Abstract
The polysaccharide capsule is a major virulence mechanism of Streptococcus pneumoniae, shielding the bacterium from phagocytes. Capsule types may differ in their abilities to resist immune defense. Antibody-mediated complement activation and opsonophagocytosis are crucial in protection against pneumococcus. Conjugate vaccine trials suggest imperfect protection against 19F. We have previously shown that significantly more anti-19F than anti-6B antibody is needed for killing in the opsonophagocytic assay (OPA). In this study, we explored whether the amount of C3 deposited on serotype 6B and 19F pneumococcal strains reflects their sensitivity to opsonophagocytosis. We compared clinical 6B and 19F nasopharyngeal, middle ear, and blood isolates as well as reference OPA strains (n = 16) for their sensitivity to opsonophagocytosis and C3 deposition. Sixfold anticapsular antibody concentrations were required for 50% opsonophagocytic killing of 19F compared to that of 6B strains. Serotype 19F was more resistant to C3 deposition than 6B. Complement deposition and opsonophagocytosis were dependent on the concentration of anticapsular antibodies. Differences between pneumococcal serotypes in antibody-mediated protection may partly be explained by the abilities of the capsules to resist complement deposition. These findings support previous studies suggesting that higher antibody concentrations to the capsular polysaccharide are needed for protection against disease caused by serotype 19F than that caused by 6B.
Worldwide, Streptococcus pneumoniae (pneumococcus) is an important cause of local respiratory tract infections, otitis media, and serious invasive diseases, especially in young children and the elderly (14, 15). Pneumococcal strains expressing certain capsular serotypes appear to be better able to cause disease than others; a limited number of the >90 serotypes account for the majority of infections (4, 11, 26).
Serotypes 6B and 19F were two of the most common serotypes isolated from middle ear infections in the Finnish Otitis Media (FinOM) Cohort Study (26). In the FinOM Vaccine Trial, the efficacy of two 7-valent pneumococcal conjugate vaccines (PCVs; CRM197 and OMPC conjugates) was assessed in prevention of acute otitis media (AOM) (9, 27). Neither vaccine offered good protection against AOM caused by serotype 19F (25% and 37% efficacy), but both efficiently protected against serotype 6B (84% and 79% efficacy). The poor efficacy against 19F AOM could not be explained by inferior immunogenicity as measured by the enzyme immunoassay (EIA); the anti-19F antibody concentrations after vaccination with the OMPC conjugate were higher than the anti-6B concentrations (6, 27). Lower efficacies of conjugate vaccines against AOM caused by 19F have also been reported in other studies (2, 39, 43).
Protection against invasive pneumococcal diseases has been reported to cover all the serotypes included in the vaccine formula (2, 31, 40, 56), but a case-control study in the United States suggests less-efficient protection against 19F (87%) than against 6B (94%) (57). Vaccine failures caused by 19F were observed in both AOM and invasive disease with PncCRM in the Northern California Kaiser Permanente study (2). According to our previous data from the FinOM Vaccine Trial, up to five times more geometric mean antibody concentration (GMC) was needed for 50% killing of the 19F strain compared to that of the 6B strain (7) in the in vitro functional opsonophagocytic assay (OPA). Similar results suggesting that higher antibody concentrations are required for opsonophagocytic killing of 19F than for 6B have also been reported with 11-valent conjugates (50, 59).
The OPA measures the functional activity of serum antibodies, i.e., the ability of antibodies to enhance phagocytosis of encapsulated pneumococci (47). Preopsonization of bacteria with antibodies results in activation of the complement system, which leads to opsonization of the pneumococcal surface with C3b and iC3b, enabling intake of pneumococci by phagocytic cells through complement receptor-mediated phagocytosis (10, 48). Complement is an essential component of immunity in eliminating invasive pneumococci from the host. The ability of pneumococci to evade complement attack contributes greatly to the pathogenicity of the bacterium (22, 54). The capsule is the major virulence factor. Capsular polysaccharide forms an inert shield that prevents recognition of opsonins adherent to the bacterial cell wall by phagocytic cells (38).
Several pneumococcal virulence proteins inhibit complement-mediated host protection (21). Pneumococcal surface protein A (PspA) and C (PspC) act in synergy by inhibiting complement activation through classical and alternative pathways (33). Nearly all clinical isolates of pneumococci have either PspA family 1 or 2 proteins (16, 17). Members from PspA families 1 and 2 have the same inhibitory effect on deposition of human complement C3, suggesting that both play similar roles in virulence (46). The family of PspC proteins comprises 11 groups of polymorphic proteins, with structural similarities encoded by alleles of the same locus (19). PspC binds factor H (20, 22, 44), a serum protein involved in downregulation of complement activation and protection of host tissues from complement attack (37). This binding is species specific; PspC interacts only with human factor H via a specific binding domain (34). As a result of the bound functionally active factor H, cleavage of C3b and decay of the alternative pathway C3 convertase is promoted on the bacterial surface, resulting in reduced C3b deposition and impaired opsonophagocytosis (22). Most members of the PspC family can bind factor H, but the efficiency of factor H binding varies among pneumococcal strains (45).
In this study, we compared the ability of pneumococcal serotypes 6B and 19F to resist opsonophagocytic killing and complement deposition and explored whether the amount of C3 deposited on the surface of the bacterium reflects its sensitivity to opsonophagocytosis. We analyzed several clinical isolates side by side with the reference strains used in the standard OPA. To distinguish the role of antibodies from the inborn complement resistance/sensitivity of the strains, we measured C3 deposition by using both normal human sera and an agammaglobulinemic serum (AGS). The ability of the strains to bind factor H was analyzed to find out if other serum proteins play a role in the serotype-dependent interaction of pneumococci with complement. The results indicate that serotype 19F is generally more resistant to opsonophagocytosis than 6B and that OPA sensitivity correlates with complement deposition but not with factor H binding, suggesting that the difference between 6B and 19F relates to inborn differences in the ability of the two capsule structures to resist complement deposition.
MATERIALS AND METHODS
Bacterial strains.
The pneumococcal strains used in this study are listed in Table 1. Each strain was isolated from a different patient. Nasopharyngeal carriage strains and middle ear isolates from AOM were collected for the FinOM Cohort Study from children under 2 years of age (26, 53). Invasive strains were blood isolates from Finnish children under 2 years of age from the national infectious disease register (National Reference Laboratory for Pneumococcus, National Public Health Institute, Oulu, Finland). Reference strains used in the standard OPA (6B strain DS2212-94 and 19F strain DS2217-94) were supplied by Centers for Disease Control and Prevention, Atlanta, GA (47).
TABLE 1.
