Abstract
Escherichia coli O157:H7 is a food-borne pathogen causing hemorrhagic colitis and hemolytic-uremic syndrome, especially in children. The main virulence factor responsible for the more serious disease is the Shiga toxin 2 (Stx2), which is released in the gut after oral ingestion of the organism. Although it is accepted that the amount of Stx2 produced by E. coli O157:H7 in the gut is critical for the development of disease, the eukaryotic or prokaryotic gut factors that modulate Stx2 synthesis are largely unknown. In this study, we examined the influence of prokaryotic molecules released by a complex human microbiota on Stx2 synthesis by E. coli O157:H7. Stx2 synthesis was assessed after growth of E. coli O157:H7 in cecal contents of gnotobiotic rats colonized with human microbiota or in conditioned medium having supported the growth of complex human microbiota. Extracellular prokaryotic molecules produced by the commensal microbiota repress stx2 mRNA expression and Stx2 production by inhibiting the spontaneous and induced lytic cycle mediated by RecA. These molecules, with a molecular mass of below 3 kDa, are produced in part by Bacteroides thetaiotaomicron, a predominant species of the normal human intestinal microbiota. The microbiota-induced stx2 repression is independent of the known quorum-sensing pathways described in E. coli O157:H7 involving SdiA, QseA, QseC, or autoinducer 3. Our findings demonstrate for the first time the regulatory activity of a soluble factor produced by the complex human digestive microbiota on a bacterial virulence factor in a physiologically relevant context.
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 causes food-borne disease ranging from uncomplicated diarrhea to hemorrhagic colitis and life-threatening complications, such as hemolytic-uremic syndrome (HUS). Shiga toxin (Stx), an AB5 toxin, is the major virulence factor of E. coli O157:H7 and is responsible for the more severe symptoms of the infection. The A subunit has N-glycosidase activity that cleaves a specific adenine in the host cell rRNA, leading to protein synthesis inhibition and ultimately to cell death. The B subunits are required for toxin binding to its eukaryotic cellular receptor, globotriaosylceramide-3. EHEC O157:H7 can produce one or both of two antigenically distinct forms of Stx, Stx1 and Stx2. Epidemiological studies, together with in vivo and in vitro experiments, have revealed that Stx2 is the most important virulence factor associated with severe human disease. Indeed, Stx2 is 1,000 times more cytotoxic than Stx1 to human renal endothelial cells, and Shiga toxin-producing Escherichia coli that produce Stx2 are more commonly associated with serious diseases than isolates producing Stx1 or Stx1 plus Stx2 (3).
The genes encoding the two subunits of Stx are located in the genomes of lambdoid bacteriophages, and their expression is under the control of the late phage promoter pR′ (37). In lysogens, expression of phage genes is repressed by the cI repressor. However, low levels of spontaneous phage induction can occur. It has been shown that most Stx2 prophages spontaneously induce more readily than lambdoid prophages that do not encode Stx (9, 24). DNA-damaging agents or inhibition of DNA replication activates the bacterial SOS response, which mediates cleavage of repressor cI of lambda bacteriophages by activated RecA, leading to induction of the phage lytic cycle. Induction of Stx prophages leads in turn to an increase in Stx production and to host cell lysis; consequently, Stx is released into the environment.
Epidemiological observations and animal models suggest that the severity of the disease is correlated to the amount of Stx produced in the gut during infection (8). Environmental and/or biological factors that may influence Stx production in vivo can thus modulate the development of hemorrhagic cases. It has been shown that E. coli O157:H7 growth is suppressed in gnotobiotic mice associated with infant intestinal microbiota (26). Thus, commensal bacteria may influence Stx concentration in the gut by inhibiting EHEC growth. Pure cultures of a number of probiotic strains have been shown to inhibit stx2 transcription in laboratory media (4). Furthermore, probiotic bifidobacteria inhibited mitomycin C-induced Stx production in the streptomycin-treated mouse model of EHEC infection without inhibiting EHEC growth (1). In both cases, the concomitant decrease in pH and increase in acetate concentration were proposed to mediate the repressive effect of probiotic strains on Stx2 synthesis. The underlying molecular mechanism was not characterized and could involve a molecular cross talk through or independently of the quorum sensing (QS). In contrast, DNase colicins produced by the colicinogenic microbiota may enhance Stx2 synthesis by inducing the SOS response in EHEC (36). However, the effect of a complex human microbiota on stx gene expression at the transcriptional level has not yet been investigated. It is critical to understand why certain ages or groups of people are more prone to HUS than others. The amount of Stx2 produced in the gut by EHEC during the infectious process probably influences the development of HUS, and the digestive environment could modulate Stx2 synthesis. The normal digestive microbiota and the microbiota-secreted molecules are major components of the digestive environment that could modulate Stx2 production. Therefore, it is necessary to investigate the role of the normal microbiota on the regulation of stx2 expression and not only that of probiotic strains.
In this study, we investigated the influence of prokaryotic molecules produced by a complex human microbiota on Stx2 synthesis by E. coli O157:H7. Using the cecal content of human microbiota-associated rats as a culture medium for E. coli O157:H7, we demonstrated that Stx2 synthesis is repressed at the transcriptional level by bacterially secreted factors. Using pure cultures of human digestive microbiota species, we showed that these factors are produced in particular by Bacteroides thetaiotaomicron. This repression is effective in the context of a physiological digestive environment and overcomes the probable activation of Stx2 synthesis exerted by host eukaryotic factors and/or the antibiotic-mediated SOS response. These findings underscore the importance of the digestive microbiota on the expression of virulence traits by EHEC and therefore on the protection afforded against severe disease such as HUS.
