Abstract
Complicated urinary tract infections (UTI) caused by Proteus mirabilis are associated with severe pathology in the bladder and kidney. To investigate the roles of two established cytotoxins, the HpmA hemolysin, a secreted cytotoxin, and proteus toxic agglutinin (Pta), a surface-associated cytotoxin, mutant analysis was used in conjunction with a mouse model of ascending UTI. Inactivation of pta, but not inactivation of hpmA, resulted in significant decreases in the bacterial loads of the mutant in kidneys (P < 0.01) and spleens (P < 0.05) compared to the bacterial loads of the wild type; the 50% infective dose (ID50) of an isogenic pta mutant or hpmA pta double mutant was 100-fold higher (5 × 108 CFU) than the ID50 of parent strain HI4320 (5 × 106 CFU). Colonization by the parent strain caused severe cystitis and interstitial nephritis as determined by histopathological examination. Mice infected with the same bacterial load of the hpmA pta double mutant showed significantly reduced pathology (P < 0.01), suggesting that the additive effect of these two cytotoxins is critical during Proteus infection. Since Pta is surface associated and important for the persistence of P. mirabilis in the host, it was selected as a vaccine candidate. Mice intranasally vaccinated with a site-directed (indicated by an asterisk) (S366A) mutant purified intact toxin (Pta*) or the passenger domain Pta-α*, each independently conjugated with cholera toxin (CT), had significantly lower bacterial counts in their kidneys ( P = 0.001) and spleens (P = 0.002) than mice that received CT alone. The serum immunoglobulin G levels correlated with protection (P = 0.03). This is the first report describing the in vivo cytotoxicity and antigenicity of an autotransporter in P. mirabilis and its use in vaccine development.
Proteus mirabilis, an etiological agent of complicated urinary tract infections (UTI) in humans, infects individuals with long-term catheterization, elderly residents in nursing homes, or individuals with postoperative wounds (35, 36). The consequences of UTI due to P. mirabilis may include catheter obstruction due to stone formation, urolithiasis, cystitis, pyelonephritis, and bacteremia (23, 26). Stone formation, resulting from urease-mediated urea hydrolysis in the urinary tract, causes severe tissue necrosis and inflammation at the site of infection (8, 11). In addition, it may also render the pathogen inaccessible to antibiotics, making infections difficult to treat. Thus, investigation of two important aspects of Proteus biology is warranted. First, identifying bacterial factors that cause cellular damage in the host would allow the possibility of antiserum-based neutralization. Second, identification of candidate antigens could lead to the development of effective vaccines against P. mirabilis. Although MR/P fimbriae and flagella are immunogenic and aid in the persistence of this pathogen in the host (3), their phase variation and antigenic variation may require that they be used in conjunction with other antigens (25, 40).
Apart from urease, hemolysin (HpmA) is the only other protein previously identified as a cytotoxin in P. mirabilis. This pore-forming secreted cytotoxin is produced by P. mirabilis during the mid-exponential or late exponential phase of growth (4, 13, 14, 39). Hemolysin, which is induced during swarmer cell differentiation of P. mirabilis and during infection (2, 6), lyses nucleated cells and erythrocytes (32, 33); however, its precise contribution to virulence has not been elucidated.
Recently, we discovered a novel bifunctional autotransporter (AT), proteus toxic agglutinin (Pta), in P. mirabilis HI4320 (1). Pta is a surface-associated, calcium-dependent, alkaline protease that exhibits time- and dose-dependent cytotoxicity with cultured epithelial cells. P. mirabilis cytotoxicity was significantly reduced in an isogenic pta mutant in vitro, and the mutant strain was also significantly outcompeted by the wild-type strain during cochallenge in a mouse model of Proteus UTI (1). In an unrelated study, our laboratory identified Pta independently as one of the immunogenic outer membrane proteins of P. mirabilis and demonstrated that Pta is expressed in vivo (27). Protease-like ATs, such as Sat in pathogenic Escherichia coli (34) or VacA in Helicobacter pylori (5), are known to elicit cytotoxic effects in vivo. Although the contribution of Pta to the pathogenesis of cystitis and pyelonephritis remains to be determined, in vitro studies (1) have provided a strong rationale to test the cytotoxicity and antigenic properties of Pta in vivo.
In this study, we compared the levels of inflammation and histopathology in the bladders and kidneys of mice infected independently with the parent strain HI4320, an isogenic pta mutant, or an hpmA mutant. We also studied the effect of an hpmA pta double mutant on the cytotoxicity of P. mirabilis both in vitro using cultured bladder epithelial cells and in vivo using a mouse model of ascending UTI. We tested the efficacy of Pta as a vaccine candidate by intranasally immunizing mice with purified Pta or with purified Pta-α (the Pta passenger domain alone) and individually testing the abilities of these preparations to protect against subsequent Proteus infection. We demonstrated that anti-Pta sera, obtained from immunized mice, neutralized the protease activity of Pta in vitro. To our knowledge, this is the first study in which the contribution of HpmA and Pta to the pathogenesis of P. mirabilis was systematically evaluated and the use of an AT in P. mirabilis as a vaccine candidate antigen was tested.
MATERIALS AND METHODS
All animal studies were performed with 6- to 8-week-old female CBA/J mice obtained from Harlan Sprague-Dawley, Indianapolis, IN. Anesthetized mice were transurethrally inoculated with 50 μl of washed P. mirabilis suspended in phosphate-buffered saline (PBS) (pH 7.2). The protocols used to perform mouse model infection studies were approved by the University of Michigan's University Committee on Use and Care of Animals (approval 08999) and have been described previously (18).
Bacterial strains and construction of mutants.
