In the respiratory system, the upper tract is colonized with commensal bacteria, whereas the lower tract is sterile. The respiratory system is continuously exposed to a variety of bacteria. To combat these intruders, the lung has developed a multifaceted system of defense. One of the most important components of the initial innate immune response in the lung against bacterial infection is the vigorous recruitment of neutrophils. However, several life-threatening bacterial lung diseases are caused by excessive neutrophil-mediated inflammation. The mechanisms underlying neutrophil accumulation during lower respiratory tract bacterial infection is learned from experimental animal models, particularly with mice (61). A better understanding of the mechanisms underlying the regulation of neutrophil influx is crucial to designing improved therapies to augment host defense and attenuate detrimental lung inflammation. The aim of this review is to highlight some of the most important recent advances in neutrophil infiltration, particularly in the roles of Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), transcription factors, chemokines, and adhesion molecules in acute lower respiratory tract bacterial infections. This minireview includes important gram-positive and gram-negative pathogens, including Streptococcus pneumoniae, Klebsiella pneumoniae, Legionella pneumophila, Haemophilus influenzae, and Staphylococcus aureus.
CLINICAL IMPORTANCE
The World Health Organization estimates that lower respiratory tract infections account for nearly 35% of all deaths from infectious diseases, causing an annual mortality of nearly 4 million adults and children (55). They cause a greater disease burden in the world than many other infections, such as human immunodeficiency virus infection and malaria (55-56). Bacteria are the most common cause of lower respiratory tract infections (55). Despite the development of broad-spectrum antibiotics, lower respiratory tract bacterial infections continue to be a major cause of morbidity and mortality in both industrialized and developing countries (55-56). Furthermore, antibiotic-resistant S. pneumoniae, H. influenzae, and S. aureus have been isolated from patients suffering from lower respiratory tract infections in recent years (11, 19, 24, 77). The emergence of antibiotic-resistant pulmonary bacteria and the growing number of immunocompromised individuals have made the treatment of these infections increasingly difficult (48, 49, 50, 51, 52, 53), emphasizing the importance of modulating host defense in treating and/or preventing severe bacterial infections without these adverse effects. In the lung, effective host defense against bacterial infection is dependent primarily upon the rapid clearance of the etiologic agent from the respiratory tract. The innate immune response is the principal pathway through which this occurs (50, 92), and its failure may result in excessive microbial colonization and subsequent infection of the lung parenchyma and septicemia.
NEUTROPHILS
In the past, several investigations have pointed to the involvement of neutrophils as key players in invoking innate immune responses (54, 65, 71, 87). Although the number of neutrophils in the peripheral blood is usually fairly constant, the host is capable of markedly increasing numbers of circulating neutrophils (29, 44), particularly through the accelerated release of neutrophils from the bone marrow reserve pool. Originally, it was noted in 1920 that lower respiratory tract bacterial infection caused the appearance of morphologically immature neutrophils in the circulation or a “left shift,” resulting from a massive release of neutrophils from the marrow (25-26, 49, 20). In addition, the fundamental role of neutrophils protecting the host against S. pneumoniae (30), K. pneumoniae (41), and L. pneumophila (82) has been demonstrated by experiments that showed that (i) selective depletion of the neutrophil cell population results in profound defects in the clearance of bacteria from the lungs and (ii) repletion of neutrophils in neutropenic mice restores host defense. Investigations have also shown that efficient neutrophil accumulation is important to induce a successful adaptive immune response in the host. For example, studies have demonstrated a significant reduction of interleukin-12 (IL-12) in the lungs of CXC chemokine receptor 2 (CXCR2)-blocked mice in response to L. pneumophila (83).
The ability of polymorphonuclear leukocytes (neutrophils) to sense bacteria and/or their components and migrate across epithelia along a chemotactic gradient is intriguing. Neutrophil sequestration is an essential antibacterial defense mechanism in the lung which involves multiple steps, including the activation of transcription factors, production of chemokines, upregulation of cell adhesion molecules, and enhancement of cell-cell interactions (Fig. 1). Elucidating the key molecules involved with innate pulmonary defense and mapping out the steps of these complex signal transduction pathways upon recognition of bacteria by pattern recognition receptors, such as TLRs and NLRs, are formidable tasks.
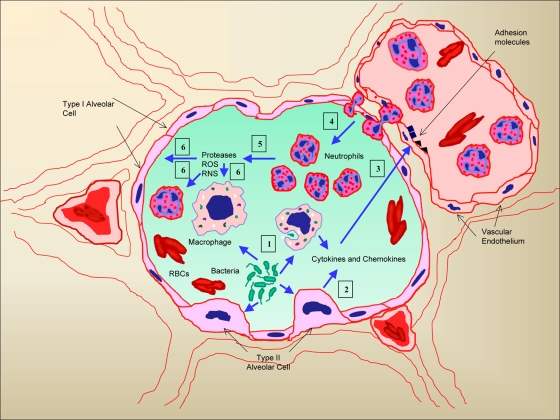
FIG. 1.
Schematic representation describing the cascade of events by which bacteria induce neutrophil sequestration and eventual lung damage associated with lower respiratory tract infection. Bacteria initially interact with the cells in the alveoli, including alveolar epithelial cells and macrophages (1), and induce the secretion of cytokines and neutrophil chemoattractants (2). These cytokines upregulate the expression of cell adhesion molecules on capillary endothelia (3) and subsequently mediate the transmigration of neutrophils into alveolar spaces based on the chemotactic gradient (4). The transmigrated neutrophils produce proteases, reactive oxygen species (ROS), and reactive nitrogen species (RNS) (5), which act on cells that are infected and induce necrotic cell death (6). Gradually, all of these events lead to extensive lung injury. RBCs, red blood cells.