MATERIALS AND METHODS
Bacterial strains.
Wild-type EHEC O157:H7 (strain EDL933) and three isogenic mutants (strains EDL933 ΔqseC, EDL933 ΔqseA, and EDL933 ΔsdiA) were used in this study. These three mutants were obtained by using the one-step PCR-based method of Datsenko and Wanner (7). Commensal E. coli isolates were isolated from fecal samples of a healthy subject and were found to be negative by PCR for stx1, stx2, and eae genes. The E. coli strain TEVS232 used for the AI-3 assay was kindly provided by V. Sperandio (32).
Fecal samples.
Fecal samples from four healthy subjects (two males and two females; 3 to 36 years old) were used in this study. All participants gave informed consent to the protocol. All subjects consumed a standard Western diet, did not receive antibiotics during the 3 months preceding the study, and had no previously diagnosed gastrointestinal disease. Freshly voided feces were obtained from all subjects, stored at 4°C under anaerobic conditions (Anaerocult; Merck, Darmstadt, Germany), and processed within 12 h. One gram of collected fecal sample was diluted 10-fold (wet weight/volume) in an anaerobic mineral solution containing 5 g/liter NaCl, 2 g/liter glucose, and 0.3 g/liter cysteine-HCl.
Conditioned LH media.
Dilutions (10−4 and 10−5) in anaerobic solutions of each fecal microbiota were inoculated in Leedle and Hespell (LH) medium (22). After growth for 24 h at 37°C under anaerobic conditions (100% CO2 in gas phase) using the Hungate technique (16), the cultures were centrifuged for 30 min at 7,000 × g and the supernatants were filtered through 0.2-μm filters. Nutrients were adjusted by the addition of 20× LB medium to a final concentration of 0.5×. pH was adjusted to 7, and the medium was then filtered. This conditioned medium and the unconditioned medium were used for in vitro growth of E. coli EDL933 at 37°C under agitation. The medium was also conditioned with fecal bacterial strains in pure culture or in association with one other strain. Each strain was grown separately under anaerobiosis for 24 h, and then 1 ml of one or two cultures was used to inoculate 40 ml of fresh medium, which was processed as described above.
Human microbiota-associated rats.
Twenty-four adult male Fisher 344 rats were used, provided by the breeding facilities of the Unit on Ecology and Physiology of the Digestive Tract of INRA (Jouy-en-Josas, France). They were born germfree and bred in germfree conditions. Rats were aged 12 weeks at the start of the experiment. They were randomly separated into three groups of eight animals in three sterile isolators. Rats were kept in pairs in standard Macrolon cages containing a bed of wood shavings. They were given free access to sterile water and a pelleted semisynthetic diet (Scientific Animal Food and Engineering, Augy, France) sterilized by γ irradiation at 40 kGy (IBA Mediris, Fleurus, Belgium). To reproduce the diversity of a human-type diet, the food contained lipids and proteins of animal and plant origins, sucrose, and cooked starch (20). Throughout the study, isolators were maintained in controlled conditions of light, temperature, and humidity. Fresh dilution (10−3) of the fecal flora from the two healthy males (age 36 and 3) were given to rats using a sterile stainless-steel stomach feeding tube (1 ml per rat) to obtain two groups of eight rats, colonized with each microbiota. The last group of eight rats was inoculated with 1 ml of anaerobic mineral solution and used as a control. The rats were bred for 2 weeks to allow the microbiota to settle in the digestive tract and the rat physiology to adapt to the new bacterial status. On day 15, the rats were sacrificed by CO2 inhalation and the ceca were removed. Total amounts of anaerobes harbored in cecal contents were enumerated by the most probable number estimation. Cecal contents from each group were then pooled and centrifuged for 30 min at 7,000 × g. Supernatants were withdrawn and filtered through a 0.2-μm filter. The filtrates were half diluted in mineral solution, and glucose was added to a final concentration of 0.2%; pH was adjusted to 7 by the addition of 10 N NaOH. The media (human microbiota-associated content [HMC] and germfree content [GFC]) were then filtered (0.2 μm) and used as culture media for the EDL933 strain.
E. coli growth conditions.
Overnight E. coli EDL933 (O157:H7) cultures in M9 medium supplemented with 0.2% glucose were diluted to a final optical density at 600 nm (OD600) of 0.02 in GFC and HMC media and incubated at 37°C under agitation. EDL933 growth was monitored by numeration on LB plates after appropriate serial dilutions.
Overnight E. coli O157:H7 and commensal E. coli cultures in the unconditioned LH medium were diluted to a final OD600 of 0.02 in unconditioned and conditioned media. E. coli growth was monitored by OD600 measurement and bacterial counts.
Stx2 measurement, stx2, stx1, and recA mRNA quantification, and stx phage particle quantification.
Amounts of Stx2 were quantified in culture supernatants using an enzyme-linked immunoassay as previously described (9). Total mRNAs were extracted as described previously (9). stx2, stx1, and recA mRNA quantifications were performed using real-time PCR, and the data were given as the absolute mRNA copy number, using tufA for normalization as previously described (9). Quantification of stx phage particles was assessed by quantitative PCR as previously described (9).
Detection of B. thetaiotaomicron in microbiota.
Total genomic DNA was purified from each microbiota, and PCR using primers specific for B. thetaiotaomicron was performed as described previously (35).
AI-3 assay.