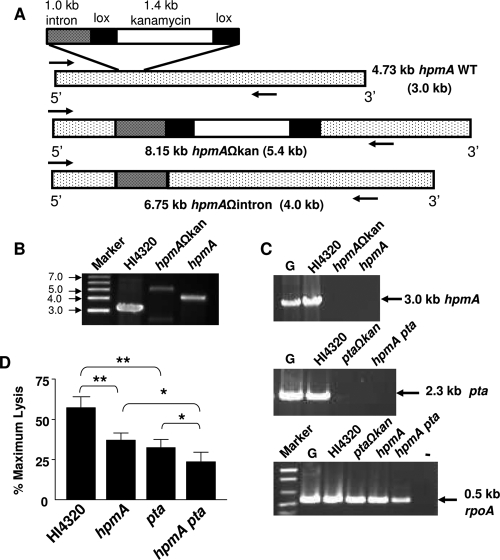
All cloning experiments were performed using Escherichia coli Top10. P. mirabilis HI4320, a human urinary tract isolate that is urease positive, hemolytic, motile, and fimbriated, was used as the parent strain in this study. An isogenic Pta (pta) mutant obtained by insertional inactivation of the gene using a TargeTron mutagenesis kit obtained from Sigma-Aldrich (catalog no. TA0010) as described previously (1) was used. A hemolysin (hpmAΩkan) mutant was also constructed using a similar approach. Primers HpmIBS (AAAAAAGCTTATAATTATCCTTACCCTCCACAACAGTGCGCCCAGATAGGGTG), HpmEBS2 (TGAACGCAAGTTTCTAATTTCGGTTGAGGGTCGATAGAGGAAGTGTCT), and HpmEBS1d (CAGATTG TACAAATGTGGTGATAACAGATAAGTCACAACACCTAACTTACCTTTCTTTGT) were designed according to the vendor's instructions. The group II intron template provided with the kit was retargeted by inserting hpmA-specific sequences by overlapping PCR using the protocol recommended by the vendor. The PCR product was cloned into the BsrGI and HindIII sites of vector pACD4K-C-loxP, which contains lox sequences flanking the kanamycin cassette. The resulting plasmid, pSAP2044, was transformed into electrocompetent P. mirabilis HI4320 carrying the T7 helper plasmid pAR1219 for insertional inactivation of hpmA. The resulting hpmAΩkan strain of P. mirabilis was designated ALM2012. Inactivation of hpmA was confirmed by observing the 2.4-kb increase in the size of the gene due to insertion of the kanamycin cassette (1.4 kb) and the adjoining intron (1.0 kb) (Fig. 1A and B). The mutant was then passaged in Luria broth several times to cure plasmid pAR1219; loss of the plasmid was confirmed by the ampicillin sensitivity of the hpmAΩkan strain. An hpmA pta double mutant was generated in two steps. First, the kanamycin cassette was excised from the hpmAΩkan strain by transforming the mutant with plasmid pQL123 (Ampr), which expresses Cre protein under an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter. Excision of the kanamycin cassette due to Cre loxP-based recombination was confirmed by PCR using primers HpmF (ATGAAATCAAAAAACTTTAAACTTTCACCC) and HpmR (TAATAGCAACGGTAATATCACTGCC), designed to amplify 3.0 kb toward the 5′ end of the hpmA gene, after which the hpmA strain was inactivated only by a 1.0-kb intron. The resulting hpmAΩintron strain was designated ALM2012B (Fig. 1A and B). We confirmed that the gene was inactivated in both cases by performing reverse transcription (RT)-PCR using primers HpmAF and HpmAR. The mutant strain was passaged in plain Luria broth several times to cure plasmid pQL123. The electrocompetent hpmA strain (Kans and Amps) was again transformed with T7 helper plasmid pAR1219 (ALM2012C) and subsequently transformed with plasmid pSAP2037 (plasmid pACD4K-C carrying group II intron retargeted to inactivate pta) (1). Inactivation of pta in the hpmA strain was confirmed by performing RT-PCR using primers PtaINTF (GGTTATATTCAAATTTACCTAGCAA) and PtaINTR (TTCAGAAGTTGGACAATTAGGTAAT), which amplified the 2.2-kb fragment toward the 5′ end of the pta gene (Fig. 1C), and by performing immunoblotting using rabbit polyclonal anti-Pta serum as described previously (1). The hpmA pta (Kanr) double mutant was designated ALM2014.
FIG. 1.
Construction of hpmA and hpmA pta mutants and estimation of cytotoxicity. (A) Schematic diagram of the 4.73-kb hpmA gene, showing the site of inactivation by a kanamycin cassette. The approximate sizes of the hpmA gene, hpmAΩkan, and hpmAΩintron are indicated; the sizes of the corresponding PCR products amplified using the internal primers HpmAF and HpmAR (arrows) are indicated in parentheses. WT, wild type. (B) Insertion and excision of the kanamycin cassette in the hpmAΩkan and hpmAΩintron strains, respectively, was confirmed by amplification of hpmA by PCR. (C) Inactivation of hpmA in the hpmAΩkan and hpmAΩintron strains (top) and inactivation of pta in the hpmA pta double mutant (middle) were confirmed by RT-PCR using gene-specific primers. rpoA, encoding RNA polymerase A, was used as the expression control (bottom). The sizes of PCR products are indicated on the right. Lane G, HI4320 genomic DNA, lane −, no RT added to the cDNA from strain HI4320. (D) P. mirabilis HI4320 and the mutants were independently cultured until mid-exponential phase (optical density at 600 nm, 0.6 to 0.7) in alkaline LB (pH 8.5) supplemented with CaCl2 and 5% glycerol. A washed suspension of bacteria (106 CFU) in PBS was laid over a confluent monolayer of bladder cells and incubated for 4 h. The extent of bladder cell lysis (determined by the quantitative LDH release assay) is expressed as a percentage of the maximum cell lysis obtained by Triton X-100 treatment. The data are the means and standard errors of three independent experiments, each conducted in triplicate. *, P < 0.05; **, P < 0.01.
Cytotoxicity assays.
The cytotoxicity of P. mirabilis HI4320 and each single and double mutant for human bladder epithelial cells was estimated by the lactate dehydrogenase (LDH) release method as described previously (1). Briefly, each P. mirabilis strain was cultured in LB (pH 8.5) to mid-exponential phase, and each washed suspension of bacteria in PBS (pH 7.5) was laid over a monolayer of human bladder epithelial cells (UMUC-3) at a multiplicity of infection of approximately 100:1. After 4 h of incubation at 37°C, the amount of LDH in the supernatant was estimated using a CytoTox membrane integrity assay kit (product no. G7890; Promega, Madison, WI) according to the vendor's instructions. Treatment of epithelial cells with 2% (wt/vol) Triton X-100 and treatment of epithelial cells with LB alone were used as positive and negative controls, respectively. The level of bladder cell lysis was expressed as a percentage of the maximal epithelial cell lysis obtained by Triton X-100 treatment.
Determination of ID50.
P. mirabilis parent strain HI4320 and each Proteus construct were cultured in LB broth overnight, harvested, washed in PBS (pH 7.2), and resuspended in PBS. The bacterial cell density was adjusted to obtain the desired inoculum. Six- to eight-week-old female CBA mice (nine mice/infectious dose/group) were transurethrally inoculated with the following doses: 5 × 106 and 5 × 107 CFU for parent strain HI4320 and the hpmA mutant; and 5 × 106, 5 × 107, and 5 × 108 CFU for the pta mutant and the hpmA pta double mutant. Each inoculum was quantified by plating serial dilutions (10−5 and 10−6) on LB agar, followed by enumeration of the CFU. One week after infection, mice were sacrificed, and bladder, kidney, and spleen tissue homogenates were plated on LB agar for enumeration of the CFU. The 50% infective doses (ID50) of the parent and mutant strains were calculated by using the method of Reed and Muench (28). The one-tailed Mann-Whitney test was used to determine the P value; a P value of <0.05 was considered significant.
Pathological evaluation.
A murine model of ascending UTI was used to evaluate the uropathogenicity of each of the single mutants and the double mutant of P. mirabilis. The following doses were used to infect mice based on the ID50 determined for each strain: each mouse received 5 × 106 CFU of P. mirabilis HI4320 or the isogenic hpmAΩkan strain or 5 × 108 CFU of the ptaΩkan strain or the hpmA pta double mutant. One week after transurethral infection, mice were sacrificed, and for each mouse the bladder, one-half of the left kidney cut longitudinally, and one-half of the right kidney cut transversely were preserved in 10% formalin (pH 7.2), embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined microscopically. The investigator was blind to the identity of the bacterial strain or mutant construct. The severity of renal pathology was expressed as a semiquantitative score for kidney damage by using the following scores for the severity of peripelvic inflammation: 0, no inflammation; 1, multifocal clusters of neutrophils (polymorphonuclear cells [PMNs]); 2, PMNs surrounding all or most of the pelvis; and 3, intense neutrophilic inflammation extending into the peripelvic tissue. The extent of spread was scored as follows: 0, no spread; 1, PMNs confined to the peripelvic region; 2, PMN clusters detectable in the papilla or peripelvic cortex; and 3, widespread extension of PMNs into the cortex or outer medulla. The severity of cystitis was scored as follows: 0, no cystitis; 1, occasional submucosal inflammatory cell infiltrates; 2, widespread submucosal inflammatory cell infiltration with minimal spread to the muscularis or epithelium; and 3, widespread inflammation with dense perivascular cuffs, transmural distribution, and intraepithelial inflammatory cells. Bladder edema was scored as follows: 0, no edema; 1, detectable edema; and 2, widespread and severe edema.
