Abstract
Many pathogens engage host cell surface glycosaminoglycans, but redundancy in pathogen adhesins and host glycosaminoglycan-anchoring proteins (heparan sulfate proteoglycans) has limited the understanding of the importance of glycosaminoglycan binding during infection. The alpha C protein of group B streptococcus, a virulence determinant for this neonatal human pathogen, binds to host glycosaminoglycan and mediates the entry of bacteria into human cells. We studied alpha C protein-glycosaminoglycan binding in Drosophila melanogaster, whose glycosaminoglycan repertoire resembles that of humans but whose genome includes only three characterized membrane heparan sulfate proteoglycan genes. The knockdown of glycosaminoglycan polymerases or of heparan sulfate proteoglycans reduced the cellular binding of alpha C protein. The interruption of alpha C protein-glycosaminoglycan binding was associated with longer host survival and a lower bacterial burden. These data indicate that the glycosaminoglycan-alpha C protein interaction involves multiple heparan sulfate proteoglycans and impairs bacterial killing. Host glycosaminoglycans, anchored by multiple proteoglycans, thereby determine susceptibility to infection. Because there is homology between Drosophila and human glycosaminoglycan/proteoglycan structures and many pathogens express glycosaminoglycan-binding structures, our data suggest that interfering with glycosaminoglycan binding may protect against infections in humans.
Streptococcus agalactiae (group B streptococcus [GBS]) commonly colonizes human mucosal surfaces and also causes devastating invasive infections, particularly in neonates. The main identified mammalian host defense against GBS is opsonophagocytosis, requiring phagocytes, complement, and specific antibody. GBS virulence determinants include surface structures such as capsular polysaccharide, cytolysin, and the protein designated d-alanylation of lipoteichoic acid (DltA) (10, 22, 25). Protecting the organism from phagocytosis, capsular polysaccharide is a high-molecular-weight polymer composed of more than 100 repeating pentasaccharide units, each with a terminal sialic acid moiety. Cytolysin promotes enhanced GBS survival during infection by contributing to phagocyte cytolysis and apoptosis and by helping shield GBS from oxidative damage (18). Deficiency in DltA is associated with increased susceptibility to phagocytosis and to antimicrobial peptides (25).
Alpha C protein (ACP) is an important GBS virulence determinant that binds to host cell glycosaminoglycan (GAG) and mediates bacterial entry into human cells (3, 4, 5) in vitro. We aimed to further characterize the host cell receptor and to determine the importance of the ACP-GAG interactions in vivo.
GAGs are highly sulfated modified polysaccharide polymers anchored in the cell membrane by a protein core as a heparan sulfate proteoglycan (HSPG). Among the most abundant cell surface GAGs are heparan sulfate (HS) and chondroitin sulfate (CS). HS consists of iduronic or glucuronic acid and N-acetylglucosamine, while CS consists of iduronic or glucuronic acid and N-acetylgalactosamine. Both HS and CS structures have variable patterns of sulfation. Several groups of core proteins have been identified, including the syndecans, which have intracytoplasmic associations allowing interactions with the actin cytoskeleton and other cellular signaling cascades, and the glypicans, which are covalently linked to membrane lipids through a glycosylphosphatidylinositol anchor. The syndecans carry both HS and CS chains, while the glypicans carry predominantly HS (7).
HSPGs are expressed widely on eukaryotic cell surfaces, where they serve important roles in diverse processes such as cell adhesion, growth factor signaling, and tumor biology. These structures also may be usurped by pathogens to enter cells or stimulate cell signaling (1, 24). Determining the contribution of a GAG or of a particular HSPG to subsequent events and infection outcome is especially complex because a cell may express multiple HSPGs and different HSPG proteins may display the same GAG (16, 33). Specifically, we found that the ME180 cell line used in prior studies expresses at least nine different HSPGs, making a genetic approach with mammals to determine which HSPG(s) binds to ACP impractical. Drosophila melanogaster is a more attractive system for determining the contribution of HSPGs to infection. This organism expresses a repertoire of GAGs similar to that of human cells (32), using three GAG polymerase genes (ttv, DEXT2 [sotv], and DEXT3 [botv]) (11, 31) that have human homologues. D. melanogaster has only three membrane-anchored proteoglycans (syndecan [sdc], dally, and dally-like protein) (15, 21, 29); these also have human homologues. The simplicity of the D. melanogaster system allowed us to investigate the contribution of ACP-GAG binding to bacterial pathogenesis. We found that this binding interaction determines infection outcome in Drosophila.
MATERIALS AND METHODS
Bacteria.
GBS strain A909 expresses ACP (19). A909/R185A expresses an ACP variant with a point mutation eliminating GAG binding (3, 4). Strains A909ΔcpsE (14), A909ΔcylE (27), and NEM316ΔDltA (NEM1636) (26) have been described previously and were grown in Todd-Hewitt broth (THB) with selective antibiotics. ACP and D2-R proteins, truncated ACP constructs containing the epitope that binds to human cells (2), were expressed as described previously (2, 5).