AI-3 was assayed using the AI-3 reporter strain TEVS232 carrying a LEE1-lacZ fusion as previously described (34).
Statistical analysis.
Student's t test was used to determine significant differences between two groups. ANOVA with the Student-Newman-Keuls test was used to analyze significant differences among multiple test groups. In both cases, a P value of ≤0.05 was considered significant.
RESULTS
Human fecal microbiota inhibits Stx2 synthesis.
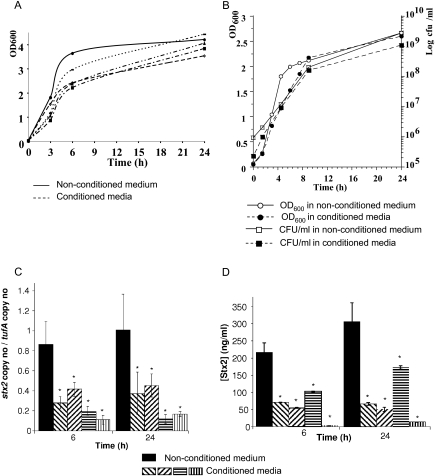
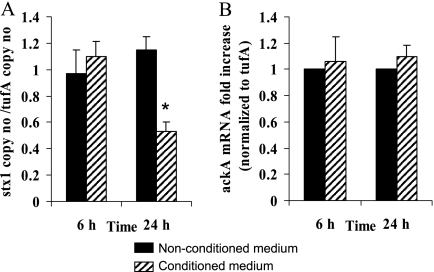
The LH medium (22) supports growth of the complex human microbiota by maintaining high bacterial diversity (5). Growth of E. coli O157:H7 (Fig. 1A) and commensal E. coli strains (Fig. 1B) was slowed in LH medium conditioned with human microbiota compared to that in the nonconditioned medium. The doubling rates of E. coli O157:H7 were 52 min and 1.1 h in the nonconditioned and the conditioned media, respectively, whereas the doubling rates of commensal E. coli were 1 h and 1.5 h in the nonconditioned and the conditioned media, respectively. After prolonged incubation, OD600 readings in conditioned and nonconditioned media were similar. A significant decrease in stx2 mRNA level and Stx2 concentration was observed in all four conditioned media at 6 h and 24 h (Fig. 1C and D). Although our study focused on Stx2 synthesis because Stx2 is more frequently associated with human disease complications, we tested stx1 expression in addition to stx2. stx1 expression was also repressed in the conditioned medium, but only at 24 h (Fig. 2A). As a control, expression of the ackA gene that encodes acetate kinase and is not carried on a prophage was unchanged in the conditioned medium (Fig. 2B).
FIG. 1.
Inhibition of Stx2 synthesis in media conditioned with human fecal microbiota. (A) Growth curves of E. coli O157:H7 strain EDL933 in nonconditioned medium and in media conditioned with the fecal microbiota of four independent individuals. For each medium, a representative growth curve of three independent experiments is shown. (B) Representative growth curves of a commensally stx-negative E. coli strain isolated from a healthy adult (laboratory collection) in nonconditioned and conditioned media. At the indicated times, stx2 mRNA levels in EDL933 (C) and Stx2 concentration in culture supernatants of EDL933 cultures (D) were determined. Data represent the mean ± the standard error of the mean (SEM) of at least three independent experiments performed in duplicate. *, a P value of <0.05 for the unconditioned medium versus conditioned medium.
FIG. 2.
Expression of stx1 and ackA. (A) Absolute quantification of stx1 mRNA in media conditioned or not conditioned with human microbiota. (B) Relative expression of the metabolic gene ackA in media conditioned or not conditioned with human microbiota. Data represent the mean ± SEM of at least three independent experiments performed in duplicate. *, a P value of <0.01 for the unconditioned medium versus conditioned medium.
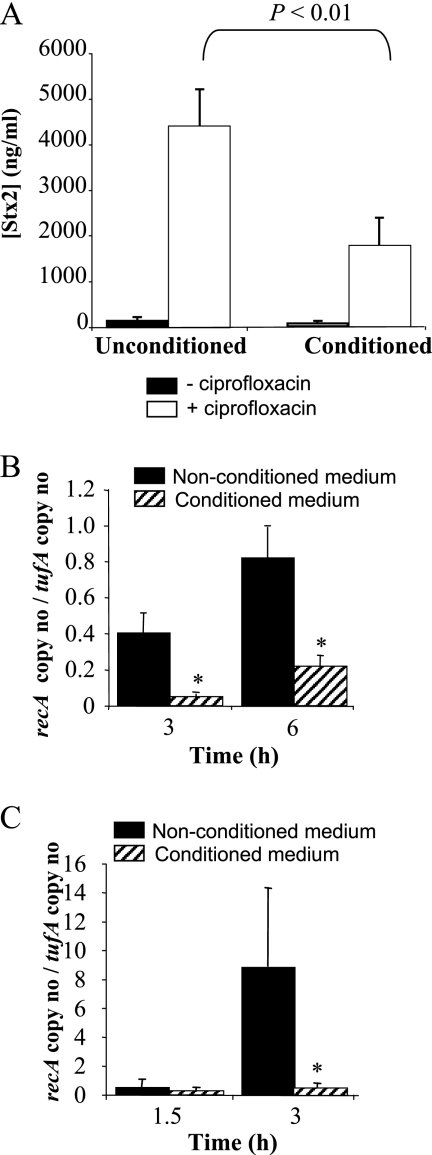
To establish whether antibiotic-induced Stx2 synthesis was also inhibited in the conditioned medium, we induced the SOS response using the quinolone antibiotic ciprofloxacin. We found that 25 ng ml−1 was sufficient to induce the SOS response. Therefore, this concentration was used in further experiments. Stx2 synthesis increased dramatically under ciprofloxacin treatment, although to a lesser extent in the conditioned medium than in the unconditioned medium (Fig. 3A). RecA is a reliable marker of the SOS response that upon activation induces self-cleavage of the phage repressor cI and the recA gene repressor LexA. We thus analyzed recA mRNA expression in conditioned and unconditioned media under exposure to ciprofloxacin and without ciprofloxacin treatment. Without ciprofloxacin induction, recA mRNA levels are low and hardly detectable before 3 h. Therefore, they were measured at 3 h and 6 h. In contrast, recA mRNA levels rapidly increase under ciprofloxacin exposure. Consequently, stx2 phages enter the lytic cycle and lyse the cells. Lysis begins around 3 h after contact with ciprofloxacin, and most of the bacterial cells are killed 3 h later (data not shown). Therefore, recA mRNA were measured at 1.5 h and 3 h after ciprofloxacin treatment. As expected, transcription of recA was induced under ciprofloxacin exposure in both media (Fig. 3B and C). However, recA induction was lower in the conditioned medium than in the unconditioned medium, both in the absence and presence of ciprofloxacin (Fig. 3B and C), suggesting that the downregulation of Stx2 synthesis in the conditioned media is consecutive to the inhibition of RecA activation.