Antigen preparation.
For vaccination, both Pta and the alpha domain of Pta alone (Pta-α) with a C-terminal six-histidine tag were individually overexpressed in E. coli BL21/plysS and purified as previously described (1). To minimize the toxic effects of the protease, the recombinant proteins were constructed with an active-site serine mutation (S365A) and were designated Pta* and Pta-α*. Purified Pta* and Pta-α* were each covalently coupled to the mucosal adjuvant cholera toxin (CT) (Sigma Chemical Co.) at a ratio of 10:1. Covalent conjugation of CT with the purified antigen was accomplished using the heterobifunctional cleavable protein cross-linker N-succinimidyl-3-(2-pyridyldithio)propionate (Pierce Biotech) as instructed by the manufacturer.
Immunization and subsequent challenge of female CBA mice.
Mice were immunized by intranasal administration of the antigen-adjuvant complex. Mice were divided into two groups (23 mice in each group) for each antigen administered. In each case the control group received CT alone, whereas the immunized group received Pta* plus CT or Pta-α* plus CT. Immediately following immunization, mice were placed on their backs until they recovered from anesthesia. The immunization schedule was as follows. On day 1, a preimmune serum sample (collected by retro-orbital bleeding) and urine were collected from each mouse. Each mouse then received 50 μg of Pta* or Pta-α* covalently coupled with 5 μg of CT in 20 μl (total volume); 10 μl of the antigen-adjuvant complex was administered into each nostril. On days 7 and 14, the mice received the first and second booster doses, respectively, which consisted of 25 μg of Pta* or Pta-α* covalently coupled with 5 μg of CT. On day 21, postimmune serum and urine samples were collected from each mouse in both groups to determine antigen-specific antibody titers. Each mouse was then transurethrally challenged with 5 × 107 CFU of P. mirabilis HI4320. On day 28, 1 week postinfection, mice were euthanized, and tissue homogenates (bladder, kidney, and spleen) were plated on LB agar plates (0.5 g/liter NaCl) to determine the number of CFU/g tissue. The lower limit of detection in this assay was 102 CFU/g tissue, and this value was assigned to samples with an undetectable level of colonization. Data were analyzed using the one-tailed Mann-Whitney test, and a P value of <0.05 was considered significant.
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was performed using 96-well plates (Costar high binding; catalog no. 9017). Each well was coated with 250 ng of antigen (Pta* or Pta-α*) dissolved in 60 mM carbonate buffer. Twofold serial dilutions of serum or urine samples were individually prepared in blocking buffer (PBS containing 10% fetal bovine serum and 0.1% sodium azide) and added to the wells. Goat anti-mouse immunoglobulin G (IgG) or goat anti-mouse IgA, each conjugated with alkaline phosphatase, was used as the secondary antibody. The substrate p-nitrophenylphosphate was used to measure the alkaline phosphatase activity by determining the A405 after a 60-min reaction. The data were expressed as direct A405 values (16, 18). All data were analyzed using Prism software (GraphPad Software, Inc.). Immunoglobulin response and bacterial load data were compared using the nonparametric Mann-Whitney tests; Spearman's rank correlation coefficient values were evaluated as previously described (15).
Serum bactericidal assay.
The bactericidal assay was performed as described previously by Hadi et al. (9). Dilutions of postimmune serum (1:2 to 1:64) in sterile PBS were prepared and transferred into sterile 96-well tissue culture plates, and 50 μl of a P. mirabilis suspension (containing approximately 104 CFU) was added to each well. The plates were covered and incubated for 120 min at 37°C. Aliquots (50 μl) were plated onto agar plates at time zero and after 120 min of incubation to enumerate the CFU at each time point, and the lowest concentration of serum that lysed 50% of the bacterial cells was determined. Rabbit polyclonal anti-Pta serum (Cacalico Biologicals) and the preimmune sera were included in each plate as positive and negative controls, respectively. Other control wells contained bacteria with PBS-bovine serum albumen and bacteria with heat-inactivated postimmune sera (56°C, 30 min).
Serum neutralization assay.
To determine whether the Pta-specific antiserum neutralized the activity of Pta, P. mirabilis HI4320 was cultured to mid-exponential phase in modified Luria broth (see above), and approximately 106 bacteria were incubated with 1:2 to 1:16 serial dilutions of each serum sample. After 3 h, a washed bacterial suspension in PBS was laid over a monolayer of bladder epithelial cells, and in each case the percentage of cell lysis was estimated using the quantitative LDH release assay as described above. The bladder cell lysis was expressed as a percentage of the maximal cell lysis obtained by treatment with Triton X-100, which was used as a positive control, and the values were normalized using the data for lysis by the pta mutant. Data for bladder cell lysis by P. mirabilis HI4320 (not incubated with serum) were used for comparison.
RT-PCR analysis of pta from clinical isolates of P. mirabilis.
Eight randomly selected P. mirabilis strains obtained from cases of pyelonephritis or catheter-associated bacteriuria or fecal isolates were used to determine the prevalence of pta. The presence of this gene was first determined by PCR amplification using primers PtaINTF and PtaINTR (see above), which were designed to amplify an internal region of pta. This was done to avoid any amplification artifacts due to a nonhomologous DNA sequence in the intergenic region upstream and downstream of pta in various isolates. Each isolate was then individually cultured in Luria broth (pH 8.5) supplemented with 5% glycerol and 10 mM CaCl2 to promote the expression of pta as described previously (1). RNA was isolated from each culture, and cDNA was synthesized using a SuperScript first-strand synthesis kit (Invitrogen). Expression of pta was determined for each strain by RT-PCR using cDNA as the template and primers PtaINTF and PtaINTR. rpoA, encoding RNA polymerase A, was used as an expression control.
RESULTS
Cytotoxicity of HpmA and Pta.
The hpmA gene was inactivated in P. mirabilis HI4320 as shown in Fig. 1A. An hpmA pta double mutant of P. mirabilis was also created using a similar approach. Inactivation of the genes in the constructs was confirmed by RT-PCR (Fig. 1B and C).
The effect of inactivation of the two cytotoxin genes on the ability of P. mirabilis to lyse bladder epithelial cells in culture was determined by the LDH release assay. Inoculation of parent strain HI4320 resulted in lysis of approximately 65% of the bladder cells (Fig. 1D). The percentage was significantly lower (P < 0.01) for cells inoculated with either the isogenic hpmA (35%) or pta (30%) mutant incubated for the same length of time. The decrease in cytotoxicity was more pronounced when bladder cells were overlaid with the hpmA pta double mutant. After 4 h of incubation only 25% of the bladder cells were lysed, as estimated by the extent of LDH release (Fig. 1D). This value was significantly (P < 0.05) lower than the lysis value obtained with either of the single mutants and less than 50% of the value obtained with the wild-type strain. These results indicated that the activities of HpmA and Pta were independent of each other but additive in that together they contributed to cytotoxicity.
Mouse colonization assay and determination of ID50.
To investigate the individual roles of HpmA and Pta in vivo, we compared the cytotoxicities of the mutants using a mouse model of Proteus UTI. The ID50 of each of the strains was determined so that we could estimate the damage to the host tissue based on the same bacterial load.
