Abstract
Bordetella colonization factor A (BcfA) is an outer membrane immunogenic protein, which is critical for efficient colonization of the murine respiratory tract. These properties of BcfA prompted us to examine its utility in inducing a protective immune response against Bordetella bronchiseptica in a mouse model of intranasal infection. Mice vaccinated with BcfA demonstrated reduced pathology in the lungs and harbored lower bacterial burdens in the respiratory tract. Immunization with BcfA led to the generation of BcfA-specific antibodies in both the sera and lungs, and passive immunization led to the reduction of B. bronchiseptica in the tracheas and lungs. These results suggest that protection after immunization with BcfA is mediated in part by antibodies against BcfA. To further investigate the mechanism of BcfA-induced immune clearance, we examined the role of neutrophils and macrophages. Our results demonstrate that neutrophils are critical for anti-BcfA antibody-mediated clearance and that opsonization with anti-BcfA serum enhances phagocytosis of B. bronchiseptica by murine macrophages. We show that immunization with BcfA results in the production of gamma interferon and subclasses of immunoglobulin G antibodies that are consistent with the induction of a Th1-type immune response. In combination, our findings suggest that the mechanism of BcfA-mediated immunity involves humoral and cellular responses. Expression of BcfA is conserved among multiple clinical isolates of B. bronchiseptica. Our results demonstrate the striking protective efficacy of BcfA-mediated immunization, thereby highlighting its utility as a potential vaccine candidate. These results also provide a model for the development of cell-free vaccines against B. bronchiseptica.
Respiratory pathogens are a major cause of morbidity and mortality in humans and animals, making the development of efficacious vaccines that protect against these infections a top priority. Bordetellae are small, aerobic, gram-negative coccobacilli that colonize the respiratory tracts of humans and animals (31). Bordetella pertussis infects only humans and causes the acute respiratory disease whooping cough (6). Bordetella parapertussis strains can be divided into two genetically distinct types: those which infect humans, causing a pertussis-like illness, and those which cause respiratory infections in sheep (22, 38). Bordetella avium infects mainly commercially grown turkeys and wild and domesticated birds (43, 45). In contrast, Bordetella bronchiseptica has a broader host range and is considered a cocontributor to a number of respiratory syndromes in agriculturally important and food-producing animals, pets, and nonhuman primates (17). B. bronchiseptica is also a primary etiological agent and/or a predisposing factor that results in porcine reproductive and respiratory disease complex, pneumonia and atrophic rhinitis in swine, infectious tracheobronchitis (i.e., kennel cough) in dogs, and bronchopneumonia in sheep, guinea pigs, rats, mice, rabbits, cats, and nonhuman primates (5, 31). According to the 2000 National Animal Health Monitoring System (NAHMS) survey, respiratory disease was the greatest cause of mortality in swine, accounting for 28.9% of nursery deaths and 39.1% of deaths in grower/finisher pigs. The annual economic impact of atrophic rhinitis and porcine reproductive and respiratory disease complex in the United States alone is estimated to be about $17 million and $40 million, respectively. B. bronchiseptica is also capable of infecting humans, mostly immunocompromised individuals with AIDS or cystic fibrosis (14, 26, 46, 52), although it was recently isolated from an immunocompetent individual (39).
Currently available and proposed vaccines against this pathogen include live, attenuated, heat-killed, or genetically modified bacteria (2, 30, 32, 48, 49). Problems associated with these various whole-cell vaccination approaches include the following: persistence of the vaccine strain in animals, poor induction of an antibody response and/or protective immunity, and retention of some of the virulence characteristics by the vaccine strains (2, 30, 32, 48, 49). The genetic mutations that result in the attenuation of many of the commercially available live, attenuated vaccines are unknown, making it likely that these strains may revert to virulent forms because of survival pressures in the host, such as coinfections with other pathogenic organisms. B. bronchiseptica can predispose animals to other infectious agents or exacerbate disease symptoms. For example, B. bronchiseptica colonization leads to increased severity of canine parainfluenza virus 2 infections and predisposition of pigs and rabbits to subsequent Pasteurella multocida colonization (8, 12, 15). Infection of porcine tracheal rings with B. bronchiseptica has also been shown to enhance the adherence of P. multocida bacteria (13).
Despite vaccination, animals continue to be carriers, resulting in outbreaks among herds. For laboratory animals like rats, mice, and rabbits, experimental infection with B. bronchiseptica results in a chronic and asymptomatic colonization of the upper respiratory tract. We have been able to isolate B. bronchiseptica from the rat nasopharynx even 85 days after inoculation (our unpublished results), and this bacterium has previously been reported to exist in this site for the life of the infected animals (30). Theoretically, persistent colonization of the upper respiratory tract of the animals vaccinated with live or attenuated strains can create a reservoir of infectious bacteria from which animal-animal and zoonotic transmission can occur. Although transmission of a vaccine strain to humans has not been experimentally proven, a number of such human cases have occurred in individuals exposed to infected, sick, or recently immunized farm and companion animals (20).
We propose that an effective acellular vaccination regimen capable of providing long-lasting protective immunity will limit the spread of B. bronchiseptica not only among animals in a herd but also from animals to humans. For B. pertussis, there has been a shift to acellular vaccines because of the high frequency of side effects and multiple adverse reactions associated with the whole-cell vaccines (34). Similarly, development of acellular vaccines capable of protecting against B. bronchiseptica should be given a priority.
BcfA (Bordetella colonization factor A) is an outer membrane protein which is positively regulated by the BvgAS signal transduction system (50). We were encouraged to examine the role of BcfA in protective immunity because our previously published research revealed its role in the colonization of the rat trachea. In addition, sera from rats infected with B. bronchiseptica specifically recognized BcfA (50). In the current report, we have evaluated the immunogenicity and protective efficacy of BcfA in an intranasal mouse model of respiratory infection. Both active and passive immunization with BcfA provided protection against subsequent intranasal challenge with B. bronchiseptica, which significantly correlated with the production of subclasses of immunoglobulin G (IgG) antibodies with high opsonic activity. Our results also suggested a role for a Th1-type cellular response in BcfA-mediated protection. Finally, we demonstrated that BcfA is expressed by multiple clinical isolates of B. bronchiseptica. Data presented in the current study underscore the potential utility of an acellular vaccine approach for B. bronchiseptica and highlight the importance of BcfA as a critical protective antigen against B. bronchiseptica infections.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains used in this study are listed in Table 1. The wild-type B. bronchiseptica strain RB50 and the isogenic mutant strain RKD110 (ΔbcfA) have been described previously (50). The clinical strains of B. bronchiseptica were a kind gift from T. L. Nicholson and K. Register at USDA-ARS. All the strains were maintained on Bordet-Gengou agar (Becton Dickinson Microbiology Systems) containing 7.5% defibrinated sheep blood (BG-agar). For RB50 and RKD110 (ΔbcfA), BG-agar was supplemented with 50 μg/ml of streptomycin (SM) (Research Products International Corp.). For animal inoculations, single colonies of RB50 were inoculated into Stainer-Scholte broth (47) and cultured overnight, followed by subculturing to an optical density at 600 nm (OD600) of ≈1 at 37°C.
TABLE 1.