Cells.
S2 cells were grown at 25°C in Schneider's medium with 10% fetal calf serum and 1% penicillin-streptomycin (Invitrogen). For flow cytometry, cells were incubated with Alexa Fluor 488-labeled bovine serum albumin (BSA), ACP, D2-R, or a D2-R construct with a charge-neutralizing mutation in the putative GAG-binding residue 185, 172, or 196 (prepared using a kit [Molecular Probes]) as described previously (4); at least 10,000 cells per sample were analyzed with a FACSCalibur instrument (Becton Dickinson) and Cellquest software. For inhibition studies, cells were treated with heparin or sodium chlorate as described previously (3, 4).
RNA interference (RNAi).
Primers (Table 1) were designed with guidance from the data at http://www.flyrnai.org/. Control sequences targeted the green fluorescent protein (GFP) gene (amplified from the construct encoding the GFP variant GFPuv [Clontech]), smoothened (smo), and minibrain (mnb). The Megascript kit (Ambion) was used to synthesize double-stranded RNAs (dsRNAs). After cells were washed with serum-free medium, aliquots of 105 cells in 40 μl of serum-free medium were added to 250 ng of targeting dsRNA in 10 μl of water. Cells were incubated at 25°C for 45 min for dsRNA uptake before the addition of 80 μl of Schneider's medium (with fetal calf serum and penicillin-streptomycin). Cells were stained 96 h later.
TABLE 1.
Regions targeted by dsRNA sequences
D. melanogaster.
Heterozygous mutants were generated by crossing strain OreR flies with flies carrying HSPG mutant alleles of dally, sdc, and both dally and dally-like genes. Alleles from the following sources were used.
sdc.
Bloomington Stock Center numbers 10431 = y[1] w[67c23];P{w[+mC] = lacW} Sdc[k10215]/CyO (Berkeley Drosphila Genome Project), which has a P-element insertion causing a mutation in sdc, and 8585 = w[*]; Df(2R)48, P{w[+mC] = Ubi-Sara.J}2/CyO, P{w[+mC] = ActGFP}JMR1 (12), with an sdc gene deficiency, were used.
dally.
dally80 (8), a null allele with a deletion from −724 to +415, and Bloomington Stock Center number 3024= Df(3L)h-i22, h[i22] Ki[1] m[roe-1] p[p]/TM3, Ser[1], with a dally gene deficiency, were used. A homozygous dally80 genotype leads to defects in the distribution and signaling of Hedgehog, a morphogen; dally80 homozygotes die as third-instar larvae.
dally and dally-like genes.
dally80 dlyA187 constructs (8) carried null alleles for both the dally and dally-like protein HSPGs; dlyA187 has a deletion of 26 nucleotides resulting in a reading frameshift from amino acid 205, and the product therefore lacks part of the cysteine-rich region, the entire GAG attachment domain, and the glycosylphosphatidylinositol-anchoring signal.
GAG polymerase ttv and sotv alleles were derived from strain FRTG13 ttv00681 sotv 1.8.1/CyO (a kind gift of Rahul Warrior) (6). The sotv 1.8.1 allele generates an sotv protein truncated at residue 184 and lacking the catalytic motif.
Infections.
Bacteria were grown to an optical density at 650 nm of 0.3, and the concentrations were then adjusted to approximately 1.5 × 109 CFU/ml; the exact stock concentration was determined in each experiment. Male flies between 2 and 5 days old were anesthetized with CO2 and then pricked in the dorsal thorax with a needle dipped in THB or a slurry of GBS in THB. Flies were infected in groups of 20 to 30 and then placed into vials with food, and the vials were incubated at 29°C. Kaplan-Meier plots were derived, and the median survival time for each group was determined. Survival curves were compared using a log-rank test (20). To determine the bacterial burden, 10 flies per condition were ground in 200 μl of phosphate-buffered saline (PBS) with 0.025% Triton X-100. Samples were spun at 3,000 rpm for 2 min after the addition of 800 μl PBS with 0.025% Triton X-100. Supernatant dilutions were plated; colony counts were compared using Student's t test.
RESULTS
GBS causes lethal infection in Drosophila by using virulence factors identified in mammalian models.
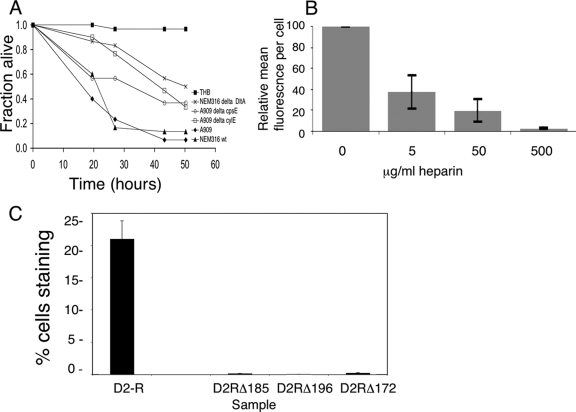
We evaluated the Drosophila model by infecting adult flies with wild-type GBS and with GBS variants carrying mutations in virulence determinants identified previously in mammalian infection models—capsular polysaccharide, cytolysin, and DltA (10, 22, 25). Flies pricked with needles dipped in THB alone survived, while those pricked with needles dipped in a slurry of wild-type GBS strains died rapidly (Fig. 1A). All of the GBS mutants were attenuated compared to wild-type GBS, demonstrating that similar virulence factors operate in Drosophila and in mammals.