FIG. 3.
Inhibition of ciprofloxacin-induced Stx2 synthesis and of recA expression in the medium conditioned with human fecal microbiota. (A) Stx2 concentration in culture supernatants at 24 h without or under ciprofloxacin treatment. *, a P value of <0.01 for the unconditioned versus the conditioned medium in the presence of ciprofloxacin. (B) recA mRNA levels in the absence of ciprofloxacin. (C) recA mRNA levels under ciprofloxacin exposure. *, a P value of <0.05 for the unconditioned versus the conditioned medium. Data represent the mean ± SEM of at least three independent experiments performed in duplicate.
Quantification of stx phage particles in supernatants confirmed these data. We found ∼10-fold more stx2 phage particles in the unconditioned (3.4 × 105·μl−1) than in the conditioned (5.1 × 104·μl−1) medium, and ciprofloxacin induced stx2 phages to a higher extent in the unconditioned (5.9 × 107·μl−1) than in the conditioned (6.5 × 106·μl−1) medium. In contrast, similar levels of stx1 phages were produced in the unconditioned and conditioned media (6.5 × 106·μl−1 and 6.3 × 106·μl−1, respectively). Ciprofloxacin slightly induced stx1 phage production in the unconditioned medium (3.7 × 107·μl−1) but not in the conditioned medium (5.7 × 106·μl−1).
B. thetaiotaomicron inhibits Stx2 synthesis.
In a first approach to characterize which bacterial species are mostly responsible for Stx2 synthesis inhibition, we conditioned the medium with pure cultures of species belonging to the three major phyla representing the human intestinal microbiota. Stx2 production was measured after 24 h of E. coli O157:H7 growth in media that were each conditioned with Bacteroides spp., Roseburia faecis, Ruminococcus callidus, Lactobacillus reuteri, Clostridium leptum, Clostridium coccoides, and Bifidobacterium breve, either in monocultures or in diassociations (Fig. 4A). The medium conditioned with B. thetaiotaomicron inhibited Stx2 synthesis by 70% compared to the nonconditioned medium (Fig. 4A). Media conditioned with R. callidus, L. reuteri, C. coccoides, or C. leptum also inhibited Stx2 synthesis, but to a lesser extent (∼30% to 50%); media conditioned with B. thetaiotaomicron in association with any one other species also inhibited Stx2 synthesis. We further confirm the inhibitory effect of media conditioned with B. thetaiotaomicron on Stx2 synthesis after 6 h and 24 h of EHEC growth (Fig. 4B). In addition, we demonstrated by PCR using primers that specifically amplify the B. thetaiotaomicron 16S rRNA genes that all four microbiota used for conditioning the media contained this species (Fig. 4C).
FIG. 4.
Inhibition of Stx2 production by factors secreted by B. thetaiotaomicron. (A) Stx2 synthesis in media conditioned with the main species of human microbiota. Stx2 concentrations were measured after 24 h of growth in conditioned media and given as a percentage of Stx2 production in the nonconditioned medium (NC). The strains used to prepare the conditioned media are as indicated: R.f, Roseburia faecis CC111; R.c, Ruminococcus callidus ATCC 27760; L.r, Lactobacillus reuteri DSM 20016; C.l, Clostridium leptum DSM 753; C.c, Clostridium coccoides DSM 935; B.sp, Bacteroides sp. strain CC134; B.b, Bifidobacterium breve IPC 6469T; and B.t, Bacteroides thetaiotaomicron VIP 5482. (B) Stx2 synthesis in B. thetaiotaomicron-conditioned medium as a percentage of Stx2 synthesis in the nonconditioned medium at 6 h and 24 h (n = 4). *, a P value of <0.05 for the unconditioned versus the conditioned medium. (C) 16S rRNA genes specific to B. thetaiotaomicron is detected in the four microbiota. Total DNA was extracted from each microbiota and from pure cultures of Bacteroides species as controls, and then 10 ng was submitted to PCR using primers specific to B. thetaiotaomicron. Lanes 1 and 12, molecular mass ladder; lanes 2 to 5, PCR from human microbiota (lane 5, microbiota from the child); lane 6, PCR from pure culture of Bacteroides eggerthii; lane 7, from pure culture of Bacteroides fragilis; lane 8, from pure culture of Bacteroides ovatus; lane 9, from pure culture of Bacteroides vulgatus; lane 10, negative control (no DNA); lane 11, from pure culture of B. thetaiotaomicron. (D) Stx2 synthesis in fractionated nonconditioned and B. thetaiotaomicron-conditioned medium at 24 h. *, a P value of <0.05 for the unconditioned versus the conditioned medium. Data represent the mean ± SEM of at least three independent experiments performed in duplicate.