Strains used in this study
| Strain | Description (reference) |
|---|---|
| RB50 | Wild-type B. bronchiseptica strain (9) |
| RKD110 | RB50 derivative having in-frame chromosomal deletion of bcfA (50) |
| MBORD628 | B. bronchiseptica strain isolated from a horse in the United States |
| MBORD631 | B. bronchiseptica strain isolated from a cat in the United States |
| MBORD698 | B. bronchiseptica strain isolated from a koala in Australia |
| M584/99/1 | B. bronchiseptica strain isolated from a seal on the Scottish coast in 1999 |
| KM22 | B. bronchiseptica strain isolated from a pig in Hungary in 1993 (3) |
| MBORD685 | B. bronchiseptica strain isolated from a dog in the United States |
| SO3287-99 | B. bronchiseptica strain isolated from a sea otter in California in 1999 |
Overexpression and purification of BcfA.
For overexpression of BcfA, Escherichia coli BL21(DE3)/pLysE cells containing the previously described bcfA overexpression plasmid, pNS101, were used (50). Bacterial growth conditions, protein induction, and cell extract preparation were carried out as described previously (50). BcfA was purified using a T7 tag affinity purification kit (Novagen) as described previously (50).
Mouse immunizations.
Five- to six-week-old female C57BL/6 mice were obtained from Charles River Laboratories. All experimental procedures were performed in compliance with institutional regulations and were approved by the animal care and use committee of the Wake Forest University Health Sciences (WFUHS).
Active immunizations.
Groups of four C57BL/6 mice were intraperitoneally injected with 10 or 30 μg of purified BcfA adsorbed to 50 μg of alum (Sigma). The control group of mice received 50 μg of alum only. Three weeks later, all the mice received another dose of the respective immunogen. A week after the second immunization, mice were sedated with isoflurane (Abbott Laboratories) and challenged intranasally with 5 × 105 CFU of RB50 in 25 μl of sterile phosphate-buffered saline (PBS). The number of CFU delivered was confirmed by plating an aliquot of the inoculum on BG-agar containing SM. One and 6 days postchallenge, mice were sacrificed and the nasal septums, tracheas, and left lungs were harvested. Colonization levels were determined by homogenizing these tissues in sterile PBS and plating various dilutions of the homogenates on BG-agar containing SM. Prior to the second immunization (post-first dose) and at the time of challenge (post-second dose), blood was drawn from the tail to collect serum for enzyme-linked immunosorbent assays (ELISAs).
Passive immunizations.
BcfA hyperimmune serum was generated in rats by Covance as described previously (50). Excised pieces of standard sodium dodecyl sulfate (SDS)-polyacrylamide protein gels containing the band corresponding to the BcfA protein were utilized to immunize rats for the generation of anti-BcfA antibodies. Preimmune serum was collected from these rats prior to immunization with BcfA. Since we were not able to obtain enough preimmune serum, naïve rat serum (Invitrogen) was utilized for some of these experiments. To obtain anti-B. bronchiseptica sera, rats were intranasally infected with RB50 and convalescent phase serum was collected 30 days postinoculation.
Groups of five C57BL/6 mice were intraperitoneally injected with 200 μl of either anti-BcfA sera, anti-B. bronchiseptica sera, preimmune serum, or sterile PBS. Three to 4 h later, they were intranasally challenged with 5 × 105 CFU of RB50 in 25 μl of sterile PBS. Mice were sacrificed 3 and 7 days postchallenge, and colonization levels were determined as described above.
Lung histopathology.
The right lungs from all mice were immersed in 10% neutral buffered formalin (EMD Chemicals, Inc.) just after sacrifice for at least 24 h, trimmed, embedded in paraffin, processed routinely for histology, cut at 4 to 6 μm, stained with hematoxylin and eosin, and examined by light microscopy. The sections were scored qualitatively for inflammation and injury, degree of overall cellularity, thickness of alveolar walls, bronchiolar and vascular degeneration, edema, hemorrhage, and degree and type of inflammatory cellular infiltration by N. Kock, a board-certified veterinary pathologist, in a blind manner.
Neutrophil depletion.
RB6-8C5 hybridoma cells were a kind gift from G. Huffnagle (University of Michigan) (23, 29). Growth of these and purification of RB6-8C5 monoclonal antibodies were performed as described previously (23, 29). For in vivo neutrophil depletion, groups of five C57BL/6 mice were intraperitoneally injected with 1 mg of RB6-8C5 monoclonal antibody. Previous studies have shown that this treatment regimen is able to deplete neutrophils for 1 to 2 weeks in mice (28). A separate group of mice was injected with 1 ml of sterile PBS. To determine the efficiency of neutrophil depletion, blood collected from the tails was analyzed by IDEXX Laboratories for complete blood cell counts. The treatment was found to be 98% effective in depleting neutrophils (data not shown). One day postadministration of RB6-8C5 antibodies, mice were intraperitoneally injected with 200 μl of either anti-BcfA sera or naïve rat serum. Three to 4 h afterwards, they were intranasally challenged with 5 × 105 CFU of RB50 in 25 μl of sterile PBS. Two days postchallenge, they were sacrificed and colonization levels were determined as described above. We were unable to extend the infection of neutropenic mice with B. bronchiseptica beyond 2 days, because four of five mice succumbed to infection by 3 days.
ELISAs.
Serum and lung homogenate antibody responses to BcfA were quantified by coating 96-well flat-bottomed Nunc-Immuno plates (Nalge Nunc International) with purified BcfA protein. The plates were incubated at 4°C overnight in a humidified chamber. The wells were then washed three times with PBS containing 0.05% Tween 20 (PBST) (EMD Chemicals, Inc.). Blocking for nonspecific interactions was carried out by the addition of 200 μl of 5% milk in PBST per well, and plates were incubated at 37°C for 1 h. Lung homogenates or serum (100 μl) from immunized mice or BcfA hyperimmune serum at various dilutions was added, and plates were incubated at 37°C for 2 h. Wells were washed three times with PBST, and bound antibodies were detected using horseradish peroxidase-conjugated goat anti-mouse (Bio-Rad Laboratories) or goat anti-rat IgG (Rockland, Inc.) (1:2,000) antibodies. Plates were washed five times with PBST and 3,3′,5,5′-tetramethylbenzidine (Sigma) was used as the substrate. Absorbance at OD450 was determined using the Labsystems Multiskan Plus plate reader. Absorbance was plotted against dilution, and the end-point titer was determined as the inverse of the highest dilution, giving an OD450 reading four to five times above the background. Negative titers were plotted as zero.
Specific class and isotypes of antibodies present in BcfA hyperimmune serum and serum from BcfA-immunized mice were determined using the rat and mouse Ig isotyping ELISA kit (BD Pharmingen), respectively, according to the manufacturer's instructions. Titers of the respective isotypes in the preimmune serum from rats or serum from alum-immunized mice were assayed by ELISA, and the OD450 values from these were subtracted from values obtained for BcfA hyperimmune serum and serum from BcfA-immunized mice, respectively. The resulting values were then plotted against the dilutions, and the end-point titer was determined as the inverse of the highest dilution that gave an OD450 reading four to five times above the background. Negative titers were plotted as zero.
Opsonophagocytosis assay.