Pneumococcal strains of this study
| Strain | Serotype | Sample type | Isolated from | Multilocus sequence type | PspA family |
|---|---|---|---|---|---|
| 6B NP | 6B | Mucosal | Nasopharynx | 506 | 1 |
| 6B MEF1 | 6B | Mucosal | Middle ear fluid | 147 | 1 |
| 6B MEF2 | 6B | Mucosal | Middle ear fluid | 506 | 1 |
| 6B MEF3 | 6B | Mucosal | Middle ear fluid | 497 | 1 |
| 6B OPAa | 6B | Invasive | Blood | 176 | 2 |
| 6B INV1 | 6B | Invasive | Blood | 138 | 2 |
| 6B INV2 | 6B | Invasive | Blood | 1518 | 1 |
| 6B INV3 | 6B | Invasive | Blood | 138 | 2 |
| 19F NP | 19F | Mucosal | Nasopharynx | 309 | 1 |
| 19F MEF1 | 19F | Mucosal | Middle ear fluid | 534 | 1 |
| 19F MEF2 | 19F | Mucosal | Middle ear fluid | 3760 | 2 |
| 19F MEF3 | 19F | Mucosal | Middle ear fluid | 236 | 2 |
| 19F OPAb | 19F | Invasive | Blood | 688 | 1 |
| 19F INV1 | 19F | Invasive | Blood | 1081 | 2 |
| 19F INV2 | 19F | Invasive | Blood | 43 | 1 |
| 19F INV3 | 19F | Invasive | Blood | 43 | 1 |
Reference strain DS2212-94.
Reference strain DS2217-94.
Bacteria were prepared for the OPA as described previously (47). In short, strains were cultured in Todd-Hewitt broth supplemented with 0.5% yeast extract (THYE) until the logarithmic growth phase. Glycerol at a final concentration of 15% was added to the cultures, which were divided into aliquots and stored at −70°C.
Fresh bacterial cultures were used in flow cytometric C3 deposition and factor H binding assays. Bacteria were cultured on blood agar plates overnight at 37°C with 5% CO2. Colonies with an opaque morphology were inoculated into THYE broth and cultured to the early logarithmic growth phase. Bacteria were collected by centrifugation for 10 min. The pellet was washed with and resuspended in GVB (141 mM NaCl, 1.8 mM sodium barbital, 3.1 mM barbituric acid, pH 7.3 to 7.4, with 0.1% gelatin). Bacterial concentrations of the samples were determined from dilutions of the bacterial aliquots by measuring the optical density at 620 nm with a spectrophotometer (Spectronic Genesys; Milton Roy). Bacterial concentrations of the samples were estimated from an optical density at 620 nm versus viable count curve.
Serum samples.
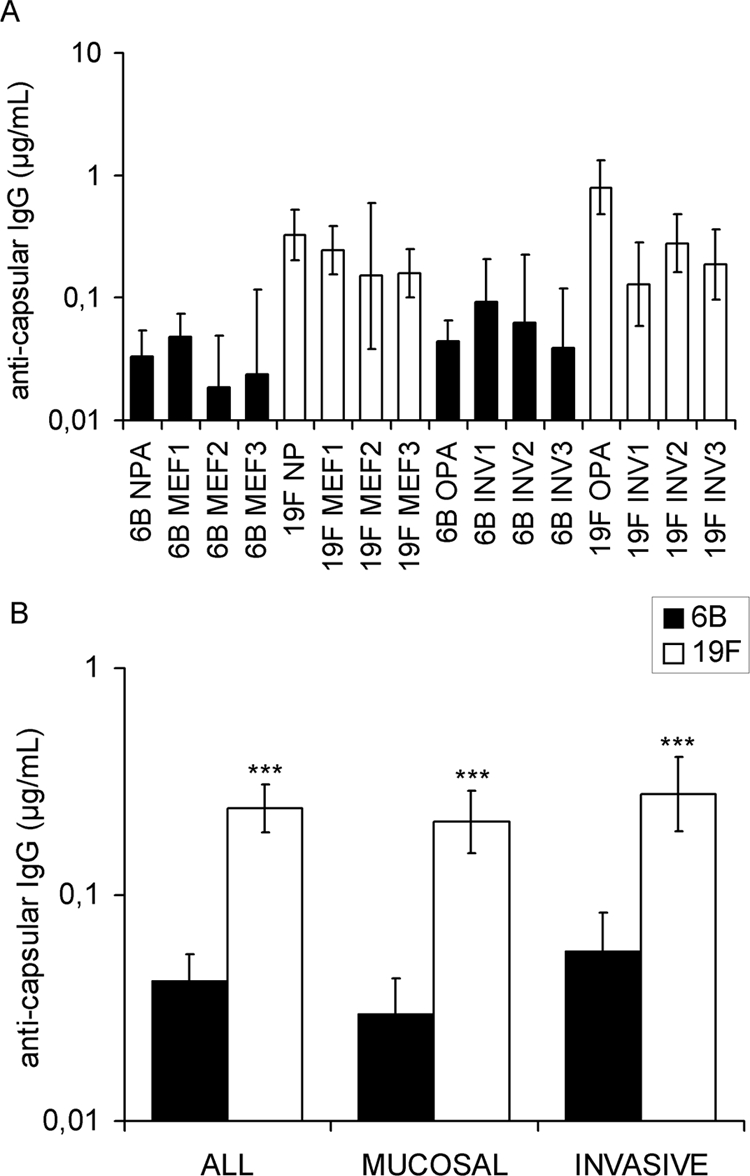
The sera used in the OPA were collected from infants immunized with 4 doses of an 11-valent PCV manufactured by Sanofi Pasteur. Written informed consents were obtained from the parents of the children participating in the PCV study before enrollment. The ethics committee of the National Public Health Institute, Helsinki, Finland, has reviewed the protocol and approved the use of the sera in this study. Serum immunoglobulin G (IgG) antibodies to capsular polysaccharides were measured with the EIA (58). Each pneumococcal strain was analyzed with 4 to 5 different infant sera; altogether, 39 different sera were used. The geometric mean antibody concentration to 6B was 5.8 μg/ml (from 3.5 to 8.1 μg/ml) and 6.6 μg/ml to 19F (from 3.2 to 12 μg/ml). Each strain was also analyzed with a control serum with a high antibody concentration to both serotypes (6B, 60 μg/ml; 19F, 57 μg/ml) drawn from an adult vaccinated with the commercial PCV, Prevenar.