As a first step to characterize the inhibitory factor, an approximate molecular weight was determined. The medium conditioned with B. thetaiotaomicron and the nonconditioned medium were subjected to ultrafiltration using filters of various molecular size cutoffs. The resulting filtrates were then examined for their inhibitory activities on Stx2 synthesis by using them to grow E. coli EDL933 for 24 h, followed by Stx2 measurement. The filtrates obtained from the conditioned medium with the smallest molecular size cutoffs, 3 kDa, resulted in retention of the inhibitory activity, whereas no inhibitory activity was observed with filtrates obtained from the nonconditioned medium (Fig. 4D).
Known QS systems are not involved in Stx2 synthesis inhibition.
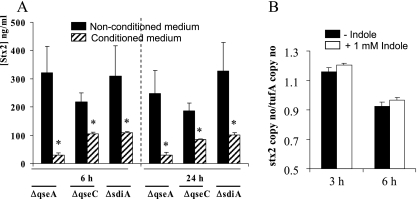
Although somewhat contradictory, previous studies have suggested that QS may control stx2 expression through AI-3 and epinephrine (2, 10, 17, 19, 33) via the QS regulators QseC (6) or QseA (34). By using the AI-3 reporter strain TEVS232, AI-3 was not detected in the medium conditioned by B. thetaiotaomicron (data not shown). Furthermore, Stx2 synthesis was inhibited to an equal degree in qseA and qseC mutants as in the wild-type strain (Fig. 5A). Indole is a bacterial signal molecule that attenuates EHEC motility, biofilm formation, and adherence to HeLa cells (2). Although quite controversial, it has been suggested that indole could signal through the QS sensor SdiA (21). Stx2 synthesis was still inhibited in the sdiA mutant (Fig. 5A) and was not affected by indole in Dulbecco's modified Eagle's medium (Fig. 5B), suggesting that indole signaling is not involved in the repression of Stx2 synthesis. Therefore, the QS pathways that have been described in EHEC O157:H7, involving QseA, QseBC, or SdiA, are not involved in Stx2 synthesis inhibition by the human microbiota-secreted molecules.
FIG. 5.
Effect of inactivation of QS pathways and of indole on Stx2 synthesis. (A) Stx2 concentrations were measured in EDL933 qseA, qseC, and sdiA isogenic mutants after 6 h and 24 h of growth in nonconditioned medium and medium conditioned with human fecal microbiota. (B) stx2 mRNA levels were measured in EDL933 after 3 h and 6 h of growth in Dulbecco's modified Eagle's medium without or with 1 mM indole. Data represent the mean ± SEM of at least three independent experiments performed in duplicate. *, P < 0.05.
Stx2 synthesis is downregulated in the cecal contents of human microbiota-associated rats.
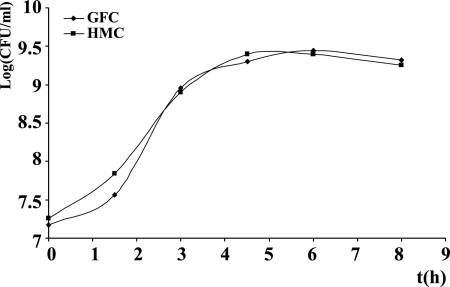
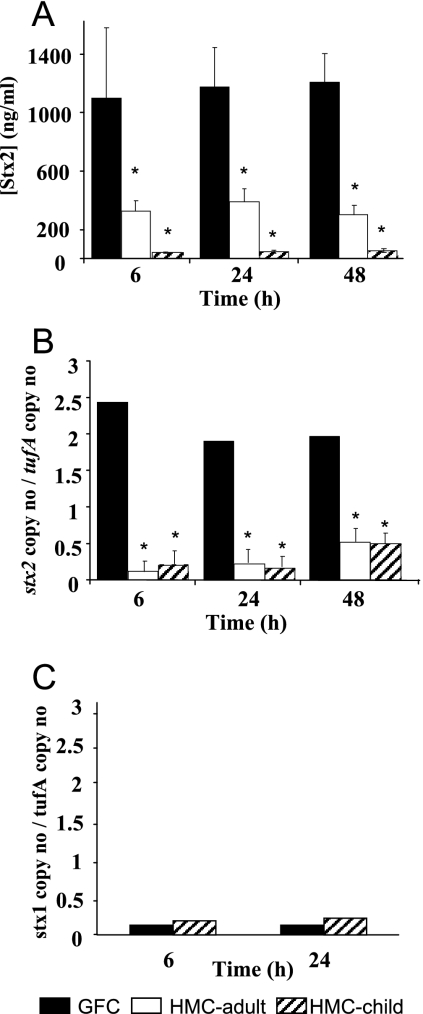
We then wanted to determine whether the repressive effect of the human microbiota on Stx2 synthesis also occurs in vivo in the digestive content by using human microbiota-associated rats versus germfree rats. We reasoned that host eukaryotic factors present in the digestive tract but absent in the LH medium, such as intestinal hormones, biliary salts, or various metabolites, could increase Stx2 synthesis by E. coli O157:H7 and bypass the repression exerted by the microbiota. Eight germfree rats were orally inoculated with an adult's human fecal microbiota, and eight others with a 3-year-old child's fecal microbiota. Seven days later, fecal strict anaerobes, facultative anaerobes, and enterobacteria were numerated to check for colonization. The level of each population was similar to the normal population levels of human feces, indicating successful colonization of the rat digestive tract by the complex human microbiota, as previously described (20). We did not directly inoculate the rats with E. coli O157:H7 because it is known that EHEC colonizes the gut of conventional rodents to a much lesser extent than the guts of germfree or streptomycin-treated rodents (27, 28). Therefore, observation of a difference in Stx2 concentration in feces or in the gut could be due to a difference in colonization and not to a repression of Stx2 synthesis. Thus, we harvested the cecal contents of the rats 7 days later for in vitro E. coli EDL933 growth after removal of the autochthonous microbiota (HMC). Cecal contents of germfree rats were used as the control medium (GFC). E. coli EDL933 growth was similar in GFC and HMC media (Fig. 6), with bacterial counts reaching approximately 109 CFU·ml−1 after 24 h. Stx2 production was decreased by 70% and 96% in the media obtained with the adult and child flora compared to the cecal contents of germfree rats, respectively (Fig. 7A). Similarly, quantification of stx2 mRNA using quantitative real-time PCR showed a decrease in the expression of this gene in the HMC medium (Fig. 7B). Interestingly, Stx2 synthesis was much higher in the cecal contents of germfree rats than in the unconditioned LH medium (compare Fig. 1 and 7), suggesting that eukaryotic host factors increase Stx2 synthesis, although factors produced by the human microbiota overcome this induction. In contrast, stx1 expression was very low in the cecal contents of rats (Fig. 7C), even lower than in LH medium (compare Fig. 2A and 7C), and was unchanged in HMC compared to that in GFC (Fig. 7C).
FIG. 6.
In vitro E. coli EDL933 growth in the sterile cecal contents of germfree rats (GFC) and of rats inoculated with human fecal microbiota (HMC). A representative growth curve of three independent experiments is shown. At 24 h, bacterial counts reached 9.1 log (CFU/ml) for both cultures.
FIG. 7.
Inhibition of Stx2 synthesis by factors produced by the human fecal microbiota in cecal contents of rats. E. coli EDL933 (4 × 106 bacteria/ml) was grown in the cecal contents of germfree rats (GFC) or in the cecal contents of human microbiota-associated rats (HMC) obtained with an adult's or child's fecal microbiota. At the indicated times, Stx2 concentration in culture supernatants (A), stx2 mRNA levels (B), and stx1 mRNA levels (C) in bacteria were determined. Data represent the mean ± SEM of three independent experiments performed in duplicate.*, a P value of <0.05 for GFC versus that for HMC.
DISCUSSION
It is well known that a number of bacterial strains of microbiotal origin exert antibacterial effects against intestinal pathogens by producing molecules such as colicins, microcins, lantibiotics, or other uncharacterized antimicrobial components. These molecules usually inhibit the growth of bacterial pathogens by damaging the cell membranes (23). Recently, it has been shown that pure DNase colicins or cocultures of EHEC with colicinogenic strains increase Stx2 production by inducing the SOS response (36). However, the human intestinal tract is colonized by approximately 1014 commensal bacterial cells consisting of more than 1,000 different intestinal species, in which enterobacteria do not represent more than 0.2% of the total bacterial number (38). Whether colicins are effective in vivo when the producers are part of this complex microbiota is not known. In another study, uncharacterized molecules produced by Lactobacillus strains affected the functionality of Salmonella enterica serovar Typhimurium flagella, but not expression of the gene encoding flagellin (23). Indeed, the influence of molecules secreted by the human digestive microbiota on transcriptional regulation of virulence factors is not well documented. A recent study demonstrates that the probiotic Lactobacillus acidophilus strain La-5 secretes in laboratory media an uncharacterized molecule that either acts as a QS signal inhibitor or directly interacts with bacterial transcriptional regulators, controlling the transcription of EHEC genes involved in colonization (25). However, there is no evidence that this molecule is produced in the digestive contents within a complex human microbiota and is active in physiological conditions. It has been shown that probiotic strains influence Stx2 synthesis in laboratory media (4) or in mice harboring a microbiota depleted of facultative anaerobes (1), probably by increasing acetate levels and decreasing pH values. But the role of the normal digestive microbiota, beyond any preventive or therapeutic strategy, has never been investigated.
The results presented in this study demonstrate that soluble factors released by the complex human fecal microbiota in a physiological digestive environment inhibit Stx2 synthesis at the transcriptional level, independently of the pH, by inhibiting the EHEC SOS response mediated by RecA and consequently stx2 phage particle release and Stx2 synthesis. This effect seems to be specific to stx expression, since expression of a housekeeping gene was not affected. It is of particular interest that the spontaneous as well as the ciprofloxacin-induced activation of RecA and release of Stx2 were inhibited in the human fecal microbiota-conditioned media. Furthermore, we have identified some members of the human microbiota able to contribute to the inhibition of Stx2 production. They belong to the major genera, such as Clostridium, Ruminococcus, or Bacteroides. The Bacteroides genus represents one of the most important taxonomic groups in the gut and accounts for 10 to 30% of the total population (14). Among this community, B. thetaiotaomicron seems to be one of the most efficient bacteria tested in this study, demonstrating a strong ability to inhibit Stx2 production. B. thetaiotaomicron is a predominant commensal gut bacterium harbored by a large part of the population (11). This bacterium has already demonstrated beneficial activities for the host through polysaccharide breakdown (31), attenuation of inflammation (18), and mucosal barrier fortification (15). However, no impact of B. thetaiotaomicron on bacterial virulence was reported until this study. Other strains seem to increase Stx2 production, although the global effect of the complex microbiota is Stx2 synthesis inhibition. Higher stx2 mRNA levels and higher levels of Stx2 release were observed in the cecum contents of rats than in the LH medium. In contrast, stx1 was very slightly expressed in the cecal contents and was not repressed by microbiota-secreted factors. This could be related to known differences in the capacity of stx1 and stx2 prophages to be induced. It has been shown that stx2 prophages are spontaneously induced at higher levels than stx1 prophages (24) and that stx1 prophages are poorly sensitive to inducing agents such as mitomycin C (29). In agreement with these data, we observed that stx2 prophages were induced 100-fold by ciprofloxacin, whereas stx1 prophages were induced less than 10-fold. We propose that host gut factors stimulate stx2 expression via induction of the stx2 phage lytic cycle and that microbiota-secreted compounds not only alleviate this activation but further repress stx2 expression via repression of recA transcription. Disruption or imbalance in the normal gut microbiota could therefore greatly affect this protective effect, while atypical microbiota could lead to greater sensitivity during EHEC infection. It is tempting to speculate that people having some perturbation of their microbiota could present a higher susceptibility to EHEC infection and are at greater risk for developing a HUS. It could be especially interesting to investigate the human microbiota composition of individuals affected by EHEC infection.
We showed that the normal microbiota of healthy people, in addition to its known antimicrobial activities, has the capacity to repress the expression of one of the most critical virulence factors of EHEC. In addition, using streptomycin-treated mice, it has been reported that stx genes can be horizontally transferred by stx phages to phage-susceptible commensal E. coli, which in turn can produce Stx2 and may participate in the pathogenesis of hemolytic syndromes (12, 13). By inhibiting the SOS response and thus the lytic induction of stx phages, the microbiota of healthy people may exert a collaterally protective effect by reducing the propagation of stx phages and thus Stx2 release in the gut. In addition, Stx2 increases adherence to intestinal epithelial cells (30). Therefore, by inhibiting Stx2 synthesis, the microbiota could limit EHEC colonization of the human gut.
Acknowledgments
We thank A. Durand, A. Garrivier, and Noémie Perrier for their technical assistance with real-time PCR and enzyme-linked immunoassay, Eve Delmas for detection of B. thetaiotaomicron in human microbiota, and V. Sperandio (Southwestern Medical Center, Dallas) for the gift of the TEVS232 strain.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 8 December 2008.
REFERENCES
- 1.Asahara, T., K. Shimizu, K. Nomoto, T. Hamabata, A. Ozawa, and Y. Takeda. 2004. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 722240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal, T., D. Englert, J. Lee, M. Hegde, T. K. Wood, and A. Jayaraman. 2007. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect. Immun. 754597-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey, C. M., M. Kostrzynska, S. Ojha, and S. Thompson. 2008. The effect of probiotics and organic acids on Shiga toxin 2 gene expression in enterohemorrhagic Escherichia coli O157:H7. J. Microbiol. Methods 73125-132. [DOI] [PubMed] [Google Scholar]
- 5.Chassard, C., V. Goumy, M. Leclerc, C. Del'homme, and A. Bernalier-Donadille. 2007. Characterization of the xylan-degrading microbial community from human faeces. FEMS Microbiol. Ecol. 61121-131. [DOI] [PubMed] [Google Scholar]
- 6.Clarke, M. B., D. T. Hughes, C. Zhu, E. C. Boedeker, and V. Sperandio. 2006. The QseC sensor kinase: a bacterial adrenergic receptor. Proc. Natl. Acad. Sci. USA 10310420-10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean-Nystrom, E. A., A. R. Melton-Celsa, J. F. Pohlenz, H. W. Moon, and A. D. O'Brien. 2003. Comparative pathogenicity of Escherichia coli O157 and intimin-negative non-O157 Shiga toxin-producing E coli strains in neonatal pigs. Infect. Immun. 716526-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sablet, T., Y. Bertin, M. Vareille, J. P. Girardeau, A. Garrivier, A. P. Gobert, and C. Martin. 2008. Differential expression of stx2 variants in Shiga toxin-producing Escherichia coli belonging to seropathotypes A and C. Microbiology 254176-186. [DOI] [PubMed] [Google Scholar]
- 10.Dowd, S. E. 2007. Escherichia coli O157:H7 gene expression in the presence of catecholamine norepinephrine. FEMS Microbiol. Lett. 273214-223. [DOI] [PubMed] [Google Scholar]
- 11.Eckburg, P. B., E. M. Bik, C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson, and D. A. Relman. 2005. Diversity of the human intestinal microbial flora. Science 3081635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamage, S. D., A. K. Patton, J. E. Strasser, C. L. Chalk, and A. A. Weiss. 2006. Commensal bacteria influence Escherichia coli O157:H7 persistence and Shiga toxin production in the mouse intestine. Infect. Immun. 741977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamage, S. D., J. E. Strasser, C. L. Chalk, and A. A. Weiss. 2003. Nonpathogenic Escherichia coli can contribute to the production of Shiga toxin. Infect. Immun. 713107-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hold, G. L., S. E. Pryde, V. J. Russell, E. Furrie, and H. J. Flint. 2002. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis. FEMS Microbiol. Ecol. 3933-39. [DOI] [PubMed] [Google Scholar]
- 15.Hooper, L. V., M. H. Wong, A. Thelin, L. Hansson, P. G. Falk, and J. I. Gordon. 2001. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291881-884. [DOI] [PubMed] [Google Scholar]
- 16.Hungate, R. E. 1969. A roll-tube method for the cultivation of strict anaerobes, p. 117-132. In D. W. G. J. R. Norris (ed.), Methods in microbiology. Academic Press, New York, NY.