The murine macrophage cell line J774A.1 and murine monocyte/macrophage cell line RAW 264.7 were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone) and 4 mM l-glutamine (Invitrogen). Approximately 2 × 105 cells were seeded into wells of a 24-well cell culture plate (Corning Incorporated) and incubated overnight with 5% CO2. For the opsonophagocytosis assay, BcfA hyperimmune serum and naïve rat serum were heat inactivated by incubation at 55°C for 15 min. RB50 or RKD110 (ΔbcfA) was grown to an OD600 of 1, and ≈2 × 106 cells were incubated with heat-inactivated BcfA hyperimmune serum (at 1% or 10%), naïve rat serum (10%), or PBS in 100 μl at 37°C for 30 min. The assay was performed by incubating macrophage cells with the above-mentioned mixture of serum or PBS containing bacteria for 1 h. This was followed by gentamicin (100 μg/ml) (Invitrogen) treatment for 1 h to kill the extracellular bacteria, washing twice with PBS to remove adherent bacteria, and lysing with water, and different dilutions were plated to enumerate the number of phagocytosed bacteria. The fold CFU for each treatment was calculated by dividing the intracellular CFU obtained from different serum groups by the CFU obtained from the PBS control. The assay was performed in triplicate and repeated two to three times.
T-cell cytokine assays.
Groups of five C57BL/6 mice were immunized at 0 and 3 weeks with two doses of either 30 μg of BcfA adsorbed to alum or 50 μg of alum only, as described above. Two and 4 weeks after administrations of the second dose, mice were sacrificed and spleens were harvested. Spleens were homogenized in RPMI medium (Invitrogen), and red blood cells were lysed using ACK lysing buffer (Lonza). Splenocytes were counted, and approximately 2 × 106 cells were added to each well of a 96-well plate in RPMI medium supplemented with gentamicin. The splenocytes were restimulated with 10 μg of purified BcfA protein or medium alone. Supernatants were harvested after 72 h of incubation and analyzed for gamma interferon (IFN-γ), interleukin 4 (IL-4), IL-10, IL-12, and tumor necrosis factor alpha (TNF-α) production using respective ELISA kits (BD OptEIA) according to the manufacturer's instructions. Using the standards provided with the kit, a standard curve was plotted for each cytokine, and the respective cytokine concentrations in the samples were derived from the standard curve.
SDS-PAGE and immunoblot analyses.
Membrane fractions were prepared from different B. bronchiseptica strains as described previously (50), resolved using SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes (Osmonics, Inc.), and probed with enriched anti-BcfA antibody (1:5,000). Anti-BcfA serum was enriched by repeated incubation with overnight cultures of RKD110 (ΔbcfA) for 3 to 4 h as described previously (50). Goat anti-rat IgG (Rockland, Inc.) conjugated to horseradish peroxidase (1:2,000) was used as the secondary antibody, and proteins were detected using the Amersham ECL system.
RESULTS
Active immunization with BcfA induces protective immunity against B. bronchiseptica infections.
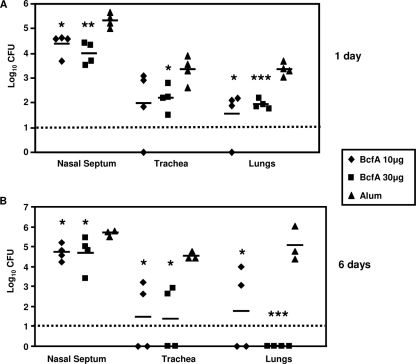
We have previously demonstrated that the outer membrane protein BcfA promotes colonization of the rat trachea (50). Our results also suggested that BcfA-specific antibodies were produced during infection of animals with B. bronchiseptica (50). Therefore, we hypothesized that the immune response elicited against BcfA will provide protective immunity against subsequent B. bronchiseptica infection. To examine this hypothesis, mice were intraperitoneally injected with two doses of either 10 or 30 μg of purified BcfA adsorbed to alum. One week after the second immunization, mice were challenged with RB50 utilizing an intranasal inoculation regimen that seeds and leads to colonization of the entire respiratory tract (30, 44). In control mice receiving only alum, high numbers of bacteria were recovered from the noses, tracheas, and lungs at 1 and 6 days following challenge (Fig. 1A and B, respectively). Three out of four control mice showed signs of bordetellosis, and one mouse succumbed to the infection at 6 days postchallenge. In contrast, mice immunized with either dose of BcfA did not display any signs of bordetellosis and had remarkably lower bacterial burdens at both time points postchallenge (Fig. 1). The reduction of colonization due to immunization with BcfA was most dramatic in the lungs and tracheas and at 6 days postinoculation. Half of the mice immunized with the lower dose and all of the mice immunized with the higher dose of BcfA had no detectable bacteria in their lungs (Fig. 1B). Both of the immunizing doses resulted in lowering of bacterial burdens in the tracheas of all of the vaccinated mice (Fig. 1B), and two of the four mice were cleared of bacteria from this site. Although immunization with BcfA conferred protection against nasal challenge, compared to the other respiratory organs, there was only a modest reduction in the bacterial burden. This observation is consistent with the fact that B. bronchiseptica is extremely difficult to clear from the nose (18). Our results thus demonstrate that immunization with BcfA elicits immunity that is protective against B. bronchiseptica infections.
FIG. 1.
Immunization with BcfA protects mice against B. bronchiseptica challenge. Mice were immunized intraperitoneally at 0 and 3 weeks with either 10 or 30 μg of BcfA adsorbed to alum or alum only. One week after the second immunization, mice were intranasally challenged with 5 × 105 CFU of RB50 in a 25-μl volume. Mice were sacrificed at 1 day (A) and 6 days (B) postchallenge, and the number of CFU was determined in the nasal septums, tracheas, and lungs. Individual symbols represent a single mouse. The dashed line represents the lower limits of CFU detection. Black bars represent mean colonization of respective groups. A statistical analysis was carried out using the unpaired two-tailed Student t test to compare the CFU obtained from the respective groups of BcfA-immunized mice to that of mice receiving alum. The asterisks indicate the range of the different P values (one asterisk, ≤0.05; two asterisks, ≤0.005; and three asterisks, ≤0.0005).
Immunization with BcfA reduces lung pathology in B. bronchiseptica-infected mice.
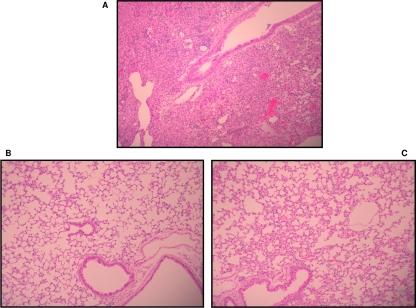
Infection of mice with B. bronchiseptica results in significant lung pathology and inflammation (21). We next sought to determine whether the reduced morbidity observed as a result of B. bronchiseptica infection in BcfA-vaccinated mice, compared to that observed in the control mice, correlated with reduced pulmonary injury. Lungs from both groups of mice were excised at 6 days following challenge with RB50 and examined microscopically. Control mice had pneumonia, characterized by extensive neutrophilic infiltration of the parenchyma (Fig. 2A), while BcfA-immunized mice had only modest cellular infiltration (Fig. 2B and C). Injury scores from both groups are given in Table 2. Those of the control mice averaged 10.0, while those immunized with 10 or 30 μg of BcfA were markedly lower at 3.0 and 2.7, respectively. This attests that immunization with BcfA considerably diminishes the pulmonary injury in mice infected with B. bronchiseptica.
FIG. 2.
Immunization with BcfA reduces lung pathology in mice challenged with RB50. Representative hematoxylin and eosin-stained lung sections harvested 6 days postchallenge with RB50 from mice immunized with alum only (A) or with 10 μg (B) and 30 μg (C) of BcfA adsorbed to alum. The sections were examined and evaluated in a blind manner. Magnification, ×10.
TABLE 2.