In the C3 deposition assay, four different sera from young, healthy, unvaccinated adults with low concentrations of antibodies to 6B and 19F (normal human sera [NHS]), a serum collected from an agammaglobulinemic patient with nondetectable antibody concentrations to 6B and 19F (AGS), and a serum from a healthy adult immunized with PCV (immune human serum [IHS]) were used as the source of complement. Concentration of IgG to 6B and 19F polysaccharides was measured with 22F-EIA as previously described (51) (Table 2). Sera were divided into small volumes and stored at −70°C to preserve intact complement activity. Once thawed, the serum was used immediately.
TABLE 2.
Antibody concentrations (μg/ml IgG) in the sera used in the C3 deposition assaya
| Serum | 6B | 19F |
|---|---|---|
| NHS 124 | 0.14 | 1.04 |
| NHS 126 | 0.28 | 2.42 |
| NHS 127 | ND | 0.99 |
| NHS 128 | 0.40 | 4.14 |
| IHS | 14.5 | 42.4 |
| AGS | ND | ND |
IHS was from an individual vaccinated with a pneumococcal conjugate vaccine; ND, nondetectable antibody concentration by 22F EIA.
In the factor H binding assay, human serum from a healthy, unimmunized adult donor was inactivated by incubating at 56°C for 30 min. The serum was divided into small aliquots and stored at −70°C before it was used as a source of factor H. Only one human serum was used in the assay; sera from different donors gave identical results in the factor H binding assay (data not shown). Sera used in the complement deposition and factor H assays were drawn from voluntary donors, and written informed consents were obtained.
MLST.
The genotypes of the pneumococcal strains used in this study were analyzed by multilocus sequence typing (MLST) (8). In MLST, bacterial strains are identified by comparing the sequences of internal fragments of seven housekeeping genes (35). Six of the strains had been sequence typed in previous studies (12, 13) and 10 were analyzed at the National Public Health Institute, Helsinki, Finland, using the same method with minor modifications (8, 52).
PspA family typing.
The PspA phenotype of the pneumococcal isolates was characterized serologically using two different rabbit antisera specific to PspA family 1 and PspA 2 proteins, as described before (36).
OPA.
The functional activity of antibodies in sera was measured by the standard OPA (47). CDC reference strains were analyzed alongside with clinical isolates using differentiated HL-60 cells (promyelotic leukemia cells, CCL240; American Type Culture Collection, Rockville, MD), which express complement receptors CR1 and CR3 (for iC3b and C3b) and a low-affinity receptor FcγRII for IgG (47). Cells were cultured as described previously (47); differentiation of the cells was allowed to occur for 5 days. Shortly, polymorphonuclear cells were allowed to phagocytose bacteria in the presence of sera containing anticapsular antibodies and baby rabbit complement (Peel-Freez Biologicals/Dynal) or, as a control, complement only. The assay was performed on microtiter plates, and duplicate samples from each well were plated on THYE agar plates. The results were interpreted as the serum dilution that resulted in 50% of bacteria being killed compared to the bacteria present in the control well in which only complement, and no antibodies, was present. Each bacterial strain was analyzed with the same positive control serum and four to five other sera. Results are expressed as the anticapsular antibody concentration that resulted in 50% killing of bacteria; GMCs of the five sera with 95% confidence intervals (95% CI) are given.
Complement C3 deposition assay.
Deposition of C3 on pneumococci was measured by flow cytometry. The bacterial concentrations were adjusted to 109 cells/ml in 20% serum diluted in GVB2+ (GVB with 0.5 mM MgCl2 and 0.15 mM CaCl2). Control samples were incubated with serum diluted in GVB-EDTA (GVB with 10 mM EDTA) or with GVB2+ without serum. Bacteria were incubated in a horizontal shaker incubator for 1 and 5 min at 37°C. The reaction was terminated by suspending bacteria in 1 ml of ice-cold GVB-EDTA. Bacteria were collected by centrifugation, washed with GVB-EDTA, and resuspended in 100 μl of GVB with anti-conjugated rabbit polyclonal anti-human C3c complement (anti-C3c)-fluorescein isothiocyanate (Dako Immunoglobulins, Denmark). The anti-C3c antibody reacts with human C3c and with the C3c part in C3, C3b, and iC3b. Bacteria were incubated with the anti-C3c antibody for 30 min on ice, washed with 1 ml of ice-cold VBS (141 mM NaCl, 1.8 mM sodium barbital, 3.1 mM barbituric acid, pH 7.3 to 7.4) and pelleted by centrifugation. Bacterial pellets were resuspended in 1 ml of VBS, and C3 on bacteria was detected by flow cytometry (FACSCalibur flow cytometer; Becton Dickinson), collecting data from 20,000 gated events. A sample with bacteria incubated with GVB2+ buffer instead of serum was used as a negative control in setting the threshold and fluorescence intensity. Intensity higher than 10 was considered positive for C3 binding. The percentage of positively staining bacteria (the percentage of bacteria above the threshold fluorescence intensity of 10 [% > FL-10]) was calculated for each sample. Each bacterial strain was analyzed on four different days with a different NHS in each analysis. In addition, each strain was analyzed once with IHS in the standard GVB2+ buffer as well as in GVB-EGTA (GVB with 10 mM EGTA), which blocks activation of complement via the classical pathway. Each strain was also analyzed once with the AGS. A sample with bacteria incubated with NHS in GVB-EDTA, which blocks both alternative and classical pathways of complement activation, was used as a negative control in each analysis.
Factor H binding assay.
Binding of factor H to pneumococci was measured by flow cytometry. Pneumococci (109 cells/ml) were incubated with 30% heat-inactivated serum diluted in GVB-bovine serum albumin (BSA) (GVB with 1% BSA). Bacteria were incubated on a horizontal shaker for 30 min at 37°C. Pellets were washed once with 1 ml GVB and incubated in 100 μl of GVB-BSA with in-house monoclonal mouse anti-human factor H antibody 196X (24) for 30 min at 22°C with shaking, followed by one wash with VBS. Secondary antibody, Alexa488-labeled goat anti-mouse IgG (Molecular Probes; Invitrogen) was added in a volume of 50 μl and incubated for 30 min at 22°C with shaking. The bacteria were washed and resuspended in VBS. Binding of factor H on bacteria was detected by flow cytometry collecting data from 20,000 gated events. A sample with bacteria incubated in buffer instead of serum was used as a negative control in setting the threshold. Geometric mean intensities of fluorescence (GMF) were analyzed from each sample. Each bacterial strain was analyzed on three different days, with the same serum.
Statistical methods.
Geometric means and 95% CI of the serum antibody concentrations required for 50% opsonophagocytic killing were calculated from the five or six sera with which each bacterial strain was analyzed. Averages of bacteria positive for C3 deposition with standard deviations were calculated from the four sera analyzed. Because all bacterial strains bound factor H, GMF and not the percentage of positive bacteria were compared and geometric means were calculated from three separate analyses. Student's t test (2-tailed, assuming unequal variance) was applied in comparisons of serotypes 6B and 19F. Differences between individual strains within the serotype were compared by one-way analysis of variance (ANOVA), followed by Tukey's honestly significant difference post hoc test when appropriate. In all analyses, P values of <0.05 were considered to indicate statistically significant differences.
RESULTS
Multilocus sequence types and PspA families of the pneumococcal isolates.
The pneumococcal isolates compared in this study were genotypically heterogeneous. Among the 16 isolates there were six different sequence types within serotype 6B and seven within serotype 19F (Table 1). All isolates expressed either PspA family 1 or family 2 proteins. Both serotypes included five isolates with a family 1 PspA and three with a family 2 PspA (Table 1).
Opsonophagocytic killing of pneumococci.
A total of 16 pneumococcal strains were analyzed in the OPA with sera from vaccinated individuals, and the GMC required for 50% killing of the bacteria was calculated. All strains of serotype 19F were more resistant to opsonophagocytic killing than any 6B strain (Fig. 1A). The difference between serotypes 6B and 19F in sensitivity to opsonophagocytosis was statistically significant in all comparisons, both between mucosal and invasive strains (Fig. 1B). On average, 5.8 times as much antibody was required for killing 19F strains as for 6B strains. Significantly more antibodies were required for killing invasive 6B strains (0.06 μg/ml) than for mucosal 6B strains (0.03 μg/ml; P = 0.017; Student's t test), while the concentrations of antibodies required for killing invasive and mucosal 19F strains were similar (0.28 μg/ml and 0.21 μg/ml, respectively). Statistically significant differences between individual strains were observed within serotype 19F (P < 0.001; one-way ANOVA). The 19F OPA strain was the least sensitive to opsonophagocytosis and required a significantly higher GMC for 50% opsonophagocytosis than 19F MEF2, MEF3, INV1, or INV3 (P < 0.05; Tukey's honestly significant difference test).
FIG. 1.
Opsonophagocytic killing of serotype 6B and 19F pneumococcal strains; each strain was analyzed with 5 to 6 different sera from vaccinated individuals. The results for individual strains (A) and for all strains, mucosal strains, and invasive strains combined (B) are presented as GMCs required for 50% opsonophagocytic killing with 95% CI. ***, P < 0.001 (Student's t test, 2-tailed with unequal variance).
Deposition of C3 on pneumococci.
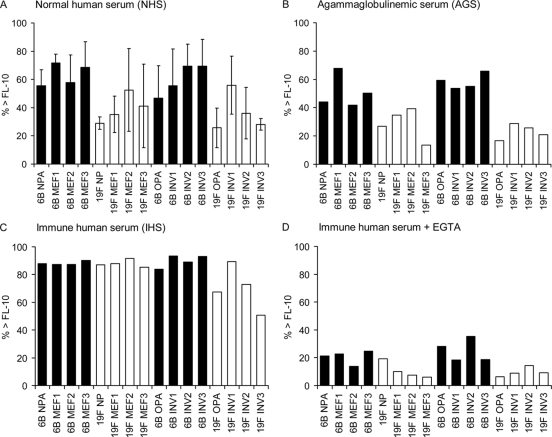
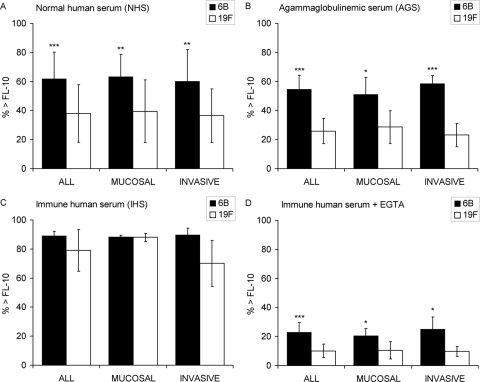
Complement deposition was measured by flow cytometry, and the percentages of bacteria positive for C3 deposition (the percentage of bacteria above the threshold fluorescence intensity of 10 [% > FL-10]) were compared. On average, the higher the concentration of the specific anticapsular antibodies in the sera, the more complement was deposited on the pneumococcal strains: the antibody concentrations to polysaccharides 6B and 19F were nondetectable in AGS, low in NHS, and high in IHS, always with more antibodies to 19F than 6B (Table 2). No complement deposition was measured with a serum diluted in buffer containing 10 mM EDTA, which indicates that C3 was deposited on bacteria as a result of complement activation, not unspecific or direct binding. There was a clear association between serotype and C3 deposition; in the absence of immune serum, more C3 was deposited on 6B than on 19F (Fig. 2). The difference was statistically significant both between mucosal and invasive 6B isolates (Fig. 3) with each of the four NHS and with AGS (Fig. 4). High percentages of bacteria positive for C3 were measured following incubation with the IHS, while complement deposition was significantly reduced on bacteria incubated with the IHS diluted in buffer containing 10 mM EGTA (P < 0.0001; Student's t test). Less C3 was deposited on 19F than on 6B strains with IHS, but the difference was not statistically significant (Fig. 2 to 4). In the presence of EGTA, significantly more C3 was deposited on 6B than on 19F strains (Fig. 2 to 4). Differences between individual strains within a serotype (in the average C3 deposition using NHS) were not statistically significant (one-way ANOVA). The amount of C3 deposited on pneumococci correlated with sensitivity to opsonophagocytosis; more C3 was deposited on 6B (with either NHS or AGS), and fewer antibodies were required for 50% opsonophagocytic killing of 6B than of 19F (Fig. 5 A). The serotype 19F OPA strain was the most resistant to complement deposition and the least sensitive to opsonophagocytosis (Fig. 5A).
FIG. 2.
Deposition of complement protein C3 on pneumococcal strains of serotypes 6B and 19F. Strain-specific average percentages of bacteria positive for C3 binding (% > FL-10) with standard deviations are given. Each pneumococcal strain was analyzed with four different NHS (A), an AGS (B), an IHS (C), and the IHS diluted in buffer containing EGTA to inhibit the classical pathway of complement activation (D).
FIG. 3.
Deposition of complement protein C3 on pneumococcal serotypes 6B and 19F. Average percentages of mucosal and/or invasive strains positive for C3 deposition (% > FL-10) with standard deviations are given. Pneumococci were analyzed with four different NHS (A), an AGS (B), an IHS (C), and the IHS diluted in buffer containing EGTA to inhibit the classical pathway of complement activation (D). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student's t test, 2-tailed with unequal variance).
FIG. 4.
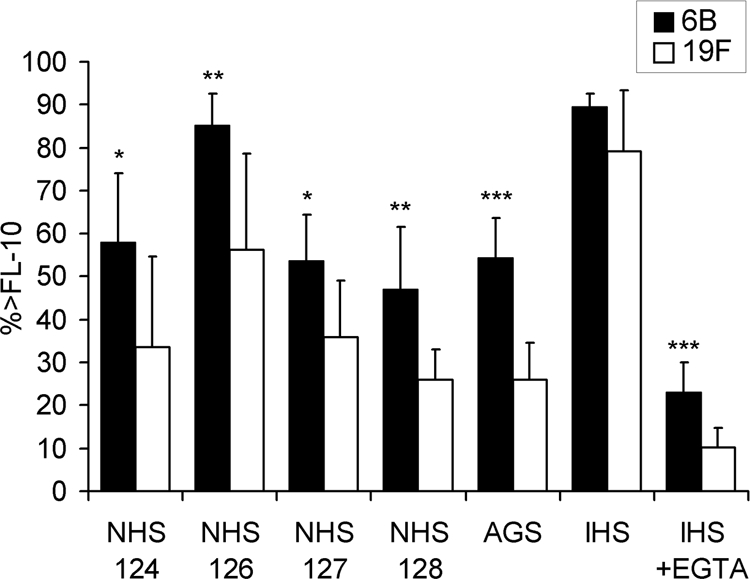
Comparison of C3 deposition on pneumococcal serotypes 6B and 19F with different sera. All serotype 6B and 19F strains (listed in Table 1) were analyzed in parallel with four different sera with low concentrations of anticapsular antibodies (NHS), an AGS, an IHS, and the IHS with 10 mM EGTA (IHS+EGTA). Average percentages of bacteria positive for C3 deposition (% > FL-10) with standard deviations are given. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student's t test, 2-tailed with unequal variance).
FIG. 5.
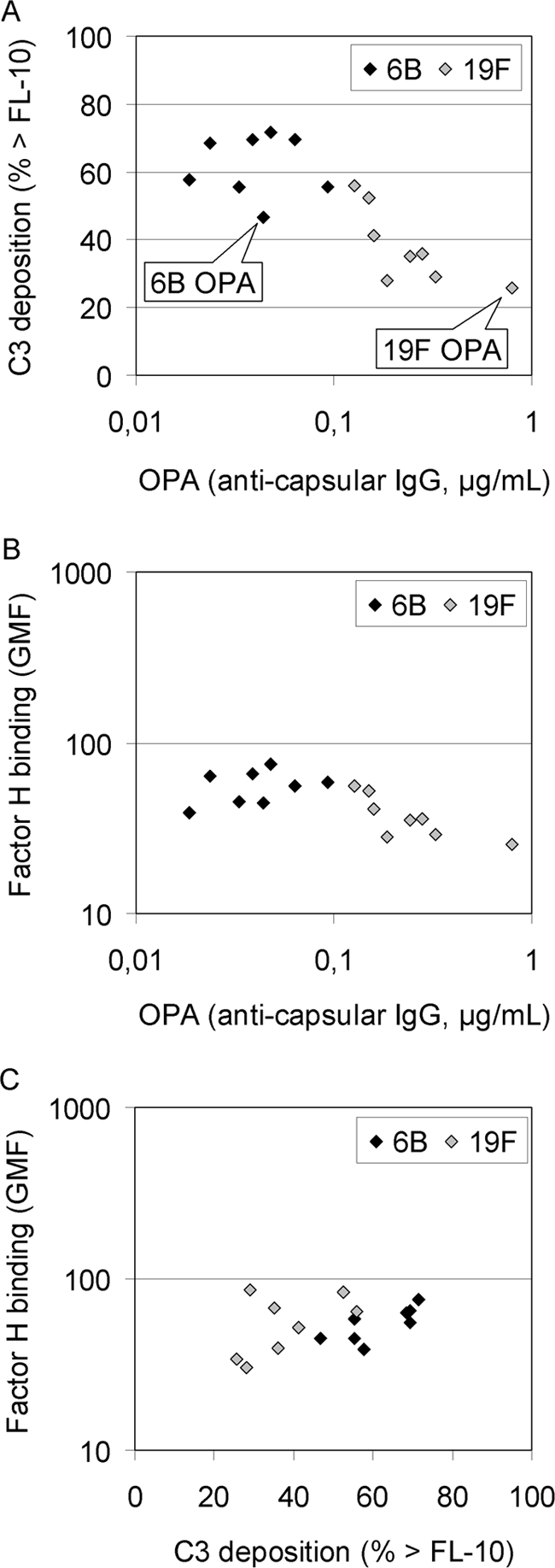
Correlation between deposition of complement protein C3 and OPA (A), factor H binding and OPA (B), and factor H binding and C3 deposition (C). All pneumococcal serotype 6B and 19F strains (listed in Table 1) were compared. The results are given as bacteria positive for bound C3 (% > FL-10; average of four NHS, GMC needed for 50% opsonophagocytic killing, and the average amount of factor H bound to bacteria [GMF]).
Factor H binding by pneumococci.
The ability of the 16 strains to bind factor H was compared because binding of factor H is a mechanism for complement evasion by pneumococcus (22). Heat-inactivated NHS was incubated with the bacteria, and binding was measured by flow cytometry. All pneumococcal strains bound factor H. Although differences could be observed between individual strains (P < 0.001; one-way ANOVA), serotypes 6B and 19F bound on average similar amounts of factor H (nonsignificant differences between serotypes). Mucosal and invasive strains of serotype 6B bound the same amount of factor H, whereas mucosal 19F strains bound significantly more factor H than the invasive 19F strains (P < 0.001; Student's t test; data not shown). Factor H binding did not correlate with the OPA results (Fig. 5B), but a weakly positive correlation existed between C3 deposition and factor H binding (Fig. 5C).
DISCUSSION
We have previously shown that two clinically prominent pneumococcal serotypes, 6B and 19F, differ with respect to the concentration of specific anticapsular antibodies required for killing in the OPA, a finding which is in agreement with the differences in clinical efficacy elicited by PCVs. In the present study, we assessed whether the differences observed between 6B and 19F reference strains in sensitivity to opsonophagocytosis can be seen between clinical strains representing the two serotypes in general and whether different susceptibilities to complement deposition could explain the differences between 6B and 19F in OPA and vaccine efficacy studies. The results suggest a clear serotype-linked difference between strains in resistance to opsonophagocytosis and complement deposition.
It appears that serotype 19F is more difficult than 6B to protect against by vaccine-induced immunity to polysaccharide antigens. Previous studies have indicated that a significantly higher concentration of antibody may be necessary to achieve protection against middle ear infection caused by serotype 19F than 6B (2). The antibody concentration associated with protection against AOM was estimated to be 0.5 μg/ml against 6B, whereas the predicted efficacy for 19F was negligible up to the highest antibody concentration tested (23). Although EIA for quantitation of antipneumococcal capsular antibodies is currently the primary method recommended by the WHO to evaluate immune responses to pneumococcal polysaccharide-based vaccines, the accumulating data from clinical trials clearly demonstrate the added value of OPA in vaccine evaluation (7, 50). Analysis of several clinical isolates of pneumococci in this study confirmed the previous finding (7) that significantly more antibody to the serotype 19F than 6B polysaccharide is required for the same level of opsonophagocytosis. Differences between strains were not statistically significant within serotype 6B, whereas the reference 19F strain (DS2217-94) was by far more resistant than any of the other 19F strains. Because the genetic background of the strain can have such a clear impact on the results received with the OPA, attention should be paid on the choice of reference strains. In order to avoid variance between laboratories applying the standard OPA, either the same strains, or strains tested against the CDC reference strains, should be used.
Serotype 19F isolates compared in this study were significantly less susceptible to complement deposition than the serotype 6B strains, which is in agreement with serotype 19F being more resistant to opsonophagocytosis. We observed small differences in complement deposition between strains within each serotype, but they were not statistically significant. The possibility that expression of pneumococcal proteins PspC and PspA could affect complement resistance of the isolates was taken into consideration by analyzing the ability of the strains to bind factor H and by serologically detecting the PspA family on the bacteria. All isolates expressed PspA and bound factor H. Differences in the efficiency of factor H binding could not account for the serotype-related differences seen in complement resistance. As expected from previous studies describing the similar roles of PspA families 1 and 2 in virulence (46), resistance to complement deposition and phagocytosis could not be accounted for by a particular PspA family, since isolates representing serotypes 6B and 19F both included the same number of strains with family 1 and family 2 PspAs. In serotype 2 and 3 strains with either family 1 or 2 PspA, both the capsule structure and the genetic background were found to influence complement deposition (1). In this study, both serotypes 6B and 19F included several different multilocus sequence types, and although differences were observed between strains within a serotype, mainly the capsule rather than the genetic background of the isolates determined their complement resistance.
The AGS and NHS used in the C3 deposition analyses contained little if any antibody to capsular polysaccharides 6B and 19F, whereas the IHS was from an immunized individual. Complement deposition was highest when using the IHS, lowest with the AGS, and intermediate with the NHS, which indicates that antibodies enhance complement deposition. Complement deposition was similar to 6B and 19F when using the IHS with a threefold concentration of anti-19F compared to that of anti-6B, suggesting that by increasing anti-19F antibody concentration, the complement resistance of 19F pneumococcal strains could be overcome. Natural- and vaccine-induced antibodies to PspA can also increase complement deposition (41). Our finding that serotype 19F isolates were more resistant to C3 deposition even in the absence of antibodies in AGS suggests that the resistance to C3 deposition cannot be attributed to a poorer susceptibility to protein antibodies or poorer quality of 19F than 6B polysaccharide antibodies. Furthermore, the differences between serotype 6B and 19F isolates in complement deposition could not be explained by differential recruitment of complement regulatory protein factor H on the bacterial surface, supporting our conclusion that the serotype 19F isolates were generally more resistant to complement due to the serotype 19F polysaccharide capsule.
The mechanism explaining the resistance of serotype 19F strains to complement deposition and opsonophagocytosis remains to be solved. As a result of complement activation, C3 forms covalent bonds with hydroxyl and amino groups on biological targets. Comparison of the chemical structures of the polysaccharides (30) reveals that the repeat unit of serotype 6B polysaccharide (galactose-glucose-rhamnose-ribitol) contains 10 hydroxyl groups, while serotype 19F (mannose-glucose-rhamnose) has only 7. However, the molecular structures immediate to the hydroxyl groups affect the efficiency of C3b binding (5). Whether the difference in the amount of hydroxyl groups, molecular composition of the polysaccharides, or other factors are responsible for the larger amount of C3b binding on serotype 6B strains remains to be seen, as it cannot be concluded based on the data we have so far.
Streptococcus pneumoniae undergoes phase variation from transparent to opaque phase involving increased expression of the capsule, teichoic acids, and virulence proteins such as PspA (28). Opaque variants are more virulent in the mouse model of sepsis (28), and more antibody is required for opsonophagocytic killing of opaque variants expressing larger amounts of capsular polysaccharides than transparent phase variants (29). Phase variation is reversible; extensive changes in gene expression are observed in different host environments (32, 42). During bacteremic phase, pneumococci most closely resemble bacteria in a broth culture (42). For this study, bacteria were cultured to the logarithmic growth phase in broth culture in order to enhance encapsulation. In the complement deposition assay, only fresh cultures were used, because differences were observed between individual strains within serotype 19F regarding tolerance to freezing. Transparent variants are more capable of colonizing the nasopharynx (55), suggesting that strains isolated from mucosal surfaces would be in the transparent phase. Both invasive and mucosal isolates were selected for this study. Mucosal isolates of 6B (but not 19F) were more susceptible to opsonophagocytic killing than the invasive strains, while complement deposition on mucosal and invasive isolates was similar. Differences between serotypes 6B and 19F in complement deposition and opsonophagocytosis were significant both between mucosal and invasive isolates, indicating that the differences cannot be explained by the 6B isolates being in a different phase.
The early studies of Hostetter suggested that the ratio of iC3b to C3d rather than the total amount of C3 deposited on pneumococci would explain differences between serotypes in resistance to opsonophagocytosis, because the most important complement receptor, CR3, recognizes iC3b but not C3d (18). The experiments suggested that serotypes 3 and 4 would enhance degradation of C3b to C3d, while serotypes 6A and 14 displayed only iC3b on their surfaces (18). Hostetter concluded that the absolute number of C3b molecules bound on bacteria could not explain differences observed in resistance of these serotypes to phagocytosis as described in literature. However, we found a clear association between the total amount of C3b detected on the bacterial surface and sensitivity of the same strains to opsonophagocytosis. Opsonophagocytic killing of pneumococci is strongly dependent on activation of the classical pathway of complement by antibodies, not direct antibody-mediated phagocytosis via Fc receptors (49). Rapid cleavage of C3b to iC3b is favorable to the pathogen as it results in decay of the alternative pathway C3 convertase and inhibits deposition of new C3b molecules on the pneumococcal surface. Breakdown of C3b to iC3b thus decreases net C3b deposition, which leads to impaired opsonophagocytosis.