Mice were independently challenged transurethrally with a range of doses, as described above, and the level of colonization obtained with each dose was determined by plating the tissue homogenates. Table 1 shows the median bacterial loads recovered from mice that received various doses of parent strain HI4320 or one of the mutant constructs. The ID50 of each strain was calculated using quantitative counts obtained for kidney homogenates for mice sacrificed 1 week after challenge, as described by others (11, 28). The ratios of the number of mice with kidney levels of >103 CFU/ml to the total number of mice challenged by inoculation were determined for a range of bacterial loads. The ID50 of the parent strain and the isogenic hpmA mutant calculated from these ratios was 5 × 106 CFU, whereas the ID50 of the pta and hpmA pta mutants was 100-fold greater (5 × 108 CFU), suggesting that Pta is an important virulence factor in P. mirabilis.
TABLE 1.
Colonization of mice by P. mirabilis HI4320 and isogenic mutants
| P. mirabilis construct | Log10 CFU/g tissue witha:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 × 106-CFU inoculum
|
5 × 107-CFU inoculum
|
5 × 108-CFU inoculum
|
|||||||
| Bladder | Kidney | Spleen | Bladder | Kidney | Spleen | Bladder | Kidney | Spleen | |
| HI4320 | 7.3 | 6.0 | 4.2 | 8.1 | 6.4 | 5.2 | ND | ND | ND |
| hpmA mutant | 6.7 (NS) | 5.4 (NS) | 4.8 (NS) | 6.8 (NS) | 6.2 (NS) | 6.0 (NS) | ND | ND | ND |
| pta mutant | 6.5 (P = 0.037) | 3.9 (P = 0.002) | 2.1 (P = 0.017) | 7.5 (NS) | 3.6 (P = 0.002) | 2.5 (P = 0.024) | 6.5 (NS) | 5.8 (NS) | 4.4 (NS) |
| hpmA pta mutant | 5.8 (P = 0.0012) | 4.1 (P = 0.0017) | 2.8 (P = 0.011) | 7.2 (NS) | 4.8 (P = 0.001) | 3.4 (P = 0.031) | 6.5 (NS) | 6.2 (NS) | 6.0 (NS) |
Median log10 CFU/g recovered from mouse tissue. The median for each construct was compared to the value for the wild type when the inoculum was 5 × 106 CFU. P values were calculated using the one-tailed Mann-Whitney U test, and a P value of <0.05 was considered significant. NS, not significant. ND, not determined.
Histopathologic evaluation of mouse bladders and kidneys.
To determine the effect of inactivation of either or both of the toxins on disease due to P. mirabilis, five groups of 6-week-old female CBA/J mice (six mice per group) were independently inoculated with P. mirabilis HI4320 or the pta, hpmA, or hpmA pta mutant by using a dose corresponding to the ID50, and the histopathology of the bladder and kidney was evaluated (Fig. 2 to 4).
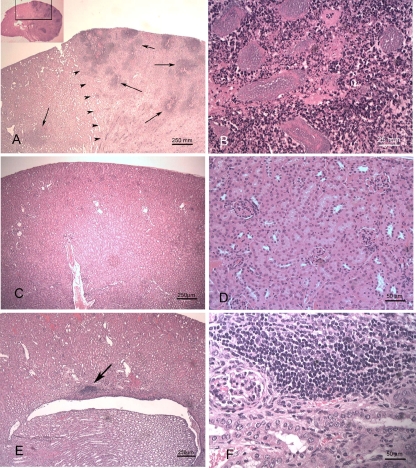
FIG. 2.
Mice were independently transurethrally inoculated with P. mirabilis HI4320 or the mutant constructs. After 7 days of infection, a kidney from each mouse was fixed, and the sections were stained for histopathological evaluation. (A) Kidney from a mouse inoculated with the hpmA mutant of P. mirabilis. Multiple foci of inflammation and necrosis are scattered throughout the cortex (arrows). The arrowheads delineate the border between more-normal tissue (left) and necrotic tissue (right). (Inset) Overview of the entire kidney, indicating the location of the section. (B) Higher magnification of panel A showing a focus of necrosis, neutrophilic inflammation, and bacterial colonies. (C) Normal kidney from an uninfected mouse. (D) Higher magnification of panel C. (E) Kidney from a mouse inoculated with wild-type P. mirabilis HI4320. A mild lymphocytic infiltrate is present adjacent to the renal pelvis (arrow). (F) Higher magnification of panel E showing lymphocytic infiltrate.
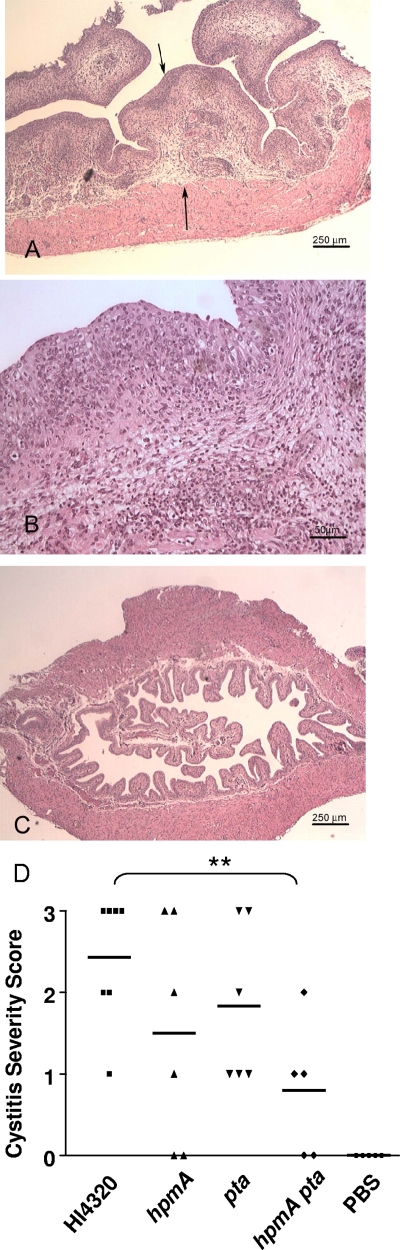
FIG. 4.
Histopathological evaluation of bladders from mice. (A) The urinary bladder obtained from a mouse after 7 days of infection with 5 × 106 CFU of parent strain HI4320 was processed for histopathological evaluation as described in the text. The mucosa is thickened with edema and inflammatory cells (arrows). (B) Higher magnification of panel A showing neutrophils within the epithelium. (C) Bladder from a mouse that received PBS as a control. (D) Cystitis severity after inoculation of various P. mirabilis constructs. The horizontal bars indicate the means. P values were calculated by the one-tailed Mann-Whitney U test. **, P < 0.01.