BcfA immunization reduces lung pathologya
| Pathology parameters | Total pathology scores (±SD) for mice treated with:
|
||
|---|---|---|---|
| 10 μg BcfA | 30 μg BcfA | Alum | |
| Consolidation | 0 | 0.27 ± 0.5 | 1.6 ± 1.1 |
| Alveolar walls | 1.7 ± 0.5 | 1.5 ± 0.5 | 1.8 ± 0.5 |
| Degeneration | 0 | 0.18 ± 0.4 | 1 ± 0.7 |
| Edema | 0.4 ± 0.5 | 0 | 1.2 ± 0.8 |
| Hemorrhage | 0.1 ± 0.4 | 0.09 ± 0.3 | 0.6 ± 0.9 |
| Alveolar/interstitial PMNs | 0.7 ± 0.5 | 0.3 ± 0.5 | 2 ± 1.0 |
| Intrabronchial PMNs | 0 | 0.09 ± 0.3 | 1 ± 0.7 |
| Perivascular/peribronchiolar lymphocytes | 0 | 0.09 ± 0.3 | 0.4 ± 0.6 |
| Alveolar macrophages | 0 | 0.2 ± 0.4 | 0.4 ± 0.6 |
| Average total score | 3 ± 1.2* | 2.7 ± 2** | 10 ± 5.4 |
Mice were immunized with 10 or 30 μg of BcfA adsorbed to alum or alum only and challenged with RB50 as described in the legend to Fig. 1. Six days postchallenge, mice were sacrificed and the right lungs were harvested and processed for hematoxylin and eosin staining. The sections were examined by N. Kock in a manner that was blind to the treatment groups. The unpaired two-tailed Student t test was used to compare pathology scores between BcfA-immunized and alum-immunized mice. *, P ≤ 0.05; **, P ≤ 0.005; PMN, polymorphonuclear leukocyte.
Immunization with BcfA induces high antibody responses.
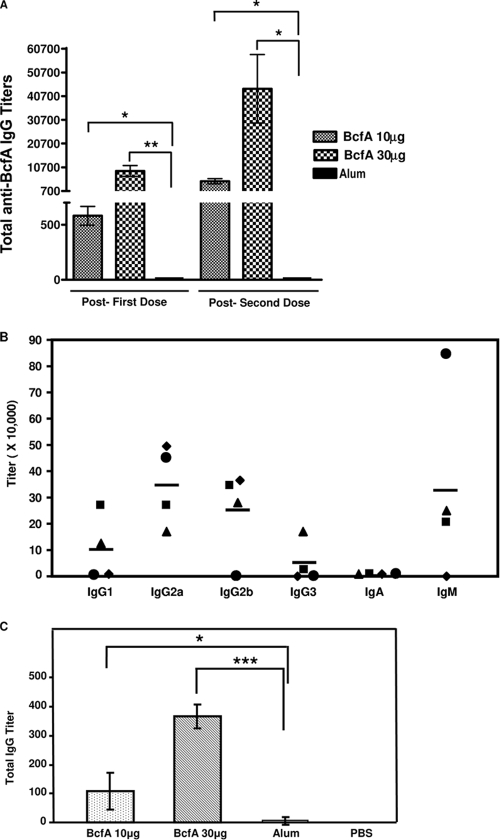
Our next objective was to evaluate whether immunization with BcfA was able to induce the production of specific antibodies in mice. A single dose of either 10 or 30 μg of BcfA elicited high levels of anti-BcfA IgG in the sera (Fig. 3A). After administration of the second dose of BcfA, IgG levels were much higher, indicating a booster effect. In control mice, the anti-BcfA IgG titers were at undetectable or background levels (Fig. 3A). While immunization with BcfA resulted in the induction of specific IgM antibodies in three of the immunized mice, IgA antibodies were not detected (Fig. 3B). We also determined the levels of the different subclasses of anti-BcfA IgG antibodies. While IgG1, IgG2b, and IgG3 anti-BcfA antibodies were detected in the sera of some of the BcfA-immunized mice, IgG2a was detected in all of the immunized mice.
FIG. 3.
Anti-BcfA antibody titers in immunized mice. Anti-BcfA antibody titers in sera (A and B) and lung homogenates (C) collected from mice immunized with 10 μg or 30 μg of BcfA adsorbed to alum or alum only. (A) Mouse serum was collected 3 weeks subsequent to delivery of the first dose (post-first dose) and immediately before challenge with RB50 (post-second dose). Total anti-BcfA IgG titers were determined by ELISA, using BcfA as an antigen. Values represented are mean titers from three to five mice, and bars represent ± standard deviation. A statistical analysis was carried out using the unpaired two-tailed Student t test. The asterisks indicate the range of the different P values (one asterisk, ≤0.05; two asterisks, ≤0.005). (B) Mouse serum was collected immediately before challenge with RB50. Titers of anti-BcfA-specific IgA, IgM, and the different IgG isotypes were measured utilizing specific ELISA kits as described in Materials and Methods. Each symbol represents an individual mouse. Black bars represent mean titer. (C) Total anti-BcfA IgG titers was determined from the lung homogenates of individual mice immunized with 10 μg or 30 μg of BcfA adsorbed to alum, alum only, or PBS. Lung homogenates were prepared 6 days postchallenge with RB50 as described in Materials and Methods. Values represented are mean titers from three to four mice, and bars represent ± standard deviation. A statistical analysis was carried out using the unpaired two-tailed Student t test. The asterisks indicate the range of the different P values (one asterisk, ≤0.05; three asterisks, ≤0.0005).
We also assayed lung homogenates from immunized mice 6 days postchallenge with RB50 for antibody production. Whereas BcfA-immunized mice generated a dose-dependent antibody response in the lungs after infection with RB50, mice which received only alum or PBS had considerably lower levels of anti-BcfA antibodies (Fig. 3C). Therefore, these results are consistent with the conclusion that clearance of B. bronchiseptica from the lower respiratory tract correlates with the presence of high levels of specific antibodies in both the sera and the lung homogenate.
Passive transfer of anti-BcfA antibodies provides protection against B. bronchiseptica challenge.
A critical role for anti-Bordetella antibodies in both vaccine- and infection-induced immunity against B. bronchiseptica has been demonstrated (18, 27). We hypothesized that passive immunization with anti-BcfA antibodies would protect mice against B. bronchiseptica challenge. For production of hyperimmune serum, rats were immunized with excised polyacrylamide gel fragments containing the recombinant BcfA protein, and preimmune and immune rat sera were generated as described in Materials and Methods. We have previously shown that this polyclonal serum specifically recognizes BcfA (50). ELISA showed that while antibody titers specific for BcfA were very high (≈1:70,000) in immune rats, the levels of these specific antibodies were undetectable in preimmune rats.
