneous caseum on gross inspection.
FIG. 1.
CFU counts at intrathoracic sites. Abbreviations: Rt Lung, right lung parenchyma; Lt Lung, left lung parenchyma; Cavity, center of cavitary lesion; Wall, wall of cavitary lesion; Ty/MLN, thymus and mediastinal lymph nodes; R, Ravenel; AF, AF2122. The thymus and mediastinal lymph nodes were classified together because of the difficulty in separating these closely located anatomical sites on dissection. P values are based on average CFU counts at each intrathoracic site compared to the cavity center.
FIG. 2.
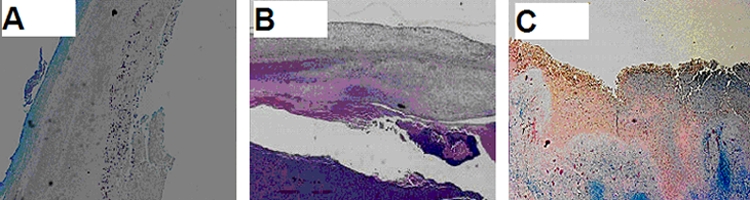
Histopathology of selected cavitary rabbit tuberculous lesions. Staining of acid-fast bacilli in paraffin-embedded tissue sections of lung cavity specimens. Magnification, ×20. (A) M. bovis Ravenel-infected rabbit showing fibrosis at the edge of the cavity wall. Bacilli were evident solely at the lining of the central cavity. (B) M. tuberculosis H37Rv-infected rabbit displaying a fibrotic wall with bacilli in the liquefied center. (C) M. tuberculosis CDC1551 ΔsigC mutant-infected rabbit with necrotic and fibrotic areas throughout the cavity. Bacilli are visible in all parts of the cavitary lesion.
Clearly defined cavity walls with various amounts of necrosis and fibrosis were seen in each rabbit that produced cavities. Necropsy specimens obtained later in infection (>50 days) were more likely to fall into types C and D of Yamamura's spectrum of lung cavity classification: type A, necrosis or loss of tissue substances in the central part accompanied by no granulation tissue in the surrounding region; type B, caseation and liquefaction in the central part with beginning of fibroblastic proliferation around a lesion; type C, caseation and liquefaction in the central part accompanied by the fibroblastic proliferation and connective formation in surrounding tissue; type D, greater fibroblastic proliferation and connective tissue formation (35).
M. bovis uniquely generates extrapulmonary dissemination.
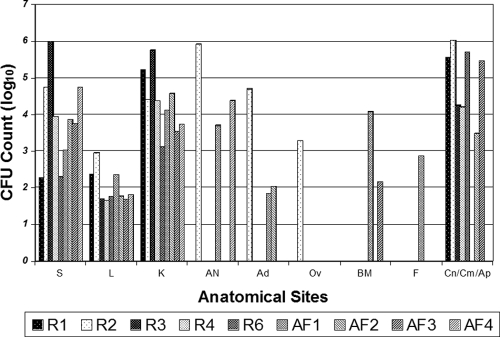
M. bovis Ravenel and AF2122 both uniquely disseminated to all observed intrapulmonary and extrapulmonary sites (Fig. 3). Despite the cavity formation seen when other mycobacterial species and strains were used, uniform spreading from primary lesions was seen only in rabbits infected with M. bovis. Although M. bovis Ravenel and AF2122 led to numerous visualizable extrapulmonary tuberculomas, splenic lesions were seen in only one M. bovis Ravenel-infected rabbit and two M. bovis AF2122-infected rabbits on gross pathology (Fig. 4). However, CFU counts were most abundant in the spleen and thus confirm the early extrapulmonary dissemination of tubercle bacilli through the blood and key reticuloendothelial organs (6). Visible tuberculomas and histopathologic granulomas were noted in the bilateral kidneys, cecum, and appendices. Few abdominal lesions were seen in the gastrointestinal tract, with the exception of the appendiceal/cecal area. A stomach wall granuloma was seen in a rabbit infected with M. bovis Ravenel and AF2122. CFU counts confirmed gross pathological findings, with elevated counts measured in the liver, kidney, and selected gastrointestinal sites (cecum and appendix). The organs and sites that showed the lowest CFU counts were the liver, ovaries, bone marrow, and feces.
FIG. 3.
CFU counts at extrapulmonary locations at necropsy. Single-animal CFU counts for the spleen (S), liver (L), kidney (K), abdominal lymph node (AN), adrenal gland (Ad), ovary (Ov), bone marrow (BM), feces (F), colon (Cn), cecum (Cm), and appendix (Ap) are shown. Results for rabbits infected with M. bovis Ravenel (R) and AF2122 (AF) are shown because these strains were most likely to result in extrapulmonary dissemination.
FIG. 4.
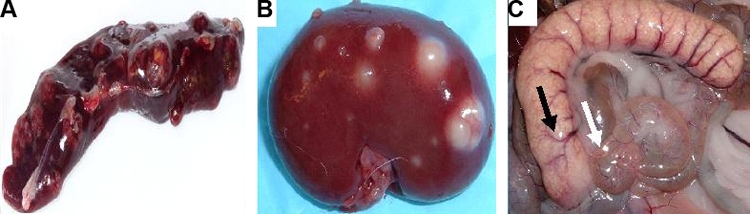
Gross pathology of extrapulmonary specimens on necropsy. (A) Spleen of a rabbit (AF4) infected with M. bovis AF2122. (B) Kidney of a rabbit (R1) infected with M. bovis Ravenel. (C) Appendiceal/cecal region of a rabbit (R3) infected with M. bovis Ravenel. The appendix (black arrow) and cecum (white arrow) are shown. Despite the cavity formation seen with other mycobacteria species and strains, only rabbits infected with M. bovis showed uniform spreading from primary lesions.
Limited extrapulmonary dissemination was noted at the gross pathology level among rabbits infected with M. tuberculosis and M. bovis BCG. Microbiology of CFU counts in non-M. bovis-infected rabbits corresponded relatively well to the absence of extrapulmonary gross pathology. Two notable exceptions included two M. tuberculosis CDC1551-infected rabbits that had CFU counts of 2- to 3-log units in the normal-appearing abdominal lymph nodes that were sampled. M. tuberculosis H37Rv also resulted in CFU counts of approximately 3-log units in a grossly unremarkable kidney of the single rabbit that died prematurely of pneumothorax.
Both M. bovis-infected and non-M. bovis-infected rabbits show intrapulmonary dissemination.
Multiple lesions were noted at pulmonary sites of the ipsilateral (right upper and middle lobes) and contralateral (left) lung segments of M. bovis-infected rabbits. CFU counts of the intrathoracic compartments confirmed gross pathological findings (Fig. 5). M. bovis-infected rabbits yielded greater than 3- to 4-log unit CFU counts at all selected pulmonary sites. M. bovis Ravenel- and AF2122-infected rabbits displayed similar CFU count profiles. The highest total CFU counts were measured in the inner cavitary contents, which yielded greater than 106 bacilli in M. bovis Ravenel-infected rabbits and greater than 107 bacilli in M. bovis AF2122-infected rabbits.
FIG. 5.
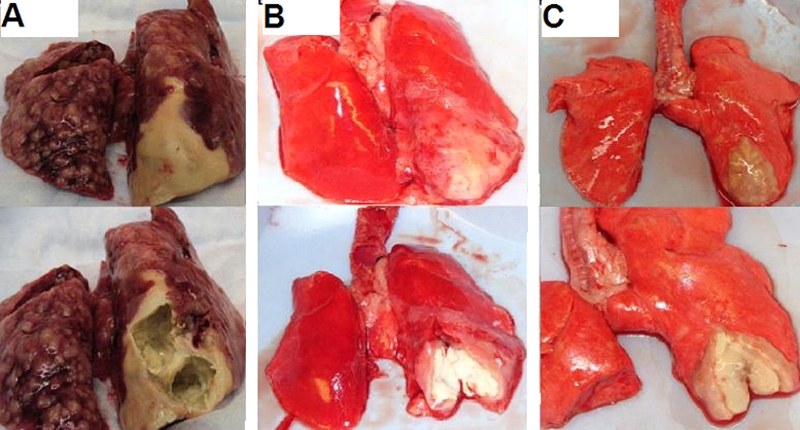
Gross pathology of selected lung specimens at necropsy. Specimens shown in the upper and lower parts of each panel are paired. The lower parts show dissected cavity lesions. Panels: A, M. bovis Ravenel, rabbit R6; B, M. tuberculosis H37Rv, rabbit H1; C, M. tuberculosis CDC1551 ΔsigC mutant, rabbit SC1. In each panel, the most significant cavitary areas are located at the primary site of infection, in the right lower lobe.
All non-M. bovis bacilli showed intrapulmonary dissemination to the ipsilateral and/or contralateral upper lobe lung segments. M. tuberculosis CDC1551 and H37Rv were the only organisms to spread to the thymus and mediastinal lymph nodes. Cavitary lesions found in non-M. bovis bacilli showed colony counts that ranged from 104 to 108 CFU. The highest CFU counts were found in the cavity centers within the softened “solid” caseum. Difficulty in dissecting the cavity wall from the inner cavity contents was also evident in these rabbits, and therefore the cavity CFU counts may have been partially reflective of both anatomical sites. Granulomas were also visualized inside a uniquely thick cavity wall (intramural cavity lesion) in two M. tuberculosis CDC1551-infected rabbits and three of four M. tuberculosis CDC1551 ΔsigC-infected rabbits. Despite the suspected decreased virulence, ΔsigC mutants were the only non-M. bovis bacilli to yield a majority of infections with this thick-walled pattern of gross pathology.
Gene expression of bacilli in rabbit cavitary lesions.
To define the microenvironment in the cavitary lesions, the transcriptional profiles of the Rv0467 (icl) and Rv3133c (dosR) genes were evaluated in RNA samples isolated from log-phase and early stationary-phase cultures of in vitro-grown M. tuberculosis CDC1551. The icl gene, which encodes isocitrate lyase, is involved in utilizing lipids as a carbon source and, along with its isoform icl2, is required for in vivo growth and virulence (19, 20). The two-component response regulator DosR is hypothesized to play a leading role in the adaptation of M. tuberculosis to hypoxia/anaerobic growth (22, 25). Both of these genes are involved in modulating functions believed to be closely associated with the establishment and maintenance of chronic and/or latent M. tuberculosis infection in the human host. Transcript levels of these genes were assessed in two rabbits infected with M. tuberculosis CDC1551 in both cavity luminal contents and wall samples. The in vivo expression levels were compared to those of in vitro-grown bacteria in log phase and stationary phase. As shown in Table 1, levels of both icl and dosR were significantly upregulated in the cavity and wall specimens. This observation suggests that the intragranulomatous milieu is hypoxic and also that the bacilli are expressing enzymes of the glyoxalate shunt, essential for carbon anaplerosis in the Krebs cycle during growth on substrates containing C2 units such as fatty acids. A modest increase in the levels of these transcripts was observed in the cavity and wall specimens obtained from M. bovis AF2122-infected rabbits as well (data not shown).
TABLE 1.
Bacterial gene expression in the cavity microenvironment
| Gene | Mean relative transcript level ± SEMa
|
|||
|---|---|---|---|---|
| Log-phase in vitro culture | Stationary-phase in vitro culture | Intraluminal contents of caseating cavity | Cavity wall | |
| icl | 1 | 0.86 ± 1.26 | 83.69 ± 40.3 | 25.56 ± 10.4 |
| dosR | 1 | 261.23 ± 171.9 | 14.48 ± 9.5 | 5.53 ± 4.13 |
Relative icl and dosR transcript levels in stationary-phase cultures, scrapings of the intraluminal contents of caseating cavities, and fragments of the granuloma walls of M. tuberculosis CDC1551-infected rabbits are shown. The transcript levels of the respective genes in log phase were assigned a value of 1. The results are representative of measurements from two infected rabbits, with at least two replicates in each set.
DISCUSSION
This report describes a reproducible animal model of TB cavity pathogenesis and disseminated secondary lesions. Patients with cavitary TB are the key contributors to TB transmission (13). In humans, cavitary disease has also been found to be an independent risk factor for disease relapse following 6 months of directly observed therapy. The baseline risk of relapse was 2%, while those with cavitary disease and sputum smear positivity after 2 months of therapy (intensive phase) had a relapse rate of 22% (29). Moreover, elevated bacillary titers in cavities increase the probability of establishing drug-resistant bacterial populations (2, 9). Lung cavities are thus the biological foundation for multidrug-resistant and extensively drug-resistant TB. Therefore, this model may have considerable value for assessing strategies to attenuate disease transmission and also for testing the efficacy of chemotherapeutic regimens to prevent the emergence of resistance.
In our study, all rabbits underwent presensitization with five subcutaneous injections with 107 heat-killed M. bovis bacilli in incomplete Freund's adjuvant prior to infection. Through an elegant series of experiments, Yamamura et al. showed the importance of sensitization to reduce the time and improve the probability of cavity formation. A mixture that included heat-killed bovine tubercle bacilli was injected subcutaneously at various intervals over a period of 5 to 7 days. Rabbits were inoculated by infections through the thoracic wall after confirming the acquisition of a cutaneous DTH reaction with bovine- and human-type mycobacterial bacilli. Cavities were noted to be generated in less time and more reliably in animals that underwent presensitization (34, 35). Investigations into the individual components of tubercle bacilli leading to cavity formation showed that a mixture of mycobacterial lipids and proteins could generate cavities. With proteins alone, phenotypically distinct cavities were generated if rabbits were properly presensitized. Yamamura et al. later showed that mycobacterial proteins combined with synthetic adjuvants could predictably generate cavity formation (38). Interestingly, he also demonstrated that desensitization to such mycobacterial lipoproteins inhibited the creation of these cavities (37).
In our study, we infected rabbits with inocula of 5,000 to 18,000 bacilli. Such high-dose infection may raise questions regarding the specificity of the cavitation response and whether the pulmonary cavities would have formed regardless of presensitization. Three rabbits were selected to undergo high-dose infection with either M. bovis AF2122 or Ravenel (greater than 104 bacilli) without presensitization (unpublished results). These rabbits, although they did not form cavities, underwent greater intra- and extrapulmonary dissemination than their sensitized counterparts. Pulmonary involvement in all specimens yielded massive amounts of caseation throughout the region of inoculation in the right lung with sparing of solely the apical regions that contained multiple granulomas.
Our study also showed that bacterial CFU counts were most abundant in the center of the cavities. This location of increased multiplication is logical since the host immune response is limited, if not absent, in the liquefied caseum (5, 7, 17). Due to reduced macrophage function and entry in the liquefied menstruum, excessive bacillary growth can result in secondary pulmonary and extrapulmonary dissemination (6, 37). Approximately 107 to 109 bacilli can be routinely cultured from a single human pulmonary cavity (4). In our case, M. bovis infections generated cavitary CFU counts of 106 to 109 bacilli, while M. bovis BCG and non-M. bovis species showed CFU counts that ranged from 104 to 108 bacilli. The fewest cavity CFU were observed in rabbits infected with the attenuated ΔsigC mutant. The diminished CFU counts obtained with the ΔsigC mutant suggest that transcriptional adaptation by the bacterium is required for full survival in the cavity environment.
It is well known that the virulence of M. bovis is far greater than that of M. tuberculosis in rabbits challenged with low-dose infection (8). Rabbits are relatively resistant to M. tuberculosis and are better able to contain the infection via host immune response (12, 18, 39). Such distinctions in the pathogenesis of mycobacterial species was confirmed in our study in which rabbits were infected with high doses of both M. bovis and M. tuberculosis bacilli. Extrapulmonary dissemination was almost exclusively noted among rabbits infected with M. bovis Ravenel and AF2122. Indeed, with limited exceptions, non-M. bovis bacilli generally did not show extrapulmonary dissemination. Nonetheless, intrapulmonary secondary lesions could be generated with all of the mycobacteria used in this model. On gross pathology, it was the ipsilateral and contralateral lungs' secondary lesions due to the more virulent M. bovis species that were most apparent. All M. bovis species uniformly led to greater than 104 bacilli in the lungs of every infected rabbit.
To determine the nature of the intragranulomatous environment in cavitary lesions, we carried out a transcriptional analysis of two bacterial genes likely to be central to the regulation of M. tuberculosis physiology in human TB. Both the icl and dosR transcripts were found to be substantially induced, consistent with the above hypothesis. Lipids have long been thought to play key roles in the pathogenesis of M. tuberculosis. There is growing evidence that host-derived lipids provide nutrition whereas pathogen-derived lipids mediate immune suppression (10). It has been suggested that fatty acids are likely to be a major carbon source for energy metabolism in M. tuberculosis in chronically infected lung tissues (3). The more recent observation of the impaired persistence of an M. tuberculosis Δicl mutant highlighted the role of the glyoxylate shunt, an anaplerotic pathway that bypasses the CO2-generating steps of the Krebs cycle and enables bacteria to synthesize carbohydrates and replenish Krebs cycle intermediates from fatty acid-derived acetyl coenzyme A (19). Hypoxic conditions within the human host have been closely linked to the phenomenon of TB latency (31). Rv3133c/DosR, a transcription factor of the two-component response regulator class, has been shown to be the primary mediator of a hypoxic signal within M. tuberculosis, and its role in dormancy has been extensively studied (15, 22, 24, 25, 26, 31). We therefore conclude from the transcription analyses that bacilli in the cavitary environment experience low oxygen tension and are probably utilizing fatty acids as an energy source (30).
One limitation of our work was that we did not investigate the necessity of infection with live bacilli for the formation of lung cavities. Future experiments that measure cavity formation with either heat-killed mycobacteria or antigen-coated polystyrene beads may be insightful for our rabbit model. Such experiments would allow us to determine the contribution of the host immune response to lesion development. The host's involvement in cavitary pathogenesis is well known from studies showing attenuation of cavity formation with the use of immunosuppressive agents (36). Another limitation was the sole focus on cavitary lesions for gene expression data, as opposed to such sites as solid granulomas. Future studies may include comparing the molecular environment of granulomas and cavitary lesions to determine the factors that contribute to cavity formation.
In summary, this is the first study to use a bronchoscopic form of infection to produce cavities in the rabbit model. Through the use of presensitization and direct bronchoscopic instillation of bacilli, we observed cavities with M. tuberculosis, M. bovis, and mutants of these parental strains. Attenuated mutant strains (M. tuberculosis CDC1551 ΔsigC and M. bovis BCG) even displayed classic cavity formation, with notably fewer culturable bacilli, which resembled the gross pathology and histopathology of more classically virulent species like M. bovis. Moreover, M. bovis species could be phenotypically differentiated from non-M. bovis species by their unique capacity for extrapulmonary dissemination.
Acknowledgments
The support of NIH grants AI36973, AI37850, and AI30036 and a senior scholar award from the Ellison Foundation is gratefully acknowledged.
We thank Jonathan Osborne for his generous assistance with the animal experiments.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 8 December 2008.
REFERENCES
- 1.Behr, M. A., S. A. Warren, H. Salamon, P. C. Hopewell, A. Ponce de Leon, C. L. Daley, and P. M. Small. 1999. Transmission of Mycobacterium tuberculosis from patients smear negative acid fast bacilli. Lancet 353444-449. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard, J. S. 1996. Molecular mechanisms of drug resistance in Mycobacterium tuberculosis. Annu. Rev. Biochem. 65215-239. [DOI] [PubMed] [Google Scholar]
- 3.Bloch, H., and W. Segal. 1956. Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J. Bacteriol. 72132-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canetti, G. 1955. The tubercle bacillus. Springer Publishing Company, Inc., New York, N.Y.
- 5.Dannenberg, A. M., Jr., and M. Sugimoto. 1976. Liquefaction of caseous foci in tuberculosis. Am. Rev. Respir. Dis. 113257-259. [DOI] [PubMed] [Google Scholar]
- 6.Dannenberg, A. M., Jr., and J. F. Tomashefski, Jr. 1988. Pulmonary diseases and disorders. McGraw-Hill, New York, N.Y.
- 7.Dannenberg, A. M., Jr. 1993. Immunopathogenesis of pulmonary tuberculosis. Hosp. Pract. 2851-58. [DOI] [PubMed] [Google Scholar]
- 8.Dannenberg, A. M., Jr. 2001. Pathogenesis of pulmonary Mycobacterium bovis infection: basic principles established by the rabbit model. Tuberculosis 8187-96. [DOI] [PubMed] [Google Scholar]
- 9.David, H. L. 1970. Probability of distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl. Microbiol. 20810-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrt, S., and D. Schnappinger. 2007. Mycobacterium tuberculosis virulence: lipids inside and out. Nat. Med. 13284-285. [DOI] [PubMed] [Google Scholar]
- 11.Grosset, J. 2003. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob. Agents Chemother. 47833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helke, K. L., J. L. Mankowski, and Y. C. Manabe. 2006. Animal models of cavitation in pulmonary tuberculosis. Tuberculosis 86337-348. [DOI] [PubMed] [Google Scholar]
- 13.Iseman, M. D. 2000. A clinician's guide to tuberculosis, p. 51-62. Lippincott Williams & Wilkins, Philadelphia, PA.
- 14.Jasmer, R., P. Nahid, and P. C. Hopewell. 2002. Latent tuberculosis infection. N. Engl. J. Med. 3471860-1866. [DOI] [PubMed] [Google Scholar]
- 15.Karakousis, P. C., T. Yoshimatsu, G. Lamichhane, S. C. Woolwine, E. L. Nuermberger, J. Grosset, and W. R. Bishai. 2004. Dormancy phenotype displayed by extracellular Mycobacterium tuberculosis within artificial granulomas in mice. J. Exp. Med. 200647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramnik, I., W. F. Dietrich, P. Demant, and B. R. Bloom. 2000. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 978560-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lurie, M. B. Resistance to tuberculosis: experimental studies in native and acquired defense mechanisms. Harvard University Press, Cambridge, MA.
- 18.Manabe, Y. C., A. K. Kesavan, J. Lopez-Molina, C. L. Hatem, M. Brooks, R. Fujiwara, K. Hochstein, M. L. Pitt, J. Tufariello, J. Chan, D. N. McMurray, W. R. Bishai, A. M. Dannenberg, and S. Mendez. 2007. The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis (Edinburgh) 88187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinney, J. D., K. Höner zu Bentrup, E. J. Muñoz-Elías, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russel. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406735-738. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz-Elías, E. J., and J. D. McKinney. 2005. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 11638-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nardell, E. A., and W. F. Piessens. 2000. Transmission of tuberculosis, p. 215-240. In L. B. Reichman and E. S. Hershfield (ed.), Tuberculosis: a comprehensive international approach. Marcel Dekker, New York, NY.
- 22.Park, H. D., K. M. Guinn, M. I. Harrell, R. Liao, M. I. Voskuil, M. Tompa, G. K. Schoolnik, and D. R. Sherman. 2003. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 3833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratcliffe, H. L., and W. F. Wells. 1948. Tuberculosis of rabbits induced by droplet nuclei infection. J. Exp. Med. 87575-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roupie, V., M. Romano, L. Zhang, H. Korf, M. Y. Lin, K. L. Franken, T. H. Ottenhoff, M. R. Klein, and K. Huygen. 2007. Immunogenicity of eight dormancy regulon-encoded proteins of Mycobacterium tuberculosis in DNA-vaccinated and tuberculosis-infected mice. Infect. Immun. 75941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rustad, T. R., M. I. Harrell, R. Liao, and D. R. Sherman. 2008. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS ONE 3e1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saini, D. K., V. Malhotra, D. Dey, N. Pant, T. K. Das, and J. S. Tyagi. 2004. DevR-DevS is a bona fide two-component system of Mycobacterium tuberculosis that is hypoxia-responsive in the absence of the DNA-binding domain of DevR. Microbiology 150865-875. [DOI] [PubMed] [Google Scholar]
- 27.Smith, D. W., and G. E. Harding. 1977. Animal model: experimental airborne tuberculosis in the guinea pig. Am. J. Pathol. 89273-276. [PMC free article] [PubMed] [Google Scholar]
- 28.Sun, R., P. J. Converse, C. Ko, S. Tyagi, N. E. Morrison, and W. R. Bishai. 2004. Mycobacterium tuberculosis ECF sigma factor sigC is required for lethality in mice and for the conditional expression of a defined gene set. Mol. Microbiol. 5225-38. [DOI] [PubMed] [Google Scholar]
- 29.Tuberculosis Trials Consortium. 2002. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomized clinical trial. Lancet 360528-534. [DOI] [PubMed] [Google Scholar]
- 30.Via, L. E., P. L. Lin, S. M. Ray, J. Carillo, S. S. Allen, S. Y. Eum, K. Taylor, E. Klein, U. Manujantha, J. Gonzales, E. G. Lee, S. K. Park, J. A. Raleigh, S. N. Cho, D. N. McMurray, J. L. Flynn, and E. E. Barry III. 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 762333-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voskuil, M. I., K. C. Visconti, and G. K. Schoolnik. 2004. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinburgh) 84218-227. [DOI] [PubMed] [Google Scholar]
- 32.Wells, W. F., and M. B. Lurie. 1941. Experimental airborne disease: quantitative natural respiratory contagion of tuberculosis. Am. J. Hyg. 3421-41. [Google Scholar]
- 33.World Health Organization Stop TB Partnership. 2006. The stop TB strategy. (WHO/HTM/TB/2006.368). World Health Organization, Geneva, Switzerland.
- 34.Yamamura, Y., S. Yasaka, M. Yamaguchi, K. Endo, H. Iwakura, S. Nakamura, and Y. Ogawa. 1954. Studies on the experimental tuberculous cavity. Med. J. Osaka Univ. 5187-197. [Google Scholar]
- 35.Yamamura, Y. 1958. The pathogenesis of tuberculous cavities. Adv. Tuberc. Res. 913-37. [PubMed] [Google Scholar]
- 36.Yamamura, Y., Y. Ogawa, H. Yamagata, and Y. Yamamura. 1968. Prevention of tuberculous cavity formation by immunosuppressive drugs. Am. Rev. Respir. Dis. 98720-723. [DOI] [PubMed] [Google Scholar]
- 37.Yamamura, Y., Y. Ogawa, H. Maeda, and Y. Yamamura. 1974. Prevention of tuberculous cavity formation by desensitization with tuberculin-active peptide. Am. Rev. Respir. Dis. 109594-601. [DOI] [PubMed] [Google Scholar]
- 38.Yamamura, Y., H. Maeda, Y. Ogawa, and T. Hashimoto. 1986. Experimental pulmonary cavity formation by mycobacterial components and synthetic adjuvants. Microbiol. Immunol. 301175-1187. [DOI] [PubMed] [Google Scholar]
- 39.Yoder, M. A., G. Lamichane, and W. R. Bishai. 2004. Cavitary pulmonary tuberculosis: the Holey Grail of disease transmission. Curr. Sci. 8674-81. [Google Scholar]