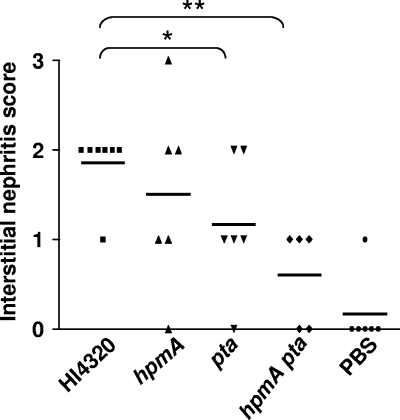
Colonization of the kidneys by P. mirabilis strain HI4320 was accompanied by neutrophilic interstitial nephritis centered on the peripelvic renal cortex and in some cases extending into and destroying the surrounding renal parenchyma (Fig. 2E and F) compared to the results to uninfected mice (Fig. 2C and D). Similar pathology was observed for the hpmA mutant (Fig. 2A and B), and in some cases bacterial colonies were present in the lesions (Fig. 2B). The intensity and extent of inflammation in kidneys ranged from none to severe, and scores of 0 to 3 were obtained as described in Materials and Methods (Fig. 3). The levels of severity and extents of kidney inflammation were similar in mice colonized by wild-type P. mirabilis and mice colonized by the hpmA mutant. However, in mice colonized by the pta mutant or the hpmA pta double mutant, both the severity and the extent of nephritis were significantly less (P < 0.05) than the severity and the extent of nephritis in mice colonized by the wild-type strain (Fig. 3). Uninfected mice had no nephritis (Fig. 3).
FIG. 3.
Interstitial nephritis caused by P. mirabilis HI4320 and isogenic mutants of this strain. Histopathology was scored as described in Materials and Methods. The horizontal bars indicate the means. P values were calculated by the one-tailed Mann-Whitney U test. *, P < 0.05; **, P < 0.01.
Cystitis in infected mice was characterized by transmural neutrophilic inflammation with epithelial transcytosis of neutrophils and marked submucosal edema (Fig. 4A and B). The intensity and extent of inflammation in the bladders ranged from none to severe, and scores of 0 to 3 were obtained as described in Materials and Methods. The hpmA pta double mutant caused significantly less inflammation than wild-type strain HI4320 (P < 0.01) (Fig. 4D). Uninfected mice had no cystitis (Fig. 4C and D). Uncompromised urease activity in each of these infections was indicated by the presence of crystal deposits (i.e., stones) that were observed in the majority of the bladder cross sections (data not shown).
Protection against P. mirabilis challenge provided by vaccination with Pta.
AT proteins have been used as effective vaccines against bacterial infections. The App proteins from Neisseria meningitidis, pertactin and BrkA from Bordetella pertussis, and Hap from Haemophilus influenzae are examples of ATs that have provided immune protection against subsequent challenge with the corresponding pathogens. Taking into account the immunogenic properties of Pta (27) and its role in colonization of the host, we asked whether vaccination of mice with Pta provides protection against subsequent challenge with P. mirabilis. CT, which was successfully used in our laboratory as an adjuvant for intranasal vaccination of mice with MrpH antigen (an adhesin of M/RP fimbria) (17), was used as a mucosal adjuvant.
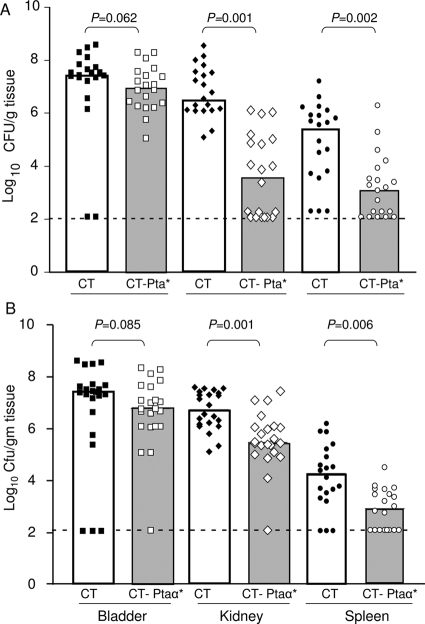
Vaccination with Pta* reduced the level of P. mirabilis infection in the bladder of mice (Fig. 5A); the median number of CFU/g was about 1 log10 lower than the value for CT-immunized (naïve) mice, although the difference was not statistically significant (P = 0.062). However, the protection in the kidney and spleen was significant (P < 0.01); the median values were log10 6.3 and log10 5.6 CFU/g tissue, respectively, for the naïve group of mice and log10 3.5 and 3.0 CFU/g tissue, respectively, for immunized mice. Indeed, for 9 of 20 mice in the immunized group the bacterial counts were below the limit of detection, suggesting that there was robust protection of the upper urinary tract.
FIG. 5.
Immune protection from P. mirabilis challenge by vaccination with Pta* and Pta-α*. Mice were vaccinated and boosted with CT-Pta* (A) or CT-Pta-α* (B) as described in the text and were challenged intraurethrally on day 21 with 5 × 107 CFU P. mirabilis HI4320. The numbers of P. mirabilis CFU in bladders, kidneys, and spleens of naïve mice (which received CT alone) and mice immunized with CT-Pta-α* or CT-Pta* were determined 1 week after bacterial challenge. Each symbol indicates the log10 CFU/g of tissue from an individual mouse. Samples in which colonization was undetectable were given a value of 2.1 log10 CFU/g of tissue (the limit of detection). The bars indicate medians. P values were determined using the one-tailed Mann-Whitney U test; a P value of <0.05 was considered significant.
Since the passenger/alpha domain (Pta-α) is the functional moiety of the AT and directly interacts with the host, we asked if vaccination with Pta-α alone protects mice from P. mirabilis infection. Two groups of mice (22 mice in each group) were intranasally vaccinated either with CT alone or with a Pta-α*-CT conjugate. Pta-α provided protection similar to that observed with Pta*. Mice challenged with P. mirabilis following immunization and boosting had significantly lower (P < 0.01) bacterial loads in their kidneys and spleens than the naïve mice (Fig. 5B). Thus, we concluded that the alpha/passenger domain alone can stimulate a strong immune response that significantly reduces colonization by P. mirabilis.
Correlates of protective immunity.
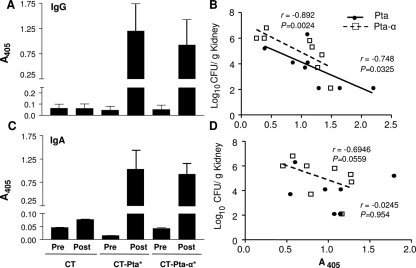
Sera from mice immunized with Pta* or Pta-α* were tested to determine their antibody responses. After immunization and boosting, the serum IgG titers (Fig. 6A) and urine IgA titers (Fig. 6C) for mice vaccinated with Pta*-CT or Pta-α*-CT were significantly higher (P < 0.01) than the titers for sera and urine from the preimmune samples or from mice that received CT alone (Fig. 6A and C), suggesting that there was a strong immune response against the antigen. The immune response (serum IgG in Pta*- and Pta-α*-immunized mice) also showed a significant inverse correlation (P = 0.0024 and P = 0.0325 for Pta*- and Pta-α*-immunized mice, respectively) with the bacterial load (that is, when the antibody levels were low, the numbers of CFU were high, and when antibody levels were high, the numbers of CFU were low or bacteria were undetectable), as determined by Spearman's rank correlation coefficient values (Fig. 6B and D), suggesting that both the magnitude and the specificity of the antibody response were critical correlates of protection. The correlation varied from moderate for mice immunized with Pta-α* (P = 0.0559) to none with Pta* (P = 0.954). There was no difference in the IgM titers for any group (data not shown), suggesting that there was efficient and complete class switching of immunoglobulins.
FIG. 6.