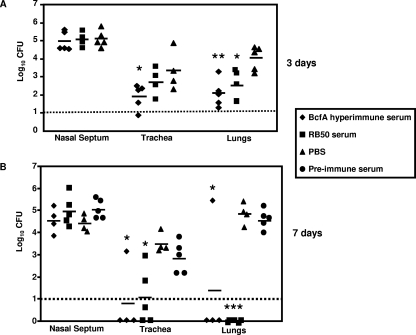
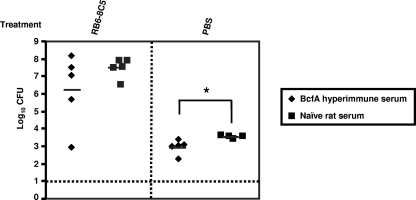
For passive immunization experiments, groups of five mice were intraperitoneally injected with either the BcfA hyperimmune serum, preimmune rat serum, convalescent-phase serum (from rats infected with B. bronchiseptica for 30 days), or sterile PBS 3 to 4 h prior to challenge with 5 × 105 CFU of B. bronchiseptica. Three and 7 days postchallenge, mice were sacrificed and bacterial burdens in the different respiratory organs were enumerated as described in Materials and Methods. At 3 days postchallenge, mice that received the BcfA-specific serum harbored lower bacterial burdens in both their tracheas and lungs than mice that received only PBS (Fig. 4A). At 7 days postchallenge, the numbers of bacteria recovered from these sites were further reduced, and in some animals, bacteria were not detected (Fig. 4B). Consistent with previous reports (27), convalescent-phase serum from B. bronchiseptica-infected rats also resulted in the lowering of bacterial numbers in the lungs at 3 days postchallenge and the clearing of infection from both the tracheas and lungs of some mice at 7 days postchallenge (Fig. 4A and B). Control mice, which received preimmune rat serum or PBS prior to challenge, showed high bacterial burdens in the lower respiratory tract. In contrast to that observed in the tracheas and lungs, adoptive transfer of either the BcfA hyperimmune serum or anti-B. bronchiseptica serum had no significant effect on nasal septum colonization. These results thus suggest that the protection from B. bronchiseptica infections observed in the lungs and tracheas was mediated at least partially by antibodies generated in response to immunization with BcfA. Furthermore, these results also demonstrate that passively transferred BcfA antibodies are efficient in protecting mice against B. bronchiseptica challenge, similar to those generated during an infection with the wild-type bacteria.
FIG. 4.
Effect of adoptive transfer of BcfA-specific sera on respiratory tract colonization. Mice were intraperitoneally injected with anti-BcfA hyperimmune serum, convalescent-phase anti-RB50 serum, preimmune serum, or sterile PBS. Three to 4 h later, mice were intranasally challenged with 5 × 105 CFU of RB50 in a 25-μl volume. Three (A) and 7 (B) days postchallenge, mice were sacrificed and bacterial colonizations in the nasal septums, tracheas, and lungs were determined. Dashed line represents lower limits of CFU detection. Individual symbols represent a single mouse. Black bars represent mean colonization of respective group. The unpaired two-tailed Student t test was used to determine statistical significance. The groups of mice receiving anti-BcfA hyperimmune serum and convalescent-phase anti-RB50 serum were compared to those groups receiving sterile PBS. The asterisks indicate the range of the different P values (one asterisk, ≤0.05; two asterisks, ≤0.005; three asterisks, ≤0.0005). Differences between the CFU obtained from preimmune-treated mice and PBS-treated mice were not statistically significant.
Anti-BcfA sera are opsonic.
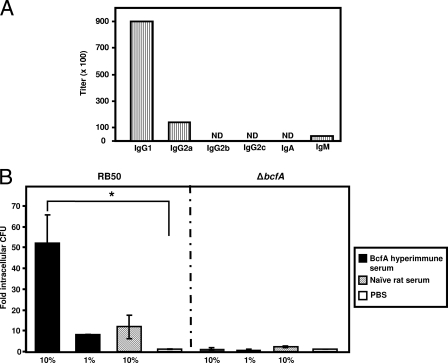
To better understand the mechanism of passive protection, we next determined the levels of antibodies other than IgG and the various IgG isotypes present in the rat sera utilized for passive protection. While significant levels of IgM were detected in the anti-BcfA rat sera, no IgA was detected. Isotyping analysis also revealed that while IgG1 was the predominant isotype, IgG2a was present at lower levels (Fig. 5A).
FIG. 5.
Opsonization with anti-BcfA serum enhances the phagocytosis of RB50 by J774 murine macrophages. (A) Characterization of anti-BcfA antibodies in BcfA-specific hyperimmune serum. Titers of different antibody classes and IgG isotypes were measured using the specific ELISA kit as described in Materials and Methods. The titers obtained were normalized to the titers of the preimmune serum. ND, not detectable. (B) Approximately 2 × 106 CFU of RB50 or RKD110 (ΔbcfA) was incubated with either 1% or 10% heat-inactivated anti-BcfA hyperimmune serum, 10% naïve rat serum, or sterile PBS at 37°C for 30 min, followed by incubation with 2 × 105 J774 cells for 1 h. Extracellular bacteria were killed by treatment with 100 μg/ml of gentamicin for 1 h, followed by washing twice with sterile PBS to remove adherent bacteria. The cells were lysed, and the CFU of phagocytosed bacteria were determined by plating on BG-agar containing SM. Results are expressed as fold CFU of intracellular bacteria over the PBS treatment and are representative of three independent experiments performed in triplicates. Bars represent means ± standard deviation. Statistical analysis was carried out using the unpaired two-tailed Student t test. An asterisk indicates the P value of ≤ 0.05.
The presence of IgG1, IgG2a, and IgM in the immune serum has been correlated with high opsonic activity (42). We examined the efficiency of BcfA hyperimmune rat serum to promote opsonization and phagocytosis of B. bronchiseptica by J774 murine macrophage cells (Fig. 5B) and RAW 264.7, the murine monocyte/macrophage cells. These cells are frequently utilized to study Bordetella pathogenesis and for phagocytic assays (24, 25, 41, 51). Naïve rat serum or PBS was used as negative control. Opsonization with BcfA-specific sera increased the efficiency of uptake of RB50 by J774 macrophages compared to incubation with naïve rat serum or PBS (Fig. 5B). In contrast, there were no significant differences in the uptake of the RKD 110 (ΔbcfA) strain on opsonization with either the BcfA-serum or naïve rat serum and PBS, thereby confirming the specificity of BcfA antibody-mediated opsonization. Similar results were obtained with the RAW 264.7 cells (data not shown). These results therefore suggest that one of the mechanisms for the observed BcfA-mediated protection is increased opsonization of B. bronchiseptica for phagocytosis.
Neutrophils are critical for anti-BcfA antibody-mediated bacterial clearance.
Neutrophils are a vital component of the immune responses responsible for clearing B. bronchiseptica infections, since neutropenic mice succumb to B. bronchiseptica infections within 1 to 4 days postinoculation (21, 28). We hypothesized that neutrophils will be a critical component of the BcfA-induced protective immunity. To evaluate this possibility, we rendered mice neutropenic by injecting the anti-Gr1 antibody (RB6-8C5), which specifically depletes neutrophils without affecting resident and circulating macrophages and lymphocytes (11, 28). Groups of four to five mice were administered either PBS or the RB6-8C5 antibody, followed by adoptive transfer of either naïve rat sera or the anti-BcfA sera from rats. Subsequently, these mice were inoculated with RB50. We examined only the lungs of these mice since the effect of passive transfer of serum on bacterial clearance is greatest in the lower respiratory tract (27) (Fig. 4). Consistent with results presented in Fig. 4A, PBS-injected mice which received BcfA-specific sera had significantly lower numbers of bacteria in the lungs than those that received the naïve rat serum (Fig. 6). In contrast, neutropenic mice (injected with RB6-8C5) that received BcfA-specific sera harbored comparatively greater numbers of bacteria in the lungs. These mice harbored greater than 1,000-fold more CFU in the lungs than those observed in PBS-treated control mice. As expected from previous results (28) (Fig. 4A and B), neutropenic mice which received naïve rat serum also harbored high bacterial burdens in the lungs (Fig. 6). The failure of adoptively transferred BcfA-specific sera to provide protection against B. bronchiseptica challenge in neutropenic mice suggests that neutrophils are required for anti-BcfA antibody-mediated clearance of B. bronchiseptica from the lower respiratory tract.