The classical pathway is the dominant pathway in eliminating pneumococcal infection (3). Therefore, it was not surprising that inhibition of the classical pathway of complement activation resulted in significantly reduced deposition of C3b on bacteria. The classical pathway can be activated not only by specific IgG and IgM, but with natural IgM (3, 60) and serum proteins detecting pathogen-associated structures and interacting with C1q (25, 61). Chelation of Ca2+ by EGTA blocks activation of the classical pathway, whether it is triggered by antibodies or serum proteins. In this study, more C3 was deposited on 6B than on 19F when the classical pathway was inhibited, which may indicate different susceptibilities of the serotypes to innate immune mechanisms.
In summary, we have demonstrated that pneumococcal strains of serotypes 6B and 19F have different susceptibilities to complement deposition and antibody-mediated phagocytosis. Our results suggest that strains with the 19F capsule are naturally more resistant to complement deposition than serotype 6B strains and that more anticapsular antibodies are required for efficient opsonophagocytosis of serotype 19F strains. The different susceptibilities of serotypes to complement deposition, opsonophagocytosis, and resultant antibody-mediated protection should be taken into account when guidelines for serological correlates for vaccine efficacy evaluations are made.
Acknowledgments
Leena Tikkanen is acknowledged for skillful assistance in the laboratory analyses and statistician Mika Lahdenkari for advice in statistical analysis of the data at the National Public Health Institute, Helsinki, Finland. We thank P. Helena Mäkelä for critical reading of the manuscript. We thank Tarja Kaijalainen for bacterial work at the National Public Health Institute, Oulu, Finland. Susan Hollingshead and David Briles from the University of Alabama at Birmingham, Birmingham, Alabama, are thanked for kindly supplying the anti-PspA rabbit sera.
This work was partly supported by GlaxoSmithKline Biologicals (GSK Bio). Helena Käyhty has provided consultancies on advisory boards for GSK Bio, has had travels paid by GSK Bio as an invited speaker or expert at symposia, and has received honoraria from GSK Bio.
Editor: A. Camilli
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Abeyta, M., G. G. Hardy, and J. Yother. 2003. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect. Immun. 71218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, S., H. Shinefield, B. Fireman, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19187-195. [DOI] [PubMed] [Google Scholar]
- 3.Brown, J. S., T. Hussell, S. M. Gilliland, et al. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 9916969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler, J. C., R. F. Breiman, H. B. Lipman, J. Hofmann, and R. R. Facklam. 1995. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978-1994: implications for development of a conjugate vaccine. J. Infect. Dis. 171885-889. [DOI] [PubMed] [Google Scholar]
- 5.Capel, P. J., O. Groeneboer, G. Grosveld, and K. W. Pondman. 1978. The binding of activated C3 to polysaccharides and immunoglobulins. J. Immunol. 1212566-2572. [PubMed] [Google Scholar]
- 6.Ekström, N., H. Ahman, J. Verho, et al. 2005. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 73369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekström, N., M. Vakevainen, J. Verho, T. Kilpi, and H. Kayhty. 2007. Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 751794-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 1443049-3060. [DOI] [PubMed] [Google Scholar]
- 9.Eskola, J., T. Kilpi, A. Palmu, et al. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344403-409. [DOI] [PubMed] [Google Scholar]
- 10.Fearon, D. T., and W. W. Wong. 1983. Complement ligand-receptor interactions that mediate biological responses. Annu. Rev. Immunol. 1243-271. [DOI] [PubMed] [Google Scholar]
- 11.Gray, B. G., and J. H. C. Dillon. 1986. Clinical and epidemiologic studies of pneumococcal infection in children. Pediatr. Infect. Dis. 5201-207. [DOI] [PubMed] [Google Scholar]
- 12.Hanage, W. P., K. Auranen, R. Syrjanen, et al. 2004. Ability of pneumococcal serotypes and clones to cause acute otitis media: implications for the prevention of otitis media by conjugate vaccines. Infect. Immun. 7276-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanage, W. P., T. H. Kaijalainen, R. K. Syrjanen, et al. 2005. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect. Immun. 73431-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30100-121. [DOI] [PubMed] [Google Scholar]
- 15.Hausdorff, W. P., G. Yothers, R. Dagan, et al. 2002. Multinational study of pneumococcal serotypes causing acute otitis media in children. Pediatr. Infect. Dis. J. 211008-1016. [DOI] [PubMed] [Google Scholar]
- 16.Hollingshead, S. K., L. Baril, S. Ferro, J. King, P. Coan, and D. E. Briles. 2006. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J. Med. Microbiol. 55215-221. [DOI] [PubMed] [Google Scholar]
- 17.Hollingshead, S. K., R. Becker, and D. E. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 685889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hostetter, M. K. 1986. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J. Infect. Dis. 153682-693. [DOI] [PubMed] [Google Scholar]
- 19.Iannelli, F., M. R. Oggioni, and G. Pozzi. 2002. Allelic variation in the highly polymorphic locus pspC of Streptococcus pneumoniae. Gene 28463-71. [DOI] [PubMed] [Google Scholar]
- 20.Janulczyk, R., F. Iannelli, A. G. Sjoholm, G. Pozzi, and L. Bjorck. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 27537257-37263. [DOI] [PubMed] [Google Scholar]
- 21.Jarva, H., T. S. Jokiranta, R. Wurzner, and S. Meri. 2003. Complement resistance mechanisms of streptococci. Mol. Immunol. 4095-107. [DOI] [PubMed] [Google Scholar]
- 22.Jarva, H., R. Janulczyk, J. Hellwage, P. F. Zipfel, L. Bjorck, and S. Meri. 2002. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8-11 of factor H. J. Immunol. 1681886-1894. [DOI] [PubMed] [Google Scholar]
- 23.Jokinen, J. T., H. Ahman, T. M. Kilpi, P. H. Makela, and M. H. Kayhty. 2004. Concentration of antipneumococcal antibodies as a serological correlate of protection: an application to acute otitis media. J. Infect. Dis. 190545-550. [DOI] [PubMed] [Google Scholar]
- 24.Jokiranta, T. S., P. F. Zipfel, J. Hakulinen, et al. 1996. Analysis of the recognition mechanism of the alternative pathway of complement by monoclonal anti-factor H antibodies: evidence for multiple interactions between H and surface bound C3b. FEBS Lett. 393297-302. [DOI] [PubMed] [Google Scholar]
- 25.Kang, Y. S., Y. Do, H. K. Lee, et al. 2006. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell 12547-58. [DOI] [PubMed] [Google Scholar]