Correlation of IgG and IgA titers with protection. The antibody responses in mice to intranasal vaccination with CT-Pta*, with CT-Pta-α*, or with CT alone were determined by an indirect ELISA. Serum IgG and urine IgA titers in pre- and postimmune sera and urine collected from each group of immunized and naïve mice were determined using a 1:512 dilution of serum or urine. Goat anti-rabbit IgA or IgG conjugated with alkaline phosphatase was used as the secondary antibody. The alkaline phosphatase activity was determined at A405 60 min after addition of the substrate. The means and standard errors for serum IgG (A) and urine IgA (C) titers for eight mice (with each experiment performed in duplicate) are indicated. The bacterial load recovered from immunized mice 1 week after challenge was tested to determine correlations with Pta*- or Pta-α*-specific serum IgG (B) and urine IgA (D) responses. Both the anti-Pta and anti-Pta-α serum IgG titers were inversely correlated with the bacterial load, whereas the inverse correlation was significant only for Pta-α*-specific urine IgA and was not significant for Pta*. P values were calculated for correlation coefficients, and a P value of <0.05 was considered significant. Spearman's rank correlation coefficients (r) are indicated. The diagonal lines indicate linear regression.
Neutralization of Pta by anti-Pta sera.
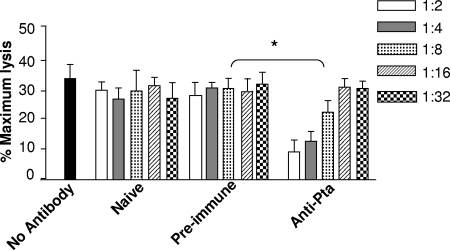
Since Pta* stimulated a strong antibody response and provided significant protection against P. mirabilis infection, we determined whether anti-Pta serum was bactericidal or neutralizing. The bactericidal activities of serial dilutions (1:2 to 1:32) of antisera obtained from different groups of mice were tested as described in Materials and Methods. No antiserum-based bacterial cell lysis was observed even when cells were incubated with the highest concentration (1:2) of the sera tested (data not shown), indicating that there was no direct anti-Pta serum-mediated bactericidal activity. However, for P. mirabilis HI4320 cells preincubated for 3 h with anti-Pta sera there was a significant decrease (P < 0.05) in the ability of the bacteria to lyse cultured bladder cells compared to the cytotoxicity of the same strain that was not incubated with antisera or to the cytotoxicity of bacteria incubated with preimmune serum or sera from mice that received CT alone (Fig. 7). Indeed, the residual cytotoxicity of P. mirabilis incubated with Pta-specific sera for bladder cells was only about 10% of the maximal cytotoxicity (lysis) observed (Fig. 7). The lowest concentration of anti-Pta sera that reduced the bladder cell lysis (by P. mirabilis) by 50% was 1:8. These results indicated that anti-Pta sera not only recognized Pta on the homologous parent strain but also compromised the activity of the toxin. No significant reduction in the extent of bladder cell lysis was observed when bladder cells were incubated with P. mirabilis treated with the lower concentrations (1:16 and 1:32) of the same serum (Fig. 7). These results suggest that the mode of protection against Proteus infection in the Pta*-vaccinated mice may involve antibody-based neutralization of Pta.
FIG. 7.
Neutralization of Pta by anti-Pta sera. LDH release was used as a measure of bladder cell lysis after inoculation of P. mirabilis preincubated for 3 h with different dilutions of sera. The y axis indicates bladder cell lysis expressed as a percentage of the maximum lysis (after treatment with 2% [wt/vol] Triton X-100) normalized to the bladder cell lysis by the pta mutant. No antibody, strain HI4320 incubated with no serum; Naive, strain HI430 preincubated with sera collected from CT-immunized mice on day 21; Pre-immune, strain HI430 preincubated with sera collected from mice on day 1 prior to vaccination with Pta*; Anti-Pta, strain HI430 preincubated with sera collected on day 21 from mice immunized with Pta*. The one-tailed Mann-Whitney U test was used to determine P values. *, P < 0.05.
Prevalence and expression of pta in P. mirabilis.
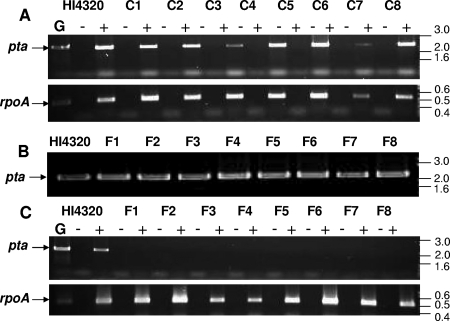
The vaccination data suggested that Pta can be used as an effective candidate vaccine to protect against P. mirabilis infection. Since ATs are pathogen specific, we wanted to know whether Pta is present in pathogenic as well as commensal strains of P. mirabilis. Each strain was independently cultured, and the genomic DNA and cDNA were isolated. Amplification by PCR showed that pta was present in all fecal, catheter-associated, and pyelonephritis (data not shown) isolates tested (Fig. 8B). Whereas pta was expressed in all the pyelonephritis (data not shown) and catheter-associated strains tested (Fig. 8A), none of the fecal isolates showed any detectable transcript, as determined by RT-PCR (Fig. 8C). This finding was in contrast to the data obtained for mrpH (encoding an adhesin of MR/P fimbria) (18) or the urease activities that were observed in all P. mirabilis isolates, suggesting that Pta is pathogen specific.
FIG. 8.
Prevalence of Pta in P. mirabilis isolates. Eight catheter-associated strains (C1 to C8) and eight fecal isolates (F1 to F8) were independently cultured to mid-exponential phase in LB (pH 8.5) supplemented with 10 mM CaCl2 and 5% glycerol. (A) Expression of pta in catheter-associated strains was determined by RT-PCR using pta-specific primers; cDNA obtained from each strain was used as a template. (B) Prevalence of pta in the genomes of fecal isolates as determined by PCR of genomic DNA. (C) Expression of pta in the strains as determined by RT-PCR using cDNA obtained from each isolate and pta-specific primers. rpoA, encoding RNA polymerase A, was used as an expression control. cDNA from strain HI4320 was used as the reference. Lane G, genomic DNA from strain HI4320 used as a template; lanes −, no RT added; lanes +, RT added. Sizes (in kb) are indicated on the right.
DISCUSSION
Complicated UTI caused by P. mirabilis are characterized by severe necrosis of the kidney and bladder epithelium, pelvic inflammation, and stone formation in the bladder and kidney (23, 36, 37). Hemolysin, urease, and Pta are the cytotoxins characterized so far in this uropathogen (1, 24, 38, 39) that have been implicated in this damage in the host. In this study, we demonstrated that inactivation of hpmA or pta alone or inactivation of both hpmA and pta significantly reduces the cytotoxicity of P. mirabilis with cultured bladder epithelial cells (Fig. 1D). The effects of the mutations in vivo also exhibited this general trend. Using histopathological examination of the kidneys and bladders from mice inoculated with the mutant strains (hpmA or pta), we observed a moderate decrease in interstitial nephritis or the severity of cystitis. A significant decrease (P < 0.01) in pathology (compared to that of the parent strain), however, was observed only when both toxin genes were inactivated (Fig. 3 and 4D). Thus, we identified similar, yet independent, roles for the two toxins in P. mirabilis infection. This was in contrast to the activity of HlyA from a uropathogenic strain of E. coli; in this organism inactivation of hylA alone was directly associated with a decrease in the shedding of uroepithelium in a mouse model of UTI (31). Nevertheless, we also observed a certain degree of residual pathology (necrosis of pelvic epithelium, cystitis, or interstitial nephritis) in mice inoculated with the hpmA pta double mutant (Fig. 3 and 4D) that could be attributed to other factors in Proteus, including urease.