FIG. 6.
Neutrophils are required for anti-BcfA antibody-mediated clearance of B. bronchiseptica. Mice were intraperitoneally injected with 1 mg of RB6-8C5 antibody or PBS. One day later, mice were intraperitoneally injected with 200 μl of anti-BcfA serum or naïve rat serum, followed by intranasal challenge with 5 × 105 CFU of RB50 in a 25-μl volume. Mice were sacrificed 2 days postchallenge, and bacterial colonization in the lungs was determined. Dashed line represents lower limits of CFU detection. Black bars represent mean colonization of respective group. The unpaired two-tailed Student t test was used to determine statistical significance. Asterisks indicate a P value of ≤ 0.05.
BcfA induces the production of high levels of IFN-γ in ex vivo-stimulated splenocytes.
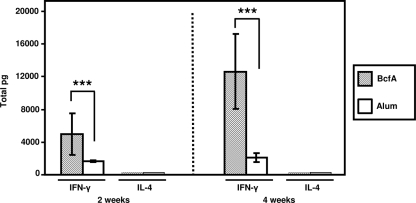
In order to characterize the cellular responses stimulated by BcfA vaccination, we examined BcfA-induced cytokine production by splenocytes ex vivo. Groups of five mice received two successive (3-week interval) doses of either 30 μg of BcfA adsorbed to alum or alum alone. Two and 4 weeks following the administration of the second dose, spleens were excised and processed as described in Materials and Methods, followed by stimulation with 10 μg of BcfA. The culture supernatant was collected and analyzed for the production of IFN-γ (a prototype Th1 cytokine), IL-4 (a prototype Th2 cytokine), IL-10, IL-12, and TNF-α. BcfA-stimulated splenocytes, collected 2 weeks after immunization with BcfA, secreted large amounts of IFN-γ and low levels of IL-4 (Fig. 7, left). Four weeks postimmunization with BcfA, there was an approximately threefold increase in the production of IFN-γ by splenocytes exposed to BcfA (Fig. 7). However, IL-4 was not detected. Very little IL-10, IL-12, or TNF-α was detected in the cultures of splenocytes from BcfA-immunized mice incubated with BcfA. Restimulation of spleen cells from mice infected with alum alone produced either very low or undetectable levels of all the cytokines tested (Fig. 7). These data are consistent with the conclusion that immunization with BcfA elicits a Th1-biased immune response with high levels of IFN-γ production, which has been previously reported to contribute to efficient clearance of B. bronchiseptica infection (19, 37).
FIG. 7.
BcfA-induced production of IFN-γ and IL-4 in splenocytes. Mice were immunized at 0 and 3 weeks with either 30 μg of BcfA adsorbed to alum or 50 μg of alum only. Two and 4 weeks postimmunization, splenocytes were harvested and restimulated with 10 μg of purified BcfA protein. Supernatants were harvested 3 days poststimulation and analyzed for IFN-γ and IL-4 production using respective ELISA kits. Restimulations were carried out in triplicate for each individual mouse, and means ± standard deviations of individual mice are represented. The unpaired two-tailed Student t test was performed to determine statistical significance. Asterisks indicate a P value of ≤ 0.0005.
BcfA expression is prevalent in clinical isolates of B. bronchiseptica.
The sequenced laboratory strains and clinical isolates of the three classical Bordetella spp. vary in the expression of different virulence factors (4, 16). In order to strengthen the utility of BcfA-based therapeutics for treatment of B. bronchiseptica infections, it is critical to determine if BcfA is expressed by circulating clinical isolates of B. bronchiseptica. We performed SDS-PAGE and immunoblot analyses on the membrane fractions of a number of B. bronchiseptica strains isolated from a variety of animal species. Using the affinity-enriched anti-BcfA serum, we detected the presence of a protein band in all the B. bronchiseptica strains tested here, which corresponded to the BcfA protein from RB50 (Fig. 8).
FIG. 8.
Expression of BcfA among clinical isolates of B. bronchiseptica. Membrane fractions of respective strains were prepared as described in Materials and Methods and subjected to SDS-PAGE, followed by immunoblot analyses utilizing anti-BcfA serum. For MBORD628, the amount of protein loaded corresponds to 0.03 times the amount loaded for RB50.
DISCUSSION
There is an urgent need to search for new less-virulent or less-aggressive vaccines for B. bronchiseptica. Similar to the switch from whole-cell vaccines to cell-free vaccines for B. pertussis, there exists a strong rationale for the use of acellular vaccines containing defined antigens for B. bronchiseptica. A prerequisite toward development of these vaccines is a complete understanding of the antigens which elicit a protective immune response. In this report, we have examined the protective efficacy of the protein antigen, BcfA, in a murine respiratory challenge model of B. bronchiseptica. Immunization of mice with purified BcfA resulted in the lowering of bacterial burdens as early as 1 day postchallenge and complete clearance of B. bronchiseptica infection from the lower respiratory tract 6 days subsequent to challenge.
The observed protection due to immunization with BcfA appeared to be mediated by antibodies specific to BcfA. Active immunization with BcfA resulted in the production of high titers of IgG and IgM anti-BcfA antibodies in the sera of mice, suggesting the induction of a strong systemic response. In addition, IgG to BcfA was also detected in the lungs after challenge with RB50. Although it is possible that the lung IgG may be derived from systemic IgG reaching the lungs by passive diffusion, the presence of BcfA-specific antibodies in the lungs is important, since it shows the generation of a localized antibody response in the actual site of B. bronchiseptica colonization. Moreover, the BcfA immune serum but not the preimmune serum protected mice in the passive immunization and challenge experiments. While adoptive transfer of immune serum from mice infected or vaccinated with B. bronchiseptica can clear bacteria from the lower respiratory tract (18), there are limited data available on the efficacy of antibodies against individual protein components in protection against B. bronchiseptica infections. Strikingly, we observed that the hyperimmune serum generated against purified BcfA was as efficient as anti-Bordetella antibodies in mediating this protection.
We found that anti-BcfA antibodies of IgG2a and IgG1 subclasses either were produced in mice as a result of vaccination with BcfA or were the major antibody isotypes present in the hyperimmune serum. Although the exact mechanism by which different IgG subclasses protect against B. bronchiseptica is not clear, in general these antibodies induce efficient opsonization and phagocytosis of the bacteria. Indeed, we found that anti-BcfA serum led to greater internalization of B. bronchiseptica cells by macrophages. These data thus suggest that one of the mechanisms of BcfA-mediated protection is by induction of large amounts of specific antibodies with high opsonic activity, which leads to enhanced phagocytosis.