TLRs.
TLRs are germ line-encoded pattern recognition receptors, and more than 11 members have been identified (2, 3). Several TLRs recognize different bacterial products (2, 3): TLR2 recognizes specific components of Mycobacterium spp. (lipoarabinomannan), fungi (zymosan), and gram-positive bacteria (lipoteichoic acid, lipoproteins); TLR4 recognizes endotoxin or lipopolysaccharide (LPS) (36); TLR5 recognizes and is activated by bacterial flagellin; and TLR9 recognizes unmethylated DNA containing CpG motifs. Investigations have shown that TLRs are important for pulmonary innate defense against bacterial pathogens. For example, TLR4 has been shown to be required for effective pulmonary innate immunity against H. influenzae (88) and K. pneumoniae (13). Furthermore, C3H/HeJ mice, which have defective TLR4 signaling, displayed increased mortality, impaired bacterial clearance, and delayed expression of inflammatory cytokines in response to intrapulmonary K. pneumoniae challenge (75). It has also been demonstrated that TLR4 plays a limited role in antibacterial defense in response to a low dose of pneumococci (22). However, TLR4 mutant mice are not more susceptible than control mice to pulmonary infection due to L. pneumophila, suggesting that other TLRs are essential for the initiation of immunity against this pathogen (46). Furthermore, TLR2 has been shown to be important for host defense against L. pneumophila (7, 28, 33), S. pneumoniae (21), and Porphyromonas gingivalis (1), whereas TLR2 and TLR4 have been shown to be significant for host defense against Acinetobacter baumannii pneumonia (43). Moreover, TLR5 has been identified as an important molecule to induce neutrophil recruitment to the lung at an early time point (4 h) during L. pneumophila infection (32). Further, TLR9 plays an essential role in host defense against both extracellular pathogens, such as K. pneumoniae (9) and S. pneumoniae (4), and intracellular pathogens, such as L. pneumophila (10), via a mechanism that is not associated with neutrophil influx in the lungs. These studies have further demonstrated that TLR9-induced recruitment of myeloid dendritic cells to the lung is such a mechanism (9-10). Further investigations suggest that innate host responses against both extracellular and intracellular bacterial pathogens, including Pseudomonas aeruginosa and L. pneumophila, are more noticeably impaired in mice that are deficient in signaling via multiple TLRs than in mice that are deficient in a single TLR (7, 33, 73, 78, 80, 89). These more remarkable phenotypes in multiple-TLR-gene-deficient mice are most likely due to the redundancy in TLR signaling cascades. Another explanation for these phenotypes of multiple-TLR-gene-disrupted mice is that the TLRs can interact either synergistically or sequentially to maximally amplify protective responses against a specific bacterium. In this context, it has been shown that innate signals produced early (4 h) in response to challenge with K. pneumoniae are markedly reduced in mice with defective TLR4 signaling but that later responses (16 h) remain intact (13, 89).
The specificity of TLR signaling is due in part to the recruitment of adaptor molecules (52, 68). There have been five TIR domain-containing TLR adaptor proteins discovered to date: myeloid differentiation marker 88 (MyD88), TIR domain-containing adaptor protein (TIRAP) (also known as MyD88 adaptor-like protein, or MAL), TIR domain-containing adaptor-inducing beta interferon (TRIF), and TRIF-related adaptor molecule (TRAM). A role for SARM, the fifth adaptor protein, has only recently been described (14). MyD88 is recruited to the TLR complex by TIRAP and plays a key role in the early activation and translocation to the nucleus of nuclear factor κB (NF-κB) (52, 68) in the TLR2 and TLR4 signaling cascades, whereas MyD88 is directly activated by other TLRs in the absence of TIRAP (2, 3, 52, 68). The TLR4-induced MyD88-independent cascade, which results in late NF-κB activation and downstream expression of type 1 interferons, is mediated by TRIF (2, 3, 52, 68). It is now well documented that TLR adaptor molecules play an essential role in pulmonary host defense against bacterial pathogens. For example, it has been shown that MyD88 is critical for host defense against P. aeruginosa (73, 78, 80), S. pneumoniae (4), and H. influenzae (91) but not S. aureus (78). Furthermore, MyD88 is important for host defense against L. pneumophila (7, 33). Experiments with TIRAP-, MyD88-, and TRIF-deficient mice demonstrated a reduced immune response to intrapulmonary Escherichia coli challenge, indicating that both the MyD88-dependent and MyD88-independent cascades contribute to the recognition of this pathogen (38, 40). Data obtained from TIRAP gene-deficient mice showed an attenuated host response to K. pneumonia but not to P. aeruginosa, demonstrating that the TIRAP-mediated cascade is important for innate host defense against selected microbes (41). Table 1 summarizes the roles of TLRs and their adaptors in lower respiratory tract bacterial infections.
TABLE 1.
Roles of TLRs and TLR adaptors in acute lower respiratory tract bacterial infections
| TLR or TLR adaptor | Infection-causing bacterium (reference[s]) | Phenotypea
|
|||
|---|---|---|---|---|---|
| Survival | Neutrophil influxb | Bacterial burdenc | Bacterial disseminationd | ||
| TLRs | |||||
| TLR2 | Acinetobacter baumannii (43) | ↓ | ