Abstract
Treponema denticola is considered to be an agent strongly associated with periodontal disease. The lack of an animal infection model has hampered the understanding of T. denticola pathogenesis and the host's immune response to infection. In this study, we have established an oral infection model in mice, demonstrating that infection by oral inoculation is feasible. The presence of T. denticola in the oral cavities of the animals was confirmed by PCR. Mice given T. denticola developed a specific immune response to the bacterium. The antibodies generated from the infection were mainly of the immunoglobulin G1 subclass, indicating a Th2-tilted response. The antibodies recognized 11 T. denticola proteins, of which a 62-kDa and a 53-kDa protein were deemed immunodominant. The two proteins were identified, respectively, as dentilisin and the major outer sheath protein by mass spectrometry. Splenocytes cultured from the infected mice no longer produced interleukin-10 and produced markedly reduced levels of gamma interferon relative to those produced by naïve splenocytes upon stimulation with T. denticola. Mandibles of infected mice showed significantly greater bone resorption (P < 0.01) than those of mock-infected controls.
Treponema denticola is highly implicated as one of the causative agents in periodontal disease in humans (7, 31). The organism is the predominant spirochete identified within the gingival crevice and developing periodontal pocket of various forms of periodontitis (30), infected root canals, and acute alveolar abscesses (28, 29). The organism has been reported to possess several putative virulence factors, such as attachment factors (6, 12, 15), proteolytic activities (13, 20, 34), and an immunosuppressive factor (14, 27). However, the actual role of these factors in the pathogenesis of T. denticola has yet to be proven, because of the lack of an oral infection model in animals. A T. denticola subcutaneous abscess model was described previously, but the model has many fundamental differences from periodontal diseases (15). As well, the host response to T. denticola oral infections is largely unknown. For other periodontal pathogens, such as Porphyromonas gingivalis, animal infection models have been established for a number of years (2). By use of these models, insights into the pathogenesis of P. gingivalis have emerged. A Th1-biased immune response to P. gingivalis infection appears to be responsible for periodontal bone loss (1, 10, 32). In addition, immunization of mice and rats with components of P. gingivalis protected against periodontal bone loss (8, 9, 22). Recently, a T. denticola oral infection model using rats was described; however, the immune response was not adequately investigated, and bone loss was only marginal (16).
The purpose of this study is to establish an oral infection model in mice with T. denticola as the infectious agent. The model will serve as a good starting point to promote understanding of the pathogenesis of periodontal disease caused by T. denticola and the host immune responses to T. denticola. The model will allow investigations of the prevention and treatment of T. denticola infections to be pursued.
MATERIALS AND METHODS
Bacteria and growth conditions.
Treponema denticola ATCC 35405 was grown in prereduced GM-1 broth (3) in anaerobic jars for 3 days at 37°C. Anaerobiosis was achieved by the “GasPak” Plus anaerobic system (Becton Dickinson and Company, Sparks, MD). Culture purity was determined by phase-contrast microscopy, and cell number was determined using a Helber bacteria counting chamber (Hawksley Medical and Laboratory Equipment, Sussex, United Kingdom). Following growth, the culture was centrifuged (10,000 × g, 10 min), and the cells were resuspended in phosphate-buffered saline (PBS) supplemented with 6 mM l-cysteine HCl to 4 × 1010 bacteria/ml. The PBS-cysteine solution was prepared fresh, boiled for 5 min, and quickly chilled on ice prior to its use for resuspending the bacteria.
Preparation of protein samples.
Lipoproteins and other hydrophobic proteins were extracted from T. denticola using Triton X-114 as described by Sela et al. (26) with modifications. A T. denticola (ca. 2.4 × 1010 CFU) suspension in 1 ml PBS containing 4% (vol/vol) Triton X-114 and 1 mM phenylmethylsulfonyl fluoride was rotated at 4°C. After 18 h, the suspension was centrifuged (20,000 × g, 30 min, 4°C). The pellet was extracted with another milliliter of PBS-Triton X-114-phenylmethylsulfonyl fluoride for 2 h. The suspension was again centrifuged. The pellet was saved and kept at −80°C. The supernatants from the two rounds of extraction were pooled, warmed to 37°C, and centrifuged (15,000 × g, 5 min, 25°C). The top aqueous layer was carefully discarded. The bottom detergent layer was washed by mixing with 1 ml of ice-cold PBS, warmed to 37°C, and centrifuged. The washing process was repeated two more times. The final detergent layer (ca. 0.4 ml) was mixed with 8 volumes of cold acetone and kept at 4°C for 24 h. The precipitated proteins were collected by centrifugation (14,000 × g, 5 min, 4°C), washed once with cold acetone and twice with hexane-isopropanol (3:2, vol/vol), air dried, and redissolved in 0.2 ml of 20 mM Tris buffer (pH 7.5). The protein solution was stored at −80°C in aliquots.
T. denticola infection.
An oral infection study was carried out using two groups of 3-week-old female C57BL/6 mice (n = 10; Charles River Laboratory, St. Constant, Quebec, Canada). Prior to infection, the animals were fed 500 μg/ml kanamycin drinking water for 3 days, followed by regular (no kanamycin) drinking water for 1 day. On the day of infection (day 1), the animals were sedated with ketamine and xylazine, and each animal in the first group was given 1 × 109 T. denticola organisms. This was achieved by pipetting 12.5 μl of the T. denticola suspension to the left half of the oral cavity and another 12.5 μl of the suspension to the right half of the oral cavity. Animals in the second group were mock infected with a PBS-cysteine solution. The infection process was repeated on days 8, 11, 15, 18, 22, and 25. The animals were euthanized on day 71. Blood was collected before infection and at euthanasia. To monitor the infection, oral swabs were obtained before infection and at days 32, 50, and 71. The swabs were vortexed in 50 μl of PBS and stored at −80°C. At euthanasia, spleens were recovered and placed in cold Hanks' balanced salt solution (Invitrogen Life Technologies, Burlington, Ontario, Canada) for the splenocyte stimulation assay described below. Also at euthanasia, the mandibles were recovered from the animals for the assessment of bone loss as described below.
Assessment of alveolar bone loss.
The mandibles were boiled 1 min in water, the flesh was removed, and the mandibles were immersed in 2% (wt/vol) KOH overnight. The mandibles were then washed twice with water and immersed in 3% (vol/vol) H2O2 at room temperature. After 6 h, the mandibles were washed twice with water, stained with 0.1% (wt/vol) methylene blue for 1 min, rinsed with water, and air dried.
A digital image of each half mandible was captured with a Zeiss Tessovar photomicrographic camera equipped with a Nikon D80 camera body at a magnification of ×8. A millimeter scale in plane with each half mandible was digitally imaged at the same time so that the measurements could be standardized for each image. Each half mandible was aligned so that the buccal and lingual cusps were superimposed. Subsequently, the distance from the cementoenamel junction to the crestal bone was estimated on both the midbuccal and mesiobuccal aspects of the first molar by using ImageJ software (version 1.39t), downloaded from the U.S. National Institutes of Health website (http://rsb.info.nih.gov/ij). The measurements were made by a single examiner, and the samples were number coded but blinded to the examiner.
PCR.
Oral swabs were thawed and vortexed for 1 min, and 5-μl samples were used in PCR. T. denticola-specific primers and PCR conditions were identical to those described by Siqueira et al. (29). The PCR generated an expected 316-bp fragment of the T. denticola 16S rRNA gene.
Antibody response.
Antigen-specific immunoglobulin G (IgG) antibody titers in sera were determined by an end point dilution enzyme-linked immunosorbent assay by using methods described previously (5). Briefly, twofold-diluted sera were added to polystyrene microplates coated with 1 × 108 CFU/well of T. denticola. The total anti-T. denticola IgG was detected using alkaline phosphatase-conjugated goat anti-mouse IgG (1:30,000; Sigma-Aldrich Chemical Co., Oakville, Ontario, Canada). The anti-T. denticola specific IgG1 or IgG2a antibodies were detected, respectively, by goat anti-mouse IgG1 or IgG2a antibodies (1:8,000; Southern Biotechnology Associates, Inc., Birmingham, AL), followed by alkaline phosphatase-conjugated rabbit anti-goat IgG. The titer of antibody was expressed as the reciprocal of the dilution that produced an A405 reading 0.05 higher than that of the pooled preimmune samples.
SDS-PAGE and Western blotting.
Protein samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gels using the buffer system of Laemmli (18). Proteins were stained with Coomassie blue. For Western immunoblotting, proteins were transferred to nitrocellulose membranes (33), and immunoreactive proteins were detected using the pooled mouse sera (1/2,000) followed by the alkaline phosphatase-conjugated goat anti-mouse IgG antibody. To detect bound IgG1 or IgG2a antibodies, the blots were probed with the goat anti-mouse IgG1 or IgG2a antibodies followed by the alkaline phosphatase-conjugated rabbit anti-goat IgG.
Mass spectrometry.
Following electrophoresis, the 62-kDa and 53-kDa bands were excised from the gel and digested with trypsin. The resulting peptides were separated by reverse-phase high-performance liquid chromatography and analyzed by tandem mass spectrometry on a hybrid quadrupole linear ion trap mass spectrometer (Applied Biosystems) equipped with a nanospray ion source (Proteomic Core Facility, Atlantic Research Centre, Dalhousie University). The resulting data were used to search the NCBI nonredundant protein database with the MASCOT (version 1.6b21) algorithm.
Splenocyte stimulation.
Spleens were placed on a 100-μm-pore-size cell strainer, mashed with a syringe plunger, and collected below into a sterile petri dish. The cells were separated using Ficoll Plaque Plus (Amersham Biosciences, Baie d'Urfe, Quebec, Canada) according to the manufacturer's instructions. The splenocytes were resuspended in fresh RPMI medium and seeded at 1 × 106 per well (400 μl) on plasma-treated 48-well plates (Becton Dickinson). Splenocytes were stimulated with 5 × 106 CFU of T. denticola for 48 h. Following stimulation, supernatants were saved and used for measurement of gamma interferon (IFN-γ), interleukin-13 (IL-13), and IL-10 production by enzyme-linked immunosorbent assays (eBioscience, San Diego, CA). Nonstimulated splenocytes were used as a negative control.
Statistical analysis.
The results were analyzed by a paired Student t test, and a P value of <0.05 was considered significant.
RESULTS
Oral infection with T. denticola.
Prior to infection, no T. denticola DNA was detected in the oral swabs, indicating that the organism was not present in the oral cavities of the mice (data not shown). Following infection, T. denticola DNA was clearly detected in oral swabs from mice given T. denticola but not in those from mock-infected mice (Fig. 1). Swabs from all 10 mice from the infected group were positive for T. denticola DNA by PCR at days 32, 50, and 71, while none of the animals from the uninfected group were positive. During the course of the experiment, none of the animals showed any signs of discomfort or readily recognizable illness. These results indicate that T. denticola had colonized the oral cavities of mice through the inoculation.
FIG. 1.
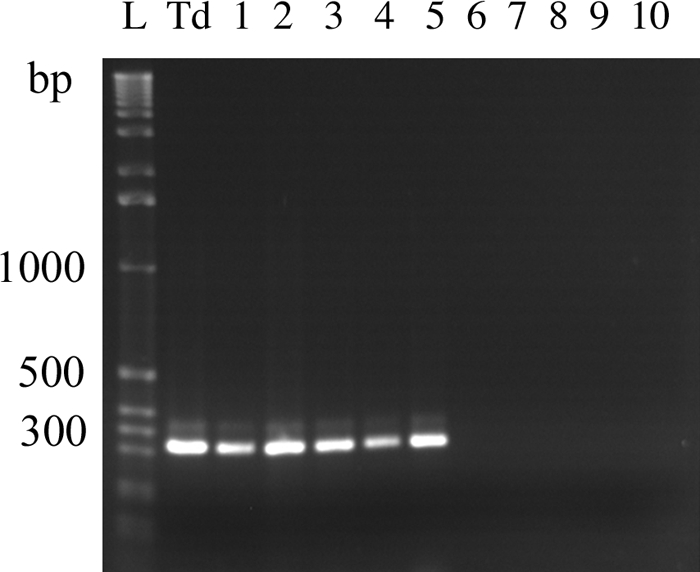
Detection of T. denticola in mice by PCR. Shown are PCR products from oral swabs, obtained on day 71 postinfection, from five T. denticola-infected mice (lanes 1 to 5) and five mock-infected mice (lanes 6 to 10). The remaining swabs from days 71 and other time points from the infected mice, but not from the mock-infected mice, showed similar PCR bands (data not shown). Lane L, 1-kb DNA ladder; lane Td, PCR product from the T. denticola culture.