In addition to the role of antibodies, we also investigated the contribution of cell-mediated immunity in BcfA-induced protection. Immunization with BcfA preferably induced a Th1 response with high IFN-γ production. Production of this cytokine has previously been shown to be important for clearance of B. bronchiseptica (19, 37). The dominant Th1 pattern observed due to vaccination with BcfA was also consistent with the titers of major BcfA-specific IgG isotypes. In all of the vaccinated mice, IgG2a was detected, whereas IgG1 was the major isotype in the hyperimmune BcfA-specific rat serum. Although the correlation between induction of different IgG subclasses and Th1/Th2 activity has not been extensively characterized, IgG2a in the mouse and IgG1 in the rat are Th1 cytokine-driven antibody subclass (7, 10, 36). Very little is known about specific cellular immune responses generated as a result of vaccination with B. bronchiseptica proteins. However, our results demonstrating the induction of a Th1 response by B. bronchiseptica BcfA are different from the Th2 response induced by component vaccines against B. pertussis. In contrast to acellular vaccines, infection with B. bronchiseptica and B. pertussis and immunization with the respective whole-cell vaccines induce a Th1-type response (1, 33, 35, 40). CD4+ Th1 cells have been demonstrated to result in more-efficient immunity to B. pertussis than CD4+ Th2 cells (1). Induction of a Th1-type response and IFN-γ production as a consequence of BcfA immunization might explain the superior protective efficacy of this protein even when administered as a single antigen.
An important prerequisite toward the development of individual protein-based therapeutics for treatment of bacterial infections is the ubiquitous demonstration of its expression among circulating isolates. We have demonstrated that BcfA is expressed by a wide variety of B. bronchiseptica strains isolated from multiple animal species. These data further strengthen the utility of BcfA as a potential vaccine candidate. Previous studies demonstrated that individual immunization with B. pertussis antigens such as Fha, pertactin, and pertussis toxin imparts reasonable protective efficacy. However, vaccines containing multiple components lead to enhanced protection compared to monocomponent vaccines (35). Similarly, we believe that despite the remarkable efficacy of BcfA, a multivalent vaccine containing other known Bordetella antigens such as Fha, pertactin, or fimbriae will be more efficacious. Future studies will have to address whether immunization with BcfA alone or that with composite vaccines will protect against infection in large animals like pigs and dogs, in which B. bronchiseptica causes disease. Finally, the broader potential utility of BcfA as a vaccine candidate is highlighted by our preliminary results, which demonstrate the presence of polypeptides that cross-react with anti-BcfA serum in the closely related human pathogens B. pertussis and B. parapertussis (our unpublished results). We are currently investigating whether these bands correspond to orthologs of BcfA and whether BcfA can confer protection against these human-adapted species. A positive outcome will be highly significant and will promote the inclusion of BcfA in the current acellular vaccines for B. pertussis.
Acknowledgments
We thank Dan Wozniak and Steven Mizel for critical reading of the manuscript. We thank T. Nicholson and K. Register for the different clinical isolates and G. Huffnagle for the gift of RB6-8C5 hybridoma cells.
Research in the laboratory of R.D. is supported by funds from the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service (grant no. 35604-16874) and the NIH (grant no. R21AI071054 and 1R01AI075081).
Editor: J. N. Weiser
Footnotes
Published ahead of print on 8 December 2008.
REFERENCES
- 1.Barnard, A., B. P. Mahon, J. Watkins, K. Redhead, and K. H. Mills. 1996. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0. Immunology 87372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bey, R. F., F. J. Shade, R. A. Goodnow, and R. C. Johnson. 1981. Intranasal vaccination of dogs with liver avirulent Bordetella bronchiseptica: correlation of serum agglutination titer and the formation of secretory IgA with protection against experimentally induced infectious tracheobronchitis. Am. J. Vet. Res. 421130-1132. [PubMed] [Google Scholar]
- 3.Brockmeier, S. L., and K. B. Register. 2007. Expression of the dermonecrotic toxin by Bordetella bronchiseptica is not necessary for predisposing to infection with toxigenic Pasteurella multocida. Vet. Microbiol. 125284-289. [DOI] [PubMed] [Google Scholar]
- 4.Buboltz, A. M., T. L. Nicholson, M. R. Parette, S. E. Hester, J. Parkhill, and E. T. Harvill. 2008. Replacement of adenylate cyclase toxin in a lineage of Bordetella bronchiseptica. J. Bacteriol. 1905502-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buonavoglia, C., and V. Martella. 2007. Canine respiratory viruses. Vet. Res. 38355-373. [DOI] [PubMed] [Google Scholar]
- 6.Carbonetti, N. H. 2007. Immunomodulation in the pathogenesis of Bordetella pertussis infection and disease. Curr. Opin. Pharmacol. 7272-278. [DOI] [PubMed] [Google Scholar]
- 7.Cazorla, S. I., P. D. Becker, F. M. Frank, T. Ebensen, M. J. Sartori, R. S. Corral, E. L. Malchiodi, and C. A. Guzman. 2008. Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi. Infect. Immun. 76324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanter, N., T. Magyar, and J. M. Rutter. 1989. Interactions between Bordetella bronchiseptica and toxigenic Pasteurella multocida in atrophic rhinitis of pigs. Res. Vet. Sci. 4748-53. [PubMed] [Google Scholar]
- 9.Cotter, P. A., and J. F. Miller. 1997. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol. Microbiol. 24671-685. [DOI] [PubMed] [Google Scholar]
- 10.Cuturi, M. C., R. Josien, D. Cantarovich, L. Bugeon, I. Anegon, S. Menoret, H. Smit, P. Douillard, and J. P. Soulillou. 1994. Decreased anti-donor major histocompatibility complex class I and increased class II alloantibody response in allograft tolerance in adult rats. Eur. J. Immunol. 241627-1631. [DOI] [PubMed] [Google Scholar]
- 11.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 1521836-1846. [PubMed] [Google Scholar]
- 12.Deeb, B. J., R. F. DiGiacomo, B. L. Bernard, and S. M. Silbernagel. 1990. Pasteurella multocida and Bordetella bronchiseptica infections in rabbits. J. Clin. Microbiol. 2870-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dugal, F., M. Belanger, and M. Jacques. 1992. Enhanced adherence of Pasteurella multocida to porcine tracheal rings preinfected with Bordetella bronchiseptica. Can. J. Vet. Res. 56260-264. [PMC free article] [PubMed] [Google Scholar]
- 14.Dworkin, M. S., P. S. Sullivan, S. E. Buskin, R. D. Harrington, J. Olliffe, R. D. MacArthur, and C. E. Lopez. 1999. Bordetella bronchiseptica infection in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 281095-1099. [DOI] [PubMed] [Google Scholar]
- 15.Eliás, B., M. Albert, S. Tuboly, and P. Rafai. 1992. Interaction between Bordetella bronchiseptica and toxigenic Pasteurella multocida on the nasal mucosa of SPF piglets. J. Vet. Med. Sci. 541105-1110. [DOI] [PubMed] [Google Scholar]
- 16.Fennelly, N. K., F. Sisti, S. C. Higgins, P. J. Ross, H. van der Heide, F. R. Mooi, A. Boyd, and K. H. Mills. 2008. Bordetella pertussis expresses a functional type III secretion system that subverts protective innate and adaptive immune responses. Infect. Immun. 761257-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopinathan, L., G. S. Kirimanjeswara, D. N. Wolfe, M. L. Kelley, and E. T. Harvill. 2007. Different mechanisms of vaccine-induced and infection-induced immunity to Bordetella bronchiseptica. Microbes Infect. 9442-448. [DOI] [PubMed] [Google Scholar]