- 26.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjanen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20654-662. [DOI] [PubMed] [Google Scholar]
- 27.Kilpi, T., H. Ahman, J. Jokinen, et al. 2003. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 371155-1164. [DOI] [PubMed] [Google Scholar]
- 28.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177368-377. [DOI] [PubMed] [Google Scholar]
- 29.Kim, J. O., S. Romero-Steiner, U. B. Sorensen, et al. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 672327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, J. S., E. R. Laskowich, R. G. Arumugham, R. E. Kaiser, and G. J. MacMichael. 2005. Determination of saccharide content in pneumococcal polysaccharides and conjugate vaccines by GC-MSD. Anal. Biochem. 347262-274. [DOI] [PubMed] [Google Scholar]
- 31.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, and N. Pierce. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 3491341-1348. [DOI] [PubMed] [Google Scholar]
- 32.LeMessurier, K. S., A. D. Ogunniyi, and J. C. Paton. 2006. Differential expression of key pneumococcal virulence genes in vivo. Microbiology 152305-311. [DOI] [PubMed] [Google Scholar]
- 33.Li, J., D. T. Glover, A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2007. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 755877-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu, L., Z. Ma, T. S. Jokiranta, A. R. Whitney, F. R. DeLeo, and J. R. Zhang. 2008. Species-specific interaction of Streptococcus pneumoniae with human complement factor H. J. Immunol. 1817138-7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiden, M. C., J. A. Bygraves, E. Feil, et al. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 953140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melin, M. M., S. K. Hollingshead, D. E. Briles, et al. 2008. Pneumococcal surface protein A families 1 and 2 in acute otitis media and nasopharyngeal carriage isolates of Finnish children. Clin. Vaccine Immunol. 151555-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meri, S. 2007. Loss of self-control in the complement system and innate autoreactivity. Ann. N. Y. Acad. Sci. 110993-105. [DOI] [PubMed] [Google Scholar]
- 38.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14801-807. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien, K. L., A. B. David, A. Chandran, et al. 2008. Randomized, controlled trial efficacy of pneumococcal conjugate vaccine against otitis media among Navajo and White Mountain Apache infants. Pediatr. Infect. Dis. J. 2771-73. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien, K. L., L. H. Moulton, R. Reid, et al. 2003. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362355-361. [DOI] [PubMed] [Google Scholar]
- 41.Ochs, M. M., W. Bartlett, D. E. Briles, et al. 2008. Vaccine-induced human antibodies to PspA augment complement C3 deposition on Streptococcus pneumoniae. Microb. Pathog. 44204-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oggioni, M. R., C. Trappetti, A. Kadioglu, et al. 2006. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol. Microbiol. 611196-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prymula, R., P. Peeters, V. Chrobok, et al. 2006. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 367740-748. [DOI] [PubMed] [Google Scholar]
- 44.Quin, L. R., S. Carmicle, S. Dave, M. K. Pangburn, J. P. Evenhuis, and L. S. McDaniel. 2005. In vivo binding of complement regulator factor H by Streptococcus pneumoniae. J. Infect. Dis. 1921996-2003. [DOI] [PubMed] [Google Scholar]
- 45.Quin, L. R., C. Onwubiko, S. Carmicle, and L. S. McDaniel. 2006. Interaction of clinical isolates of Streptococcus pneumoniae with human complement factor H. FEMS Microbiol. Lett. 26498-103. [DOI] [PubMed] [Google Scholar]
- 46.Ren, B., A. J. Szalai, O. Thomas, S. K. Hollingshead, and D. E. Briles. 2003. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 7175-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero-Steiner, S., D. Libutti, L. B. Pais, et al. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothlein, R., and T. A. Springer. 1985. Complement receptor type three-dependent degradation of opsonized erythrocytes by mouse macrophages. J. Immunol. 1352668-2672. [PubMed] [Google Scholar]
- 49.Saeland, E., J. H. Leusen, G. Vidarsson, et al. 2003. Role of leukocyte immunoglobuin G receptors in vaccine-induced immunity to Streptococcus pneumoniae. J. Infect. Dis. 1871686-1693. [DOI] [PubMed] [Google Scholar]
- 50.Schuerman, L., R. Prymula, I. Henckaerts, and J. Poolman. 2007. ELISA IgG concentrations and opsonophagocytic activity following pneumococcal protein D conjugate vaccination and relationship to efficacy against acute otitis media. Vaccine 251962-1968. [DOI] [PubMed] [Google Scholar]
- 51.Simell, B., M. Lahdenkari, A. Reunanen, H. Kayhty, and M. Vakevainen. 2008. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin. Vaccine Immunol. 151391-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sombrero, L., A. Nissinen, G. Esparar, M. Lindgren, L. Siira, and A. Virolainen. 2008. Low incidence of antibiotic resistance among invasive and nasopharyngeal isolates of Streptococcus pneumoniae from children in rural Philippines between 1994 and 2000. Eur. J. Clin. Microbiol. Infect. Dis. 27929-935. [DOI] [PubMed] [Google Scholar]
- 53.Syrjänen, R. K., T. M. Kilpi, T. H. Kaijalainen, E. E. Herva, and A. K. Takala. 2001. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J. Infect. Dis. 184451-459. [DOI] [PubMed] [Google Scholar]
- 54.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 674720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 622582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitney, C. G., M. M. Farley, J. Hadler, et al. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 3481737-1746. [DOI] [PubMed] [Google Scholar]
- 57.Whitney, C. G., T. Pilishvili, M. M. Farley, et al. 2006. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 3681495-1502. [DOI] [PubMed] [Google Scholar]
- 58.Wuorimaa, T., R. Dagan, M. Vakevainen, et al. 2001. Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J. Infect. Dis. 1841211-1215. [DOI] [PubMed] [Google Scholar]
- 59.Wuorimaa, T. K., R. Dagan, F. Bailleux, et al. 2005. Functional activity of antibodies after immunization of Finnish and Israeli infants with an 11-valent pneumococcal conjugate vaccine. Vaccine 235328-5332. [DOI] [PubMed] [Google Scholar]
- 60.Yuste, J., A. Sen, L. Truedsson, et al. 2008. Impaired opsonization with C3b and phagocytosis of Streptococcus pneumoniae in sera from subjects with defects in the classical complement pathway. Infect. Immun. 763761-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuste, J., M. Botto, S. E. Bottoms, and J. S. Brown. 2007. Serum amyloid P aids complement-mediated immunity to Streptococcus pneumoniae. PLoS Pathog. 31208-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]