FIG. 1.
Drosophila as a model for GBS infection. (A) Male flies were infected with GBS and then incubated at 29°C. Data from a representative experiment with at least 30 flies per sample are shown. By Kaplan-Meier and log-rank analyses, median survival times for flies infected with wild-type GBS strains A909 and NEM316 were 21.5 and 27 h, respectively; strains with mutations in virulence determinants identified in mammalian models (cpsE, cylE, and dltA) were attenuated in Drosophila also, with median survival times of infected flies of 43.2, 43.2, and 80.2 h, respectively (P, <0.005 for each mutant versus the parent strain). wt, wild type. (B) Fluorescence-activated cell sorter analysis shows labeled-ACP binding to S2 cells with concentration-dependent inhibition by soluble heparin. Data are shown as the MFI ± SE per cell relative to that for untreated cells, normalized to the fluorescence of cells treated with labeled BSA. With 500 μg/ml heparin, the MFI was 2.5% ± 0.2% of that for untreated cells (P = 0.001; Student's t test). (C) Fluorescence-activated cell sorter analysis shows that the level of S2 cell binding to mutant D2-R constructs with charge-neutralizing mutations in GAG-binding residues (Arg185, Arg172, and Lys196 [D2RΔ185, D2RΔ172, and D2RΔ196]) was markedly lower than the level of S2 cell binding to the native D2-R protein. The data are expressed as the mean percentage ± SE of cells staining positive for the indicated protein.
Drosophila S2 cells and human cells bind to ACP with similar characteristics.
We next investigated whether the GBS ACP binds to D. melanogaster S2 cells, which have properties of phagocytes/macrophages. S2 cells bound to fluorescently labeled ACP as well as to a truncated ACP construct (D2-R) containing the epitope that binds to human cells (2). Like binding to human cells (3), binding to S2 cells was inhibited in a concentration-dependent manner by soluble heparin (Fig. 1B) or by sodium chlorate, an inhibitor of cellular sulfate incorporation. Specifically, with 50 mM sodium chlorate, the mean fluorescence intensity (MFI) ± the standard error (SE) was 27.9% ± 1.1% of that for untreated cells (P < 0.001). These data suggest the involvement of a host cell GAG receptor. In contrast, the binding of fluorescent BSA was unchanged by these treatments. Further supporting the involvement of a host GAG receptor are results from studies showing marked reduction of binding to S2 cells by D2-R constructs with charge-neutralizing mutations in any of three GAG-binding residues predicted from the crystal structure and confirmed previously in human cell studies (4). Specifically, labeled BSA (negative control) bound 2% of cells, while D2-R, D2-R with the mutation R185A (D2-R/R185A), D2-R/R172A, and D2-R/K196A bound 20.99, 0.14, 0.22, and 0.03% of cells, respectively (Fig. 1C).
GAG polymerases are required for ACP binding to Drosophila S2 cells.
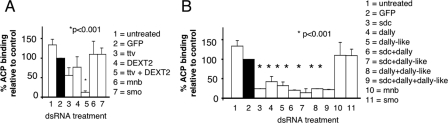
To determine which component(s) of the GAG-HSPG synthesis machinery is required for ACP-S2 cell binding, we depleted GAG expression using RNAi. We targeted the GAG polymerase genes ttv and DEXT2, whose products form a complex that adds alternating N-acetylglucosamine (GlcNAc) and glucuronic acid (GlcUA) residues to the protein-GAG linkage region after DEXT3 initiates the HS chain (11). The individual knockdown of ttv or DEXT2 expression (Fig. 2A) decreased ACP binding; ttv knockdown had a larger effect (P = 0.06 versus the control; Student's t test). When ttv and DEXT2 were both depleted, the level of ACP binding decreased to 14% of that of control cells (P < 0.01 versus the control; Student's t test). These data indicate that the ttv/DEXT2 GAG polymerase complex is critical for ACP binding to S2 cells.
FIG. 2.
GAG polymerases and HSPGs are required for the binding of ACP to S2 cells. (A) S2 cells were untreated or treated with dsRNA sequences targeting the GAG polymerase genes ttv, DEXT2 (sotv), and both ttv and DEXT2 (sotv) or with control sequences targeting the GFP gene, smo (encoding smoothened, a transmembrane receptor that binds to the segment polarity protein Hedgehog), or mnb (encoding minibrain, a serine/threonine protein kinase) prior to incubation with Alexa Fluor 488-labeled ACP. Data are shown as the MFI ± SE relative to that for the sample treated with dsRNA targeting GFP in each repetition of the experiment. The targeting of either the ttv or DEXT2 (sotv) polymerase reduced the binding of ACP compared to that by cells that were untreated or treated with control dsRNA sequences (targeting the GFP gene, mnb, or smo). The targeting of ttv (P = 0.06 versus results for the targeting of the GFP gene; Student's t test) had greater effect than the targeting of DEXT2; this effect was augmented by targeting DEXT2 in addition to ttv (P < 0.001). (B) Drosophila S2 cells were untreated or treated with dsRNA sequences targeting individual HSPGs (sdc, dally, and dally-like protein) or HSPGs in combination (sdc and dally, sdc and dally-like protein, dally and dally-like protein, and sdc, dally, and dally-like protein) or with control sequences (targeting the GFP gene, mnb, or smo) prior to incubation with Alexa Fluor 488-labeled ACP. The targeting of each HSPG alone, or of any combination of HSPGs, reduced the binding of ACP.
All Drosophila membrane HSPGs contribute to ACP-S2 cell binding.
We next assessed the contribution of HSPG(s) to ACP binding by treating S2 cells with RNAi sequences targeting the three D. melanogaster membrane HSPGs (sdc, dally, and dally-like protein) and comparing the binding of these cells to ACP with the binding of untreated cells or cells treated with control RNAi sequences. As shown (Fig. 2B), the depletion of any HSPG significantly impaired the binding of ACP to S2 cells.
GAG depletion protects Drosophila from infection with GBS.
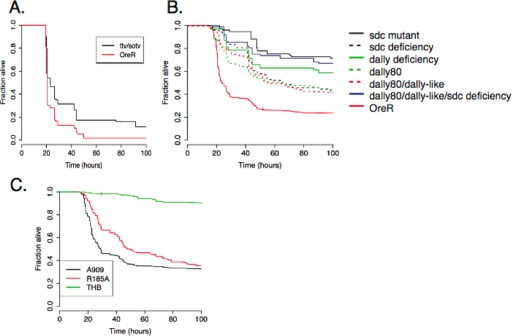
To determine whether host GAG polymerases play a role in susceptibility to infection, adult fly mutants with alterations in both ttv and DEXT2 (sotv) were generated. Compound heterozygotes were used because homozygotes do not survive to adulthood. Wild-type flies were more susceptible to infection with A909 than were ttv/sotv mutants (Fig. 3A) (median time of survival of wild-type flies, 20 h, versus 23 h for mutants [P < 0.001; log-rank test]), indicating that these host GAG polymerases promote GBS virulence.
FIG. 3.
GAG-binding activity of ACP promotes GBS virulence. (A) The products of Drosophila genes ttv and DEXT2 (sotv) form a heterodimeric GAG-polymerizing complex. We generated heterozygous ttv DEXT2 (sotv) mutants by crossing strain FRTG13 ttv00681 sotv 1.8.1/CyO with wild-type strain OreR. The Kaplan-Meier survival plot reveals that these mutants resisted lethal infection with wild-type GBS strain A909 more effectively than did wild-type OreR flies (P < 0.001). (B) We crossed mutant lines with OreR to generate flies with heterozygous mutations in the HSPGs sdc and dally, as well as flies with mutations in both dally and dally-like protein and in sdc, dally, and dally-like protein. All mutants resisted infection with A909 compared to wild-type OreR flies (P, <0.001 versus wild-type results). (C) A charge-neutralizing mutation in GBS strain A909/R185A impairs ACP-GAG binding. The Kaplan-Meier survival plot shows longer survival after fly infection with the mutant than after that with wild-type A909 (median survival times, 26.5 ± 0.04 h for A909 infection versus 48 ± 0.04 h for A909/R185A infection [P < 0.001; log-rank test]). Results for the sterile THB-injected control sample are shown. Plots show results from at least 70 flies per sample.
HSPG deficiency protects Drosophila from infection with GBS.
To determine the role of host HSPGs in susceptibility to lethal infection, we infected adult Drosophila flies expressing haploinsufficiency in sdc, dally, or the combination of dally and dally-like protein or sdc, dally, and dally-like protein. Heterozygotes again were used because homozygous mutants do not survive to adulthood. Two different alleles of sdc and of dally were included. In contrast to wild-type Drosophila, all HSPG mutants resisted infection with wild-type GBS (Fig. 3B). A strain heterozygous for mutations in all three HSPGs resisted infection more effectively than did strains with mutations in fewer HSPGs.
GAG binding of ACP determines the virulence of GBS in Drosophila.
To determine more specifically the importance of the ACP-GAG interaction in vivo, we used GBS strain A909/R185A. This strain has a single charge-neutralizing mutation in ACP that specifically eliminates GAG binding and reduces GBS entry into human epithelial cells without altering protein crystal structure or expression and without changing the bacterial growth rate or polysaccharide capsule production (4). As shown (Fig. 3C), strain A909/R185A was attenuated in Drosophila compared to strain A909 (median fly survival time following infection with A909, 26 h, versus 48 h with A909/R185A [P < 0.001; log-rank test]), indicating that the GAG-binding activity of ACP promotes GBS virulence in vivo in Drosophila.
HSPG deficiency protects Drosophila from lethal infection with GAG binding-deficient GBS.
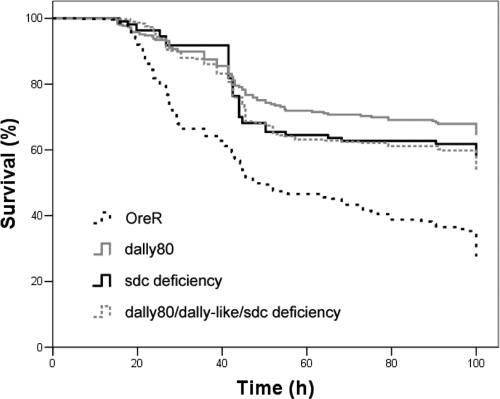
To confirm that the disruption of ACP-GAG binding underlies the survival advantage of (i) strain OreR after infection with A909/R185A compared to strain OreR after infection with A909 and (ii) HSPG mutant flies compared to wild-type flies after infection with A909, we infected adult Drosophila flies expressing haploinsufficiency in sdc, dally, or the combination of dally, dally-like protein, and sdc with GBS strain A909/R185A, the strain with a charge-neutralizing mutation that impairs GAG binding. Heterozygote flies again were used because homozygous mutants do not survive to adulthood. We hypothesized that survival times among wild-type OreR and HSPG mutant flies might be similar if direct ACP-GAG binding was the only factor influencing host survival in the earlier studies. We found that HSPG mutant hosts survived longer than wild-type OreR flies (Fig. 4). These data have several potential implications. (i) GBS may bind GAG/HSPG through non-ACP structures (or through regions of ACP outside the mutation area) that promote virulence. (ii) HSPG deficiency may also promote host survival through mechanisms independent of binding to bacterial virulence determinants.
FIG. 4.
HSPG deficiency confers a survival advantage on the host infected with GBS expressing GAG binding-deficient ACP. We crossed HSPG mutant flies with strain OreR to generate flies with heterozygous mutations in one or more HSPGs, as indicated, and then infected OreR flies and the resulting mutants with A909/R185A, the GBS strain with a charge-neutralizing mutation impairing ACP-GAG binding. Mutant flies survived longer than OreR flies (median survival times, >100 h for all mutants versus 48 h for OreR flies [P < 0.005; log-rank test]). Flies injected with broth only survived at rates above 90% (data not shown).
Interruption of GAG binding is associated with a lower GBS burden.
To determine how HSPGs promote lethal infection in Drosophila, we assessed the total bacterial burdens at 20 to 30 h after infection and found lower GBS colony counts for infections with A909/R185A, the strain carrying a charge-neutralizing mutation that impairs GAG binding, than for those with wild-type strain A909 (Fig. 5A). We also found lower numbers of GBS in the Drosophila flies with HSPG haploinsufficiency than in the wild-type hosts (Fig. 5B). Overall, the findings support the conclusion that bacterial survival is enhanced by the Drosophila GAG-bacterial ACP interaction, which promotes bacterial entry into host cells. In the absence of the Drosophila GAG-bacterial ACP interaction, bacterial killing and fly survival are favored.
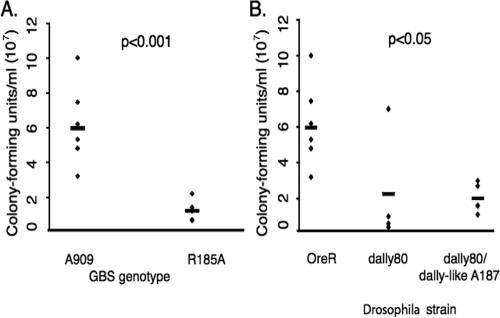
FIG. 5.
HSPG mutations are associated with lower bacterial burdens after infection. (A) Wild-type Drosophila (strain OreR) adults were infected with wild-type GBS strain A909 or strain A909/R185A, which carries a point mutation and expresses a non-GAG-binding variant of ACP. Twenty to thirty hours later, 10 flies of each type were homogenized in PBS with 0.025% Triton X-100, and the samples were plated. Infections with A909/R185A were associated with lower bacterial burdens (means, 6 × 107 CFU/ml for A909 versus 1.2 × 107 CFU/ml for A909/R185A [P < 0.001; Student's t test]). (B) OreR and HSPG mutant adult Drosophila flies were infected with GBS. By the same methods the bacterial burden in OreR flies was found to be higher than that in HSPG mutants (means, 6 × 107 CFU/ml for OreR flies versus 2.2 × 107 CFU/ml for dally80 mutants [P < 0.05] and 2.0 × 107 CFU/ml for dally80/dally-like double mutants [P = 0.004]). Each experiment was performed at least four times.
DISCUSSION
Structures that bind to host cell GAG are expressed by many pathogens. The contributions of the binding interactions to pathogenesis are difficult to study, in part because of the redundancy of both bacterial adhesins and mammalian GAG-anchoring proteins. To determine the role of the GBS GAG-binding ACP in pathogenesis, we have validated a model of GBS infection in Drosophila, an organism with less genome redundancy than mammals, by showing that virulence determinants of GBS identified in mammalian models also promote virulence in Drosophila. We also found that features of the ACP-human cell interaction (the involvement of an epitope in the D2-R region of ACP and concentration-dependent inhibition by soluble heparin or by sodium chlorate [3]) are shared by the ACP-Drosophila cell interaction.
In this model, we found that cell surface GAG, rather than a particular HSPG, confers susceptibility to infection. Specifically, the optimal binding of Drosophila cells to ACP in vitro required GAG polymerase enzymes. In vivo, deficiency in the GAG polymerase enzymes ttv and DEXT2 protected the flies from lethal infection. All three membrane-associated HSPGs were required for the full binding of ACP to Drosophila cells. At least two of these HSPGs (sdc and the glypican dally) were required for normal Drosophila susceptibility to infection in vivo, suggesting that a threshold amount of cell surface GAG may be required for normal susceptibility to infection. The ACP-GAG interaction is critical in infection outcome in this model, as a strain of GBS expressing an ACP variant with a single point mutation that eliminates ACP-GAG binding was attenuated in virulence. The prolonged survival of HSPG-deficient flies compared to that of wild-type flies after infection with this mutant GBS strain suggests that additional GAG-binding activity may occur outside of the region of our mutation—either in ACP or in other GBS structures—and/or that HSPG deficiency promotes Drosophila host survival through mechanisms independent of binding directly to bacterial virulence determinants, such as influencing immune system development. Lower burdens of bacteria were seen in infected flies in which the ACP-GAG interaction was specifically disrupted than in controls, suggesting that disrupting ACP-GAG binding enhances bacterial clearance. The in vivo findings are particularly striking in light of two factors expected to bias the results of these studies away from enhanced survival among mutant flies: (i) any mutation might be expected to weaken overall host health, increasing rather than decreasing death rates from infection, and (ii) the studies were performed using heterozygotes, which retain one functional gene. In particular, we predict that these hosts express a substantial amount of residual GAG, since there are at least three matrix HSPG core proteins in Drosophila in addition to the membrane HSPGs we have studied here. Future work toward identifying a subset of specific GAG structures that interact with ACP may facilitate the complex and technically challenging goal of measuring the levels of these particular GAGs in the wild-type and heterozygote mutant hosts.
Several explanations may account for the finding that multiple HSPGs are similarly required for the ACP-cell binding and ACP-mediated virulence results. It is possible that these HSPGs do not interact directly with ACP; instead, perhaps, they must be present in order for another unidentified host factor to develop and/or be displayed. However, the enhanced survival among Drosophila flies when the GAG-ACP interaction was disrupted by modifying residues of ACP without changing expression levels of the HSPGs suggests direct ACP-HSPG interaction. Another explanation is that each HSPG is expressed in a different organ in vivo (http://flyatlas.org), such that decreasing the expression of the predominant HSPG in a given location may dramatically diminish the total GAG available to bind to ACP in that location. For example, dally expression is highest in the brain and sdc expression is highest in the larval fat body. Site-specific HSPG expression may partially explain our findings in vivo, but the S2 cellular RNAi data indicate that each cell requires all three HSPGs for normal ACP binding. These data suggest that each HSPG binds to ACP independently or perhaps that HSPG expression patterns are mutually dependent or that the HSPGs form a complex that binds to ACP and the absence of any of them impairs the interaction.
In studies of Drosophila development, different HSPGs may perform redundant functions and may also have unique roles (13, 30). Such studies suggest that the GAG chains mediate some biological effects and that the core proteins mediate others (17, 28). For mammals, in vivo studies have been more limited. Because mammalian cells may express numerous HSPGs concurrently, distinguishing the role of GAG from that of the core protein is especially complex. In a mouse deficient in the HSPG syndecan-1, susceptibility to infection with Pseudomonas aeruginosa after intraperitoneal inoculation is normal. Susceptibility is reduced in both a model of burn-associated sepsis and a model of intranasally induced pneumonia in newborn mice but is restored by the administration of the soluble GAG heparin. These data suggest that GAG binding without specific HSPG activation may play a significant role in the outcomes of pathogenesis (9, 23), but the contributions of other syndecans, glypicans, and other HSPGs in these processes have not been described. In Drosophila, we found that ACP binding to host cell GAG, rather than to a particular HSPG, promoted bacterial survival and host demise. In this model, the interruption of ACP-GAG binding may enhance bacterial killing by keeping bacteria in the extracellular space so that they can be killed by secreted factors such as antimicrobial peptides, which are an important component of Drosophila defense against bacterial infection.
As many pathogens engage cells by binding to host GAG, the manipulation of GAG-binding interactions may protect vulnerable hosts from a broad range of infections. The Drosophila model allowed us to study a large sample of hosts with defects in multiple gene products that have redundant functions and to understand the importance of evolutionarily conserved host GAGs in determining host susceptibility to infection. This model is a useful platform on which to dissect more subtle interactions between the pathogen and the host. Further studies are warranted to determine whether GAG binding plays a similarly significant role in mammalian GBS infection outcome, as well as whether GAG-binding interactions determine the outcome of infection with other pathogens in both Drosophila and mammals.
Acknowledgments
This work was supported by National Institutes of Health grants AI059495 (to M.J.B.) and AI38424 (to L.C.M.) and by the March of Dimes Birth Defects Foundation Basil O'Connor Starter Scholar Award no. 5-FY06-580 (to M.J.B.).
We thank Norbert Perrimon, Jennifer Philips, Richard Binari, Chrysoula Pitsouli, Michele Markstein, Elliott Kieff, Dennis Kasper, Lindsay Flax, Huong Ung, David Berry, Gerald Pier, and Milan Bajmoczi for discussions, advice, and technical assistance. We thank Victor Nizet and Patrick Trieu-Cuot for GBS strains A909ΔcylE and NEM1636, respectively.
Editor: A. Camilli
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Alvarez-Dominguez, C., J. A. Vazquez-Boland, E. Carrasco-Marin, P. Lopez-Mato, and F. Leyva-Cobian. 1997. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect. Immun. 6578-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auperin, T. C., G. R. Bolduc, M. J. Baron, A. Heroux, D. J. Filman, L. C. Madoff, and J. M. Hogle. 2005. Crystal structure of the N-terminal domain of the group B Streptococcus alpha C protein. J. Biol. Chem. 28018245-18252. [DOI] [PubMed] [Google Scholar]
- 3.Baron, M. J., G. R. Bolduc, M. B. Goldberg, T. C. Auperin, and L. C. Madoff. 2004. Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. J. Biol. Chem. 27924714-24723. [DOI] [PubMed] [Google Scholar]
- 4.Baron, M. J., D. J. Filman, G. A. Prophete, J. M. Hogle, and L. C. Madoff. 2007. Identification of a glycosaminoglycan binding region of the alpha C protein that mediates entry of group B streptococci into host cells. J. Biol. Chem. 28210526-10536. [DOI] [PubMed] [Google Scholar]
- 5.Bolduc, G. R., M. J. Baron, C. Gravekamp, C. S. Lachenauer, and L. C. Madoff. 2002. The alpha C protein mediates internalization of group B Streptococcus within human cervical epithelial cells. Cell. Microbiol. 4751-758. [DOI] [PubMed] [Google Scholar]
- 6.Bornemann, D. J., J. E. Duncan, W. Staatz, S. Selleck, and R. Warrior. 2004. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development 1311927-1938. [DOI] [PubMed] [Google Scholar]
- 7.David, G., V. Lories, B. Decock, P. Marynen, J. J. Cassiman, and H. Van den Berghe. 1990. Molecular cloning of a phosphatidylinositol-anchored membrane heparan sulfate proteoglycan from human lung fibroblasts. J. Cell Biol. 1113165-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han, C., T. Y. Belenkaya, B. Wang, and X. Lin. 2004. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development 131601-611. [DOI] [PubMed] [Google Scholar]
- 9.Haynes, A., III, F. Ruda, J. Oliver, A. N. Hamood, J. A. Griswold, P. W. Park, and K. P. Rumbaugh. 2005. Syndecan 1 shedding contributes to Pseudomonas aeruginosa sepsis. Infect. Immun. 737914-7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hensler, M., G. Liu, S. Sobczak, K. Benirshcke, V. Nizet, and G. Heldt. 2005. Virulence role of group B Streptococcus beta-hemolysin/cytolysin and carotenoid pigment function in a neonatal rabbit model of early-onset pulmonary infection. J. Infect. Dis. 1911287-1291. [DOI] [PubMed] [Google Scholar]
- 11.Izumikawa, T., N. Egusa, F. Taniguchi, K. Sugahara, and H. Kitagawa. 2006. Heparan sulfate polymerization in Drosophila. J. Biol. Chem. 2811929-1934. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, K. G., A. Ghose, E. Epstein, J. Lincecum, M. B. O'Connor, and D. Van Vactor. 2004. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr. Biol. 14499-504. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, K. G., A. P. Tenney, A. Ghose, A. M. Duckworth, M. E. Higashi, K. Parfitt, O. Marcu, T. R. Heslip, J. L. Marsh, T. L. Schwarz, J. G. Flanagan, and D. Van Vactor. 2006. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron 49517-531. [DOI] [PubMed] [Google Scholar]