Alveolar bone resorption.
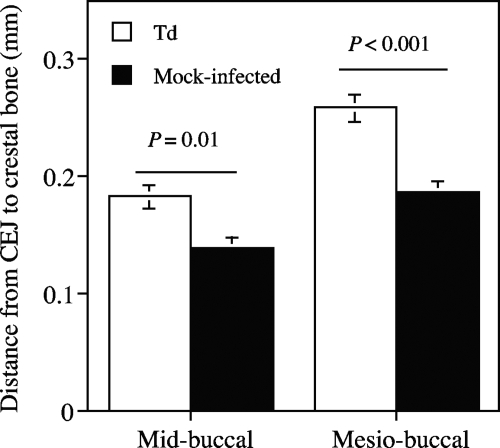
To assess whether the infection had led to alveolar bone resorption, mandibles were recovered from the animals at euthanasia and processed as described in Materials and Methods. The distance from the cementoenamel junction to the crestal bone was measured on both the midbuccal and mesiobuccal aspects of the first molar. As shown in Fig. 2, the infected mice showed greater distances at these areas than the mock-infected mice, indicating that significant bone loss had occurred.
FIG. 2.
Alveolar bone loss from T. denticola infection in mice. Mandibles from T. denticola-infected (Td) and mock-infected mice were processed, and digital images were obtained as described in Materials and Methods. Shown are the mean (± standard error) (n = 20) distances from the the cementoenamel junction (CEJ) to the crestal bone on both the midbuccal and mesiobuccal aspects of the first molar.
Immune response to infection.
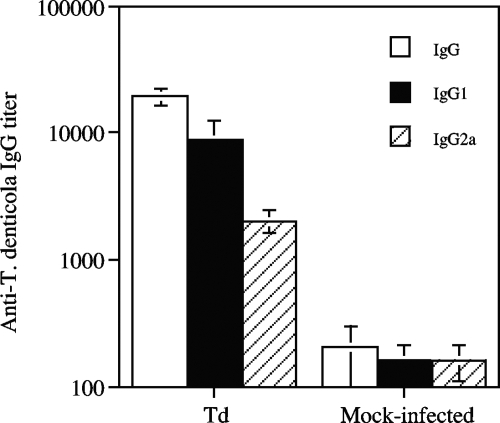
Sera from mice infected with T. denticola, but not sera from the mock-infected group, showed high titers of IgG antibody against the bacterium, indicating that specific antibody had been elicited by infection (Fig. 3). Interestingly, the anti-T. denticola IgG1 antibody titer was much higher than the IgG2a antibody titer.
FIG. 3.
Anti-T. denticola antibody responses in sera from mice following infection. Td, T. denticola-infected group. Results are means ± standard errors for five individual sera.