- 17.Jeon, B., and K. Itoh. 2007. Production of shiga toxin by a luxS mutant of Escherichia coli O157:H7 in vivo and in vitro. Microbiol. Immunol. 51391-396. [DOI] [PubMed] [Google Scholar]
- 18.Kelly, D., J. I. Campbell, T. P. King, G. Grant, E. A. Jansson, A. G. Coutts, S. Pettersson, and S. Conway. 2004. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat. Immunol. 5104-112. [DOI] [PubMed] [Google Scholar]
- 19.Kendall, M. M., D. A. Rasko, and V. Sperandio. 2007. Global effects of the cell-to-cell signaling molecules autoinducer-2, autoinducer-3, and epinephrine in a luxS mutant of enterohemorrhagic Escherichia coli. Infect. Immun. 754875-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan, A., A. Bruneau, C. Philippe, V. Rochet, A. Rouault, C. Herve, N. Roland, S. Rabot, and G. Jan. 2007. Survival and metabolic activity of selected strains of Propionibacterium freudenreichii in the gastrointestinal tract of human microbiota-associated rats. Br. J. Nutr. 97714-724. [DOI] [PubMed] [Google Scholar]
- 21.Lee, J., A. Jayaraman, and T. K. Wood. 2007. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 742. doi: 10.1186/1471-2180-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leedle, J. A., and R. B. Hespell. 1980. Differential carbohydrate media and anaerobic replica plating techniques in delineating carbohydrate-utilizing subgroups in rumen bacterial populations. Appl. Environ. Microbiol. 39709-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liévin-Le Moal, V., and A. L. Servin. 2006. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 19315-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livny, J., and D. I. Friedman. 2004. Characterizing spontaneous induction of Stx encoding phages using a selectable reporter system. Mol. Microbiol. 511691-1704. [DOI] [PubMed] [Google Scholar]
- 25.Medellin-Peña, M. J., H. Wang, R. Johnson, S. Anand, and M. W. Griffiths. 2007. Probiotics affect virulence-related gene expression in Escherichia coli O157:H7. Appl. Environ. Microbiol. 734259-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momose, Y., K. Hirayama, and K. Itoh. 2007. Effect of organic acids on inhibition of Escherichia coli O157:H7 colonization in gnotobiotic mice associated with infant intestinal microbiota. Antonie van Leeuwenhoek 93141-149. [DOI] [PubMed] [Google Scholar]
- 27.Mundy, R., F. Girard, A. J. FitzGerald, and G. Frankel. 2006. Comparison of colonization dynamics and pathology of mice infected with enteropathogenic Escherichia coli, enterohaemorrhagic E. coli and Citrobacter rodentium. FEMS Microbiol. Lett. 265126-132. [DOI] [PubMed] [Google Scholar]
- 28.Naylor, S. W., D. L. Gally, and J. C. Low. 2005. Enterohaemorrhagic E. coli in veterinary medicine. Int. J. Med. Microbiol. 295419-441. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie, J. M., P. L. Wagner, D. W. Acheson, and M. K. Waldor. 2003. Comparison of Shiga toxin production by hemolytic-uremic syndrome-associated and bovine-associated Shiga toxin-producing Escherichia coli isolates. Appl. Environ. Microbiol. 691059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson, C. M., J. F. Sinclair, M. J. Smith, and A. D. O'Brien. 2006. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc. Natl. Acad. Sci. USA 1039667-9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salyers, A. A., J. R. Vercellotti, S. E. West, and T. D. Wilkins. 1977. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl. Environ. Microbiol. 33319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 9615196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperandio, V., A. G. Torres, J. A. Giron, and J. B. Kaper. 2001. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 1835187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 1008951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teng, L. J., P. R. Hsueh, Y. H. Huang, and J. C. Tsai. 2004. Identification of Bacteroides thetaiotaomicron on the basis of an unexpected specific amplicon of universal 16S ribosomal DNA PCR. J. Clin. Microbiol. 421727-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toshima, H., A. Yoshimura, K. Arikawa, A. Hidaka, J. Ogasawara, A. Hase, H. Masaki, and Y. Nishikawa. 2007. Enhancement of Shiga toxin production in enterohemorrhagic Escherichia coli serotype O157:H7 by DNase colicins. Appl. Environ. Microbiol. 737582-7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner, P. L., M. N. Neely, X. Zhang, D. W. Acheson, M. K. Waldor, and D. I. Friedman. 2001. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J. Bacteriol. 1832081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoetendal, E. G., E. E. Vaughan, and W. M. de Vos. 2006. A microbial world within us. Mol. Microbiol. 591639-1650. [DOI] [PubMed] [Google Scholar]