Urease, a urea-inducible, cytoplasmic metalloenzyme in P. mirabilis, is essential for the persistence of P. mirabilis in the urinary tract. This enzyme enables the bacterium to use urea as a source of nitrogen, which is required for DNA and protein synthesis. Ammonia released during urea hydrolysis directly causes tissue necrosis at the site of infection. In addition, the ammonium and bicarbonate ions formed alkalinize the urinary tract and initiate precipitation of otherwise soluble Ca2+ and Mg2+ salts present in the urine (11). Prior to this report, urease was the only other protein with an established cytotoxic effect in vivo. Direct comparison of the pathology of the kidney and bladder in mice infected with a urease-negative strain of P. mirabilis with the pathology of the kidney and bladder in mice infected with the parent strain showed that there was a dramatic decrease in tissue necrosis and pelvic inflammation with the former strain (11). In this study, we propose yet another indirect role of urease activity. Pta, unlike HpmA, is a calcium-dependent, alkaline protease with a pH optimum of 8.5 to 9.0; these conditions are prevalent in a Proteus-infected urinary tract (1). Thus, Pta activity during infection is likely urease dependent. We hypothesize that the phenotype of a urease-negative strain (ureC) of P. mirabilis (12) may be in part a consequence of both a reduction in the expression and a reduction in the enzymatic activity of Pta. Thus, under nonalkaline conditions a urease-negative strain indirectly behaves like a ureC pta mutant of P. mirabilis.
A set of efficacious candidate antigens must be identified in order to develop a vaccine against Proteus infections. Flagellin (FlaA/FlaB), MR/P fimbrial protein MrpH (17, 18), recombinant Lactococcus lactis displaying Proteus fimbrial protein MrpA (30), and outer membrane protein (22) of Proteus were all previously considered as candidate antigens for a vaccine. Limitations such as the phase variability of MR/P fimbriae (and thus MrpH) and recombination of FlaA with FlaB (25, 40) mean that these proteins must be used in combination with other stable antigens as part of a multivalent vaccine. Thus, an ideal vaccine candidate should be stably expressed during infection and should contribute to virulence. Hence, we compared the colonization efficiency of each of the single mutants (hpmA or pta) with that of the parent strain to determine the individual roles of the proteins in colonization and persistence of P. mirabilis in the host. We found no role for HpmA in colonization by the pathogen (that is, the bacterial burden) in the host (Table 1), an observation consistent with that of Swihart and Welch (32). On the other hand, inactivation of pta significantly decreased (P < 0.01) the viable counts recovered from the upper urinary tract of mice compared with the data obtained with the parent strain (Table 1). Indeed, the ID50 of the isogenic pta mutant was found to be 100-fold higher than that of the parent strain. Because Pta is not only a key cytotoxin in P. mirabilis but also a surface-exposed protein that is critical for establishing an infection in the host, this protein appeared to be a logical choice for testing as a vaccine candidate.
The attenuated form of Pta was used for vaccination of mice. Immune protection against infection by mucosal pathogens, such as P. mirabilis, also requires an effective mucosal adjuvant. CT, which was successfully used as a mucosal adjuvant previously in our laboratory, was also used here. This adjuvant was covalently coupled with the antigen (Pta* or Pta-α*), a condition that promotes the critical interaction of CT with dendritic cells in the spleen and thus an effective immune response (7). As expected, immunization with Pta*-CT or Pta-α*-CT provided significant protection against Proteus UTI, especially in the upper urinary tract (Fig. 5). This protection correlated strongly with the serum IgG response elicited by immunization (Fig. 6C), but it correlated only weakly with the urine IgA response. We attribute the difference to the possible localized expression of Pta in the urinary tract. Both the mutant analysis study and the immune protection study showed that a lack of Pta or possible neutralization of Pta by serum does not significantly affect the colonization by the pathogen in the bladder, suggesting that the role of this protein in the lower urinary tract is insignificant. Hence, even though the urine IgA titer was higher in Pta*-vaccinated mice than in CT-immunized mice, the protection was not significant in the bladder, due to only basal levels of Pta expression on the surface of P. mirabilis in bladder. Hence, we propose that protection against P. mirabilis in Pta*-immunized mice may be due to serum IgG-mediated neutralization of Pta in the upper urinary tract of mice. Previous studies in our laboratory have shown that unlike infection with uropathogenic E. coli, where the antibody titer from a previous infection correlates with the short resolution time of a secondary infection (10), primary infection with P. mirabilis or immunization of naïve mice with sera obtained from immunized mice does not confer passive protection against P. mirabilis infection (19). Hence, passive protection with Pta-specific sera was not tested in this study.
ATs such as Pta are considered pathogen-specific proteins produced by gram-negative bacteria, and few of them (BrkA, Hap, and VacA) are required for virulence (20, 21, 29). Consistent with these observations, we showed that Pta was a pathogen-specific protein in P. mirabilis (Fig. 8). The reasons for a lack of pta expression in the fecal isolates were, however, not clear, but this finding provided a strong basis for use of this antigen as a vaccine against pathogenic strains of P. mirabilis; that is, commensal strains of P. mirabilis would not be affected by a Pta vaccine. This observation also suggested that strains of P. mirabilis may not be as homogeneous as once thought as genetic variation is possible, especially when pathogen-specific factors (such as ATs) are considered.
In conclusion, we established the roles of two different cytotoxins, hemolysin and proteus toxic agglutinin, in the pathogenesis of P. mirabilis. To our knowledge, this is also the first report that describes a systematic evaluation of HpmA in vivo and also provides insight into factors other than urease that contribute to host tissue damage. Identification of Pta as an effective antigen is a promising step toward our long-term goal of developing a vaccine against complicated UTIs caused by P. mirabilis. However, further investigation to identify an appropriate adjuvant for use in humans, as well as an effective antigen delivery system, is required.
Acknowledgments
We thank the histology laboratory at Michigan State University, East Lansing, for processing mouse tissue for our histopathology study.
This project was funded by Public Service Grant AI-059722 from the National Institutes of Health to H.L.T.M.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 24 November 2008.