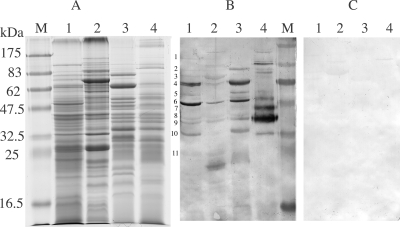
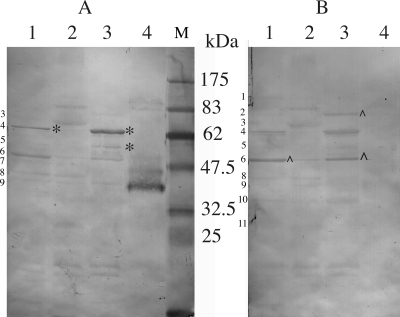
To further examine the immune response, the sera were used in Western immunoblotting against T. denticola proteins. Sera from infected animals reacted with 11 proteins from the total-cell lysate of T. denticola (Fig. 4). Among these 11 immunoreactive bands, the 62-kDa and 53-kDa proteins (Fig. 4, bands 4 and 6) showed particularly strong reactions and were deemed to be immunodominant. Mass spectrometry results showed that the 62-kDa and 53-kDa proteins were the dentilisin and major outer sheath protein (Msp), respectively (Fig. 5). The sera also reacted to all except two (Fig. 4, bands 1 and 3) of these proteins from the boiled Triton X-114 extract. The sera reacted strongly to the unboiled proteins from the Triton X-114 extract but showed less reaction to proteins from the cellular materials remaining after Triton X-114 extraction. Protein bands 1 and 3 were found in this cellular fraction. Sera from mock-infected mice had negligible reactions with the proteins.
FIG. 4.
Western blotting of immunoreactive proteins from T. denticola. (A) Coomassie blue-stained SDS-PAGE gel. (B and C) Blots reacted with sera from T. denticola-infected and mock-infected mice, respectively. Lanes 1, total T. denticola cell lysate; lanes 2, cellular materials after Triton X-114 extraction; lanes 3, lipoproteins obtained by Triton X-114 extractions; lanes 4, same materials as in lane 3, but the sample was not boiled prior to electrophoresis; lanes M, prestained protein markers (New England Biolabs, Mississauga, Ontario, Canada). The estimated molecular masses of the 11 immunoreactive bands are 103, 80, 71, 62, 57, 53, 47, 40, 37, 33, and 25 kDa.
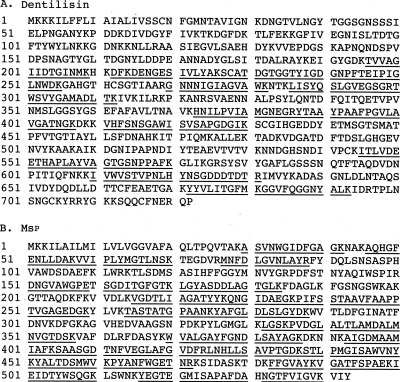
FIG. 5.
Identification of the 62-kDa and 53-kDa proteins by mass spectrometry. The matched peptides generated from trypsin digestion and identified by tandem mass spectrometry are underlined. The Mascot search results for dentilisin and Msp have scores of 1,063 and 1,590, respectively.
The antibodies generated against the proteins discussed above were of both the IgG1 and IgG2a subclasses (Fig. 6). However, the antibodies to the 62-kDa (band 4) and 57-kDa (band 5) proteins were mainly of the IgG1 subclass, while those generated against the 80-kDa (band 2) and 53-kDa (band 6) proteins were predominantly IgG2a. Interestingly, the antibody to the strongly reactive band of ca. 37-kDa in the unboiled Triton X-114 extract was exclusively IgG1.
FIG. 6.
Immunoblots of T. denticola proteins showing reactions to IgG1 (A) and IgG2a (B) antibodies generated from infection. See the legend to Fig. 4 for the protein samples used. * and ∧ indicate that proteins reacted predominantly to IgG1 or IgG2a antibodies, respectively. Lane M, prestained protein markers. The 11 immunoreactive bands identified in Fig. 4 are numbered.
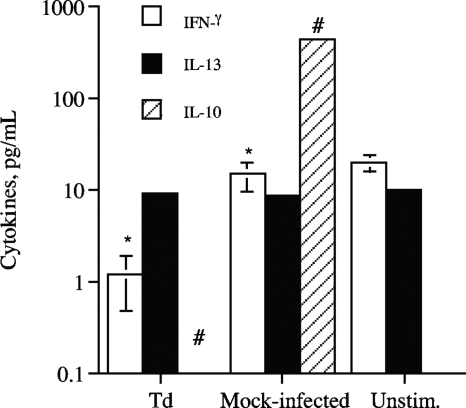
Splenocyte responses to stimulation with T. denticola were quite different for the infected and mock-infected mice (Fig. 7). Splenocytes from infected mice were no longer able to produce IL-10 and produced 1/10 the amount of IFN-γ that the mock-infected or unstimulated splenocytes produced. Surprisingly, T. denticola appeared to be unable to induce any IFN-γ production by mock-infected (naïve) splenocytes, for which the level was similar to that for the unstimulated control. On the other hand, T. denticola induced a large quantity of IL-10 from the naïve splenocytes, while the unstimulated splenocytes produced no IL-10. T. denticola had no effect on the production of IL-13.
FIG. 7.
Cytokine production by splenocytes stimulated with T. denticola. Td, splenocytes from T. denticola-infected mice; Unstim., splenocytes that were not stimulated with T. denticola. * and #, P < 0.05. Unstimulated splenocytes did not produce detectable amounts of IL-10.
DISCUSSION
In the present study, we have established a mouse model of T. denticola oral infection. We have demonstrated, following infection, the presence of T. denticola in the oral cavity, the generation of a specific antibody response, and more importantly, alveolar bone resorption. Our model exhibits a number of similarities (i.e., the ability to colonize the animal's oral cavity, the induction of a specific immune response, and the induction of alveolar bone resorption) to the rat model recently described by Kesavalu et al. (16). However, our model achieved and maintained a 100% infection rate, compared to the initial 100% infection followed by a reduced rate in the rat model. In addition, our model provides better insight into the immune response to the infection than the rat model. Taken together, oral infection by T. denticola in two species of rodents is feasible, and these models should facilitate future research to aid in the understanding of the pathogenesis of T. denticola and the development of vaccines against T. denticola.
In this mouse model, a higher titer of IgG1 than IgG2a antibody was observed, suggesting that the overall response to T. denticola oral infection is a Th2-tilted response. This is in contrast to the subcutaneous abscess model, which generated a Th1-tilted response (15). The type of immune response was not reported in the rat model (16). Coincident with the higher IgG1 antibody response is a decrease in production of the Th1 cytokine IFN-γ by splenocytes.
Western immunoblotting identified 11 immunoreactive proteins, of which the two immunodominant proteins were identified as dentilisin and Msp. The identification of dentislisin and Msp as immunodominant proteins is not a surprise, since Msp has been reported to be highly immunogenic (11), and sera from human subjects with gingivalis showed strong reactions to these two proteins (4).
The Western immunoblotting results also suggest that the immunoreactive proteins of T. denticola can be classified into four groups based on their abilities to elicit IgG1 or IgG2a antibodies. The first group (e.g., the 57-kDa protein) elicited only IgG1 antibodies; the second group (80 kDa) generated only IgG2a antibodies; the third group (62 kDa) elicited more IgG1 than IgG2a antibodies; and the fourth group (53 kDa) elicited more IgG2a than IgG1 antibodies. It is noteworthy that the 37-kDa protein in the unboiled Triton X-114 extract reacted only to the IgG1 antibody, yet the heated samples reacted to IgG2a antibodies, indicating that the epitopes generating the IgG2a antibodies are hidden in the nondenatured proteins. This information will be useful in the future when the exact role of the IgG subclass (i.e., Th1 versus Th2 immune responses) in alveolar bone loss can be established.
The results of the splenocyte stimulation assay are quite interesting. Naïve splenocytes are responsive to T. denticola stimulation in producing a large quantity of IL-10 but not IFN-γ or IL-13. Ruby et al. (23) recently reported that T. denticola can induce the production of IL-10 and a number of proinflammatory cytokines, such as IL-1β, IL-6, IL-12 p70, and tumor necrosis factor, by macrophages. Nixon et al. (21) demonstrated that T. denticola induced production of IL-6 and IL-8, but not MCP-1, by human gingival fibroblasts, while Treponema pectinovorum induced all three cytokines. Interestingly, our results showed that splenocytes from mice infected with T. denticola no longer produced IL-10 and had markedly decreased IFN-γ production upon in vitro stimulation with T. denticola.