- 14.Jones, A., R. Needham, A. Clancy, K. Knoll, and C. Rubens. 2003. Penicillin-binding proteins in Streptococcus agalactiae: a novel mechanism for evasion of immune clearance. Mol. Microbiol. 47247-256. [DOI] [PubMed] [Google Scholar]
- 15.Khare, N., and S. Baumgartner. 2000. Dally-like protein, a new Drosophila glypican with expression overlapping with wingless. Mech. Dev. 99199-202. [DOI] [PubMed] [Google Scholar]
- 16.Kim, C. W., O. A. Goldberger, R. L. Gallo, and M. Bernfield. 1994. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell 5797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkpatrick, C. A., S. M. Knox, W. D. Staatz, B. Fox, D. M. Lercher, and S. B. Selleck. 2006. The function of a Drosophila glypican does not depend entirely on heparan sulfate modification. Dev. Biol. 300570-582. [DOI] [PubMed] [Google Scholar]
- 18.Liu, G. Y., K. S. Doran, T. Lawrence, N. Turkson, M. Puliti, L. Tissi, and V. Nizet. 2004. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc. Natl. Acad. Sci. USA 10114491-14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel, J. L., L. C. Madoff, K. Olson, D. E. Kling, D. L. Kasper, and F. M. Ausubel. 1992. Large, identical, tandem repeating units in the C protein alpha antigen gene, bca, of group B streptococci. Proc. Natl. Acad. Sci. USA 8910060-10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, R. 1998. Survival analysis. Wiley, New York, NY.
- 21.Nakato, H., T. A. Futch, and S. B. Selleck. 1995. The division abnormally delayed (dally) gene: a putative integral membrane proteoglycan required for cell division patterning during postembryonic development of the nervous system in Drosophila. Development 1213687-3702. [DOI] [PubMed] [Google Scholar]
- 22.Nizet, V., R. Gibson, and C. Rubens. 1997. The role of group B streptococci beta-hemolysin expression in newborn lung injury. Adv. Exp. Med. Biol. 418627-630. [DOI] [PubMed] [Google Scholar]
- 23.Park, P. W., G. B. Pier, M. T. Hinkes, and M. Bernfield. 2001. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature 41198-102. [DOI] [PubMed] [Google Scholar]
- 24.Pethe, K., S. Alonso, F. Biet, G. Delogu, M. J. Brennan, C. Locht, and F. D. Menozzi. 2001. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 412190-194. [DOI] [PubMed] [Google Scholar]
- 25.Poyart, C., E. Pellegrini, M. Marceau, M. Baptista, F. Jaubert, M. C. Lamy, and P. Trieu-Cuot. 2003. Attenuated virulence of Streptococcus agalactiae deficient in D-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Mol. Microbiol. 491615-1625. [DOI] [PubMed] [Google Scholar]
- 26.Poyart-Salmeron, C., C. Carlier, P. Trieu-Cuot, et al. 1990. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet 3351422-1426. [DOI] [PubMed] [Google Scholar]
- 27.Pritzlaff, C. A., J. C. Chang, S. P. Kuo, G. S. Tamura, C. E. Rubens, and V. Nizet. 2001. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol. Microbiol. 39236-247. [DOI] [PubMed] [Google Scholar]
- 28.Rawson, J. M., B. Dimitroff, K. G. Johnson, X. Ge, D. Van Vactor, and S. B. Selleck. 2005. The heparan sulfate proteoglycans Dally-like and Syndecan have distinct functions in axon guidance and visual-system assembly in Drosophila. Curr. Biol. 15833-838. [DOI] [PubMed] [Google Scholar]
- 29.Spring, J., S. E. Paine-Saunders, R. O. Hynes, and M. Bernfield. 1994. Drosophila syndecan: conservation of a cell-surface heparan sulfate proteoglycan. Proc. Natl. Acad. Sci. USA 913334-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steigemann, P., A. Molitor, S. Fellert, H. Jackle, and G. Vorbruggen. 2004. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr. Biol. 14225-230. [DOI] [PubMed] [Google Scholar]
- 31.Takei, Y., Y. Ozawa, M. Sato, A. Watanabe, and T. Tabata. 2004. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development 13173-82. [DOI] [PubMed] [Google Scholar]
- 32.Toyoda, H., A. Kinoshita-Toyoda, B. Fox, and S. B. Selleck. 2000. Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu. Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. J. Biol. Chem. 27521856-21861. [DOI] [PubMed] [Google Scholar]
- 33.Zako, M., J. Dong, O. Goldberger, M. Bernfield, J. T. Gallagher, and J. A. Deakin. 2003. Syndecan-1 and -4 synthesized simultaneously by mouse mammary gland epithelial cells bear heparan sulfate chains that are apparently structurally indistinguishable. J. Biol. Chem. 27813561-13569. [DOI] [PubMed] [Google Scholar]