- 19.Gueirard, P., P. Minoprio, and N. Guiso. 1996. Intranasal inoculation of Bordetella bronchiseptica in mice induces long-lasting antibody and T-cell mediated immune responses. Scand. J. Immunol. 43181-192. [DOI] [PubMed] [Google Scholar]
- 20.Gueirard, P., C. Weber, A. Le Coustumier, and N. Guiso. 1995. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in host. J. Clin. Microbiol. 332002-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 676109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heininger, U., K. Stehr, S. Schmitt-Grohe, C. Lorenz, R. Rost, P. D. Christenson, M. Uberall, and J. D. Cherry. 1994. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr. Infect. Dis. J. 13306-309. [DOI] [PubMed] [Google Scholar]
- 23.Herring, A. C., N. R. Falkowski, G. H. Chen, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Transient neutralization of tumor necrosis factor alpha can produce a chronic fungal infection in an immunocompetent host: potential role of immature dendritic cells. Infect. Immun. 7339-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewlett, E. L., G. M. Donato, and M. C. Gray. 2006. Macrophage cytotoxicity produced by adenylate cyclase toxin from Bordetella pertussis: more than just making cyclic AMP! Mol. Microbiol. 59447-459. [DOI] [PubMed] [Google Scholar]
- 25.Huang, X. Z., and L. E. Lindler. 2004. The pH 6 antigen is an antiphagocytic factor produced by Yersinia pestis independent of Yersinia outer proteins and capsule antigen. Infect. Immun. 727212-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huebner, E. S., B. Christman, S. Dummer, Y. W. Tang, and S. Goodman. 2006. Hospital-acquired Bordetella bronchiseptica infection following hematopoietic stem cell transplantation. J. Clin. Microbiol. 442581-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirimanjeswara, G. S., P. B. Mann, and E. T. Harvill. 2003. Role of antibodies in immunity to Bordetella infections. Infect. Immun. 711719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirimanjeswara, G. S., P. B. Mann, M. Pilione, M. J. Kennett, and E. T. Harvill. 2005. The complex mechanism of antibody-mediated clearance of Bordetella from the lungs requires TLR4. J. Immunol. 1757504-7511. [DOI] [PubMed] [Google Scholar]
- 29.López, S., A. J. Marco, N. Prats, and C. J. Czuprynski. 2000. Critical role of neutrophils in eliminating Listeria monocytogenes from the central nervous system during experimental murine listeriosis. Infect. Immun. 684789-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mann, P., E. Goebel, J. Barbarich, M. Pilione, M. Kennett, and E. Harvill. 2007. Use of a genetically defined double mutant strain of Bordetella bronchiseptica lacking adenylate cyclase and type III secretion as a live vaccine. Infect. Immun. 753665-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattoo, S., and J. D. Cherry. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18326-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McArthur, J. D., N. P. West, J. N. Cole, H. Jungnitz, C. A. Guzman, J. Chin, P. R. Lehrbach, S. P. Djordjevic, and M. J. Walker. 2003. An aromatic amino acid auxotrophic mutant of Bordetella bronchiseptica is attenuated and immunogenic in a mouse model of infection. FEMS Microbiol. Lett. 2217-16. [DOI] [PubMed] [Google Scholar]
- 33.McGuirk, P., and K. H. Mills. 2002. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 23450-455. [DOI] [PubMed] [Google Scholar]
- 34.Miller, D. L., E. M. Ross, R. Alderslade, M. H. Bellman, and N. S. Rawson. 1981. Pertussis immunisation and serious acute neurological illness in children. Br. Med. J. 2821595-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills, K. H., M. Ryan, E. Ryan, and B. P. Mahon. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philips, J. R., W. Brouwer, M. Edwards, S. Mahler, J. Ruhno, and A. M. Collins. 1999. The effectiveness of different rat IgG subclasses as IgE-blocking antibodies in the rat basophil leukaemia cell model. Immunol. Cell Biol. 77121-126. [DOI] [PubMed] [Google Scholar]
- 37.Pilione, M. R., and E. T. Harvill. 2006. The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance. Infect. Immun. 741043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter, J. F., K. Connor, and W. Donachie. 1994. Isolation and characterization of Bordetella parapertussis-like bacteria from ovine lungs. Microbiology 140255-261. [DOI] [PubMed] [Google Scholar]
- 39.Rath, B. A., K. B. Register, J. Wall, D. M. Sokol, and R. B. Van Dyke. 2008. Persistent Bordetella bronchiseptica pneumonia in an immunocompetent infant and genetic comparison of clinical isolates with kennel cough vaccine strains. Clin. Infect. Dis. 46905-908. [DOI] [PubMed] [Google Scholar]
- 40.Redhead, K., J. Watkins, A. Barnard, and K. H. Mills. 1993. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect. Immun. 613190-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross, P. J., E. C. Lavelle, K. H. Mills, and A. P. Boyd. 2004. Adenylate cyclase toxin from Bordetella pertussis synergizes with lipopolysaccharide to promote innate interleukin-10 production and enhances the induction of Th2 and regulatory T cells. Infect. Immun. 721568-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlageter, A. M., and T. R. Kozel. 1990. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect. Immun. 581914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sebaihia, M., A. Preston, D. J. Maskell, H. Kuzmiak, T. D. Connell, N. D. King, P. E. Orndorff, D. M. Miyamoto, N. R. Thomson, D. Harris, A. Goble, A. Lord, L. Murphy, M. A. Quail, S. Rutter, R. Squares, S. Squares, J. Woodward, J. Parkhill, and L. M. Temple. 2006. Comparison of the genome sequence of the poultry pathogen Bordetella avium with those of B. bronchiseptica, B. pertussis, and B. parapertussis reveals extensive diversity in surface structures associated with host interaction. J. Bacteriol. 1886002-6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sloan, G. P., C. F. Love, N. Sukumar, M. Mishra, and R. Deora. 2007. The Bordetella Bps polysaccharide is critical for biofilm development in the mouse respiratory tract. J. Bacteriol. 1898270-8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spears, P. A., L. M. Temple, D. M. Miyamoto, D. J. Maskell, and P. E. Orndorff. 2003. Unexpected similarities between Bordetella avium and other pathogenic bordetellae. Infect. Immun. 712591-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spilker, T., A. A. Liwienski, and J. J. LiPuma. 2008. Identification of Bordetella spp. in respiratory specimens from individuals with cystic fibrosis. Clin. Microbiol. Infect. 14504-506. [DOI] [PubMed] [Google Scholar]
- 47.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63211-220. [DOI] [PubMed] [Google Scholar]
- 48.Stevenson, A., and M. Roberts. 2002. Use of a rationally attenuated Bordetella bronchiseptica as a live mucosal vaccine and vector for heterologous antigens. Vaccine 202325-2335. [DOI] [PubMed] [Google Scholar]
- 49.Stevenson, A., and M. Roberts. 2003. Use of Bordetella bronchiseptica and Bordetella pertussis as live vaccines and vectors for heterologous antigens. FEMS Immunol. Med. Microbiol. 37121-128. [DOI] [PubMed] [Google Scholar]
- 50.Sukumar, N., M. Mishra, G. P. Sloan, T. Ogi, and R. Deora. 2007. Differential Bvg phase-dependent regulation and combinatorial role in pathogenesis of two Bordetella paralogs, BipA and BcfA. J. Bacteriol. 1893695-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.West, N. P., H. Jungnitz, J. T. Fitter, J. D. McArthur, C. A. Guzman, and M. J. Walker. 2000. Role of phosphoglucomutase of Bordetella bronchiseptica in lipopolysaccharide biosynthesis and virulence. Infect. Immun. 684673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woolfrey, B. F., and J. A. Moody. 1991. Human infections associated with Bordetella bronchiseptica. Clin. Microbiol. Rev. 4243-255. [DOI] [PMC free article] [PubMed] [Google Scholar]