These results suggest that the responses by splenocytes are suppressed following T. denticola infection, and they are consistent with the known ability of T. denticola to suppress T-lymphocyte proliferation in response to mitogens (14, 19, 27). The suppression of IL-10 production is of particular interest, because this cytokine is needed to suppress bone resorption in a surgical exposure and endodontic bacterial infection model (24) and in a P. gingivalis oral infection model (25).
In conclusion, a mouse model of T. denticola oral infection with alveolar bone resorption has been established for the first time. The infection induces a Th2-tilted immune response that recognizes a number of T. denticola proteins.
Acknowledgments
We thank Yi-Jing Li for technical assistance, R. Ellen for providing the T. denticola strain, and P. Murray for assistance in mass spectrometry.
E. Andrian is a recipient of an IWK Postdoctoral Research Fellowship. This study was supported by the Faculty Research Fund from the Faculty of Dentistry, Dalhousie University.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Baker, P. J., M. Dixon, R. T. Evans, L. Dufour, E. Johnson, and D. C. Roopenian. 1999. CD4+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 672804-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, P. J., R. T. Evans, and D. C. Roopenian. 1994. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch. Oral Biol. 391035-1040. [DOI] [PubMed] [Google Scholar]
- 3.Blakemore, R. P., and E. Canale-Parola. 1976. Arginine catabolism by Treponema denticola. J. Bacteriol. 128616-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capone, R., H. T. Wang, Y. Ning, D. G. Sweier, D. E. Lopatin, and J. C. Fenno. 2008. Human serum antibodies recognize Treponema denticola MsP and PrtP protease complex proteins. Oral Microbiol. Immunol. 23165-169. [DOI] [PubMed] [Google Scholar]
- 5.Chan, K. G., M. Mayer, E. M. Davis, S. A. Halperin, T. J. Lin, and S. F. Lee. 2007. The roles of d-alanylation of Streptococcus gordonii lipoteichoic acid in innate and adaptive immunity. Infect. Immun. 753033-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson, J. R., and R. P. Ellen. 1990. Tip-oriented adherence of Treponema denticola to fibronectin. Infect. Immun. 583924-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellen, R. P., and V. B. Galimanas. 2005. Spirochetes at the forefront of periodontal infections. Periodontol. 2000 3813-32. [DOI] [PubMed] [Google Scholar]
- 8.Evans, R. T., B. K. Lausen, H. T. Sojar, G. S. Bedi, C. Sfintescu, N. S. Ramamurthy, L. M. Golub, and R. J. Genco. 1992. Immunization with Porphyromonas gingivalis fimbriae protects against periodontal destruction. Infect. Immun. 602926-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson, F. C., III, and C. A. Genco. 2001. Prevention of Porphyromonas gingivalis-induced oral bone loss following immunization with gingipain R1. Infect. Immun. 697959-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonçalves, R. B., O. Leshem, K. Bernards, J. R. Webb, P. P. Stashenko, and A. Campos-Neto. 2006. T-cell expression cloning of Porphyromonas gingivalis genes coding for T helper-biased immune response during infection. Infect. Immun. 743958-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haapasalo, M., K. H. Muller, V. J. Uitto, W. K. Leung, and B. C. McBride. 1992. Characterization, cloning, and binding properties of the major 53-kilodalton Treponema denticola surface antigen. Infect. Immun. 602058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haapasalo, M., U. Singh, B. C. McBride, and V. J. Uitto. 1991. Sulfhydryl-dependent attachment of Treponema denticola to laminin and other proteins. Infect. Immun. 594230-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishihara, K., H. K. Kuramitsu, T. Miura, and K. Okuda. 1998. Dentilisin activity affects the organization of the outer sheath of Treponema denticola. J. Bacteriol. 1803837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishihara, K., I. Takazoe, and K. Okuda. 1992. Immunomodulating activity of oral Treponema strains on proliferation of mouse lymphocyte. Bull. Tokyo Dent. Coll. 3345-50. [Google Scholar]