REFERENCES
- 1.Alamuri, P., and H. L. Mobley. 2008. A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol. Microbiol. 68997-1017. [DOI] [PubMed] [Google Scholar]
- 2.Allison, C., H. C. Lai, and C. Hughes. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 61583-1591. [DOI] [PubMed] [Google Scholar]
- 3.Bahrani, F. K., D. E. Johnson, D. Robbins, and H. L. Mobley. 1991. Proteus mirabilis flagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect. Immun. 593574-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun, V., and T. Focareta. 1991. Pore-forming bacterial protein hemolysins (cytolysins). Crit. Rev. Microbiol. 18115-158. [DOI] [PubMed] [Google Scholar]
- 5.Cover, T. L., and S. R. Blanke. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3320-332. [DOI] [PubMed] [Google Scholar]
- 6.Fraser, G. M., L. Claret, R. Furness, S. Gupta, and C. Hughes. 2002. Swarming-coupled expression of the Proteus mirabilis hpmBA haemolysin operon. Microbiology 1482191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grdic, D., L. Ekman, K. Schon, K. Lindgren, J. Mattsson, K. E. Magnusson, P. Ricciardi-Castagnoli, and N. Lycke. 2005. Splenic marginal zone dendritic cells mediate the cholera toxin adjuvant effect: dependence on the ADP-ribosyltransferase activity of the holotoxin. J. Immunol. 1755192-5202. [DOI] [PubMed] [Google Scholar]
- 8.Griffith, D. P., D. M. Musher, and C. Itin. 1976. Urease. The primary cause of infection-induced urinary stones. Investig. Urol. 13346-350. [PubMed] [Google Scholar]
- 9.Hadi, H. A., K. G. Wooldridge, K. Robinson, and D. A. Ala'Aldeen. 2001. Identification and characterization of App: an immunogenic autotransporter protein of Neisseria meningitidis. Mol. Microbiol. 41611-623. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins, W. J., and D. T. Uehling. 1995. Resolution time of Escherichia coli cystitis is correlated with levels of preinfection antibody to the infecting Escherichia coli strain. Urology 4542-46. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, D. E., R. G. Russell, C. V. Lockatell, J. C. Zulty, J. W. Warren, and H. L. Mobley. 1993. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect. Immun. 612748-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, B. D., C. V. Lockatell, D. E. Johnson, J. W. Warren, and H. L. Mobley. 1990. Construction of a urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 581120-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaca, W., and A. Rozalski. 1991. Characterization of cell-bound and cell-free hemolytic activity of Proteus strains. Eur. J. Epidemiol. 7159-165. [DOI] [PubMed] [Google Scholar]
- 14.Kotelko, K., W. Kaca, A. Rozalski, and M. Deka. 1983. Some biological features of Proteus bacilli. 2. Haemolytic activities of Proteus mirabilis and Proteus vulgaris strains. Acta Microbiol. Pol. 32345-351. [PubMed] [Google Scholar]
- 15.Kwissa, M., R. R. Amara, H. L. Robinson, B. Moss, S. Alkan, A. Jabbar, F. Villinger, and B. Pulendran. 2007. Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. J. Exp. Med. 2042733-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, W., M. H. Sofi, N. Yeh, S. Sehra, B. P. McCarthy, D. R. Patel, R. R. Brutkiewicz, M. H. Kaplan, and C. H. Chang. 2007. Thymic selection pathway regulates the effector function of CD4 T cells. J. Exp. Med. 2042145-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, X., J. L. Erbe, C. V. Lockatell, D. E. Johnson, M. G. Jobling, R. K. Holmes, and H. L. Mobley. 2004. Use of translational fusion of the MrpH fimbrial adhesin-binding domain with the cholera toxin A2 domain, coexpressed with the cholera toxin B subunit, as an intranasal vaccine to prevent experimental urinary tract infection by Proteus mirabilis. Infect. Immun. 727306-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, X., C. V. Lockatell, D. E. Johnson, M. C. Lane, J. W. Warren, and H. L. Mobley. 2004. Development of an intranasal vaccine to prevent urinary tract infection by Proteus mirabilis. Infect. Immun. 7266-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, X., and H. L. Mobley. 2002. Vaccines for Proteus mirabilis in urinary tract infection. Int. J. Antimicrob. Agents 19461-465. [DOI] [PubMed] [Google Scholar]
- 20.Liu, D. F., K. W. Mason, M. Mastri, M. Pazirandeh, D. Cutter, D. L. Fink, J. W. St. Geme III, D. Zhu, and B. A. Green. 2004. The C-terminal fragment of the internal 110-kilodalton passenger domain of the Hap protein of nontypeable Haemophilus influenzae is a potential vaccine candidate. Infect. Immun. 726961-6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marr, N., D. C. Oliver, V. Laurent, J. Poolman, P. Denoel, and R. C. Fernandez. 2008. Protective activity of the Bordetella pertussis BrkA autotransporter in the murine lung colonization model. Vaccine 264306-4311. [DOI] [PubMed] [Google Scholar]
- 22.Moayeri, N., C. M. Collins, and P. O'Hanley. 1991. Efficacy of a Proteus mirabilis outer membrane protein vaccine in preventing experimental Proteus pyelonephritis in a BALB/c mouse model. Infect. Immun. 593778-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mobley, H. L. T., and J. W. Warren. 1987. Urease-positive bacteriuria and obstruction of long-term urinary catheters J. Clin. Microbiol. 252216-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mobley, H. L., and G. R. Chippendale. 1990. Hemagglutinin, urease, and hemolysin production by Proteus mirabilis from clinical sources. J. Infect. Dis. 161525-530. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, C. A., and R. Belas. 1999. Genomic rearrangements in the flagellin genes of Proteus mirabilis. Mol. Microbiol. 31679-690. [DOI] [PubMed] [Google Scholar]
- 26.Nemoy, N. J., and T. A. Staney. 1971. Surgical, bacteriological, and biochemical management of “infection stones.” JAMA 2151470-1476. [PubMed] [Google Scholar]
- 27.Nielubowicz, G. R., S. N. Smith, and H. L. Mobley. 2008. Outer membrane antigens of the uropathogen Proteus mirabilis recognized by the humoral response during experimental murine urinary tract infection. Infect. Immun. 764222-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed, L. J., and H. A. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 29.Salama, N. R., G. Otto, L. Tompkins, and S. Falkow. 2001. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect. Immun. 69730-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scavone, P., A. Miyoshi, A. Rial, A. Chabalgoity, P. Langella, V. Azevedo, and P. Zunino. 2007. Intranasal immunization with recombinant Lactococcus lactis displaying either anchored or secreted forms of Proteus mirabilis MrpA fimbrial protein confers specific immune response and induces a significant reduction of kidney bacterial colonization in mice. Microbes Infect. 9821-828. [DOI] [PubMed] [Google Scholar]
- 31.Smith, Y. C., S. B. Rasmussen, K. K. Grande, R. M. Conran, and A. D. O'Brien. 2008. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect. Immun. 762978-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swihart, K., and R. A. Welch. 1990. Cytotoxic activity of the Proteus hemolysisn HpmA. Infect. Immun. 581861-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swihart, K. G., and R. A. Welch. 1990. The HpmA hemolysin is more common than HlyA among Proteus isolates. Infect. Immun. 581853-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taddei, C. R., A. Fasano, A. J. Ferreira, L. R. Trabulsi, and M. B. Martinez. 2005. Secreted autotransporter toxin produced by a diffusely adhering Escherichia coli strain causes intestinal damage in animal model assays. FEMS Microbiol. Lett. 250263-269. [DOI] [PubMed] [Google Scholar]
- 35.Warren, J. W. 1991. The catheter and urinary tract infections. Med. Clin. N. Am. 75481-493. [DOI] [PubMed] [Google Scholar]
- 36.Warren, J. W., D. Damron, J. H. Tenney, J. M. Hoopes, B. Deforge, and H. L. Muncie, Jr. 1987. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J. Infect. Dis. 1551151-1158. [DOI] [PubMed] [Google Scholar]
- 37.Warren, J. W., H. L. Muncie, Jr., and M. Hall-Craggs. 1988. Acute pyelonephritis associated with bacteriuria during long-term catheterization: a propspective clinicopathological study. J. Infect. Dis. 1581341-1346. [DOI] [PubMed] [Google Scholar]
- 38.Welch, R. A. 1991. Pore-forming cytolysins of gram-negative bacteria. Mol. Microbiol. 5521-528. [DOI] [PubMed] [Google Scholar]
- 39.Welch, R. A., C. Forestier, A. Lobo, S. Pellett, W. Thomas, Jr., and G. Rowe. 1992. The synthesis and function of the Escherichia coli hemolysin and related RTX exotoxins. FEMS Microbiol. Immunol. 529-36. [DOI] [PubMed] [Google Scholar]
- 40.Zhao, H., X. Li, D. E. Johnson, I. Blomfield, and H. L. Mobley. 1997. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol. Microbiol. 231009-1019. [DOI] [PubMed] [Google Scholar]