- 15.Kesavalu, L., S. C. Holt, and J. L. Ebersole. 1999. Lack of humoral immune response protection against Treponema denticola virulence in a murine model. Infect. Immun. 675736-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesavalu, L., S. Sathishkumar, V. Bakthavatchalu, C. Matthews, D. Dawson, M. Steffen, and J. L. Ebersole. 2007. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect. Immun. 751704-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokeguchi, S., M. Miyampto, K. Kato, T. Tanimoto, H. Kurihara, and Y. Murayama. 1994. Isolation and characterization of a 53 kDa major cell envelope protein from Treponema denticola ATCC 35405. J. Periodontal Res. 2970-78. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lee, W., L. Pankoski, A. Zekavat, and B. J. Shenker. 2004. Treponema denticola immunoinhibitory protein induces irreversible G1 arrest in activated human lymphocytes. Oral Microbiol. Immunol. 19144-149. [DOI] [PubMed] [Google Scholar]
- 20.Mäkinen, P. L., K. K. Mäkinen, and S. A. Syed. 1994. An endo-acting proline-specific oligopeptidase from Treponema denticola ATCC 35405: evidence of hydrolysis of human bioactive peptides. Infect. Immun. 624938-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nixon, C. S., M. J. Steffen, and J. L. Ebersole. 2000. Cytokine responses to Treponema pectinovorum and Treponema denticola in human gingival fibroblasts. Infect. Immun. 685284-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien-Simpson, N. M., R. D. Pathirana, R. A. Paolini, Y. Y. Chen, P. D. Veith, V. Tam, N. Ally, R. N. Pike, and E. C. Reynolds. 2005. An immune response directed to proteinase and adhesin functional epitopes protects against Porphyromonas gingivalis-induced periodontal bone loss. J. Immunol. 1753980-3989. [DOI] [PubMed] [Google Scholar]
- 23.Ruby, J., H. Rehani, and M. Martin. 2007. Treponema denticola activates mitogen-activated protein kinase signal pathways through Toll-like receptor 2. Infect. Immun. 755763-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki, H., L. Hou, A. Belani, C. Y. Wang, T. Uchiyama, R. Muller, and P. Stashenko. 2000. IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J. Immunol. 1653626-3630. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki, H., Y. Okamatsu, T. Kawai, R. Kent, M. Taubman, and P. Stashenko. 2004. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J. Periodontal Res. 39432-441. [DOI] [PubMed] [Google Scholar]
- 26.Sela, M. N., A. Bolotin, R. Naor, A. Weinberg, and G. Rosen. 1997. Lipoproteins of Treponema denticola: their effect on human polymorphonuclear neutrophils. J. Periodontal Res. 32455-466. [DOI] [PubMed] [Google Scholar]
- 27.Shenker, B. J., M. A. Listgarten, and N. S. Taichman. 1984. Suppression of human lymphocyte responses by oral spirochetes: a monocyte-dependent phenomenon. J. Immunol. 1322039-2045. [PubMed] [Google Scholar]
- 28.Siqueira, J. F., I. N. Rôças, A. Favieri, and K. R. N. Santos. 2001. Detection of putative oral pathogens in acute periradicular abscesses by 16S rDNA-directed polymerase chain reaction. J. Endod. 27164-167. [DOI] [PubMed] [Google Scholar]
- 29.Siqueira, J. F., I. N. Rôças, A. Favieri, and K. R. N. Santos. 2000. Detection of Treponema denticola in endodontic infections by 16S rRNA gene-directed polymerase chain reaction. Oral Microbiol. Immunol. 15335-337. [DOI] [PubMed] [Google Scholar]
- 30.Simonson, L. G., C. H. Goodman, and H. E. Morton. 1990. Quantitative immunoassay of Treponema denticola serovar in adult periodontitis. J. Clin. Microbiol. 281493-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Socransky, S. S., and A. D. Haffajee. 1992. The bacterial etiology of destructive periodontal disease: current concepts. J. Periodontol. 63322-331. [DOI] [PubMed] [Google Scholar]
- 32.Stashenko, P., R. B. Gonçalves, B. Lipkin, A. Ficarelli, H. Sasaki, and A. Campos-Neto. 2007. Th1 immune response promotes severe bone resorption caused by Porphyromonas gingivalis. Am. J. Pathol. 170203-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uitto, V. J., D. Grenier, E. C. Chan, and B. C. McBride. 1988. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect. Immun. 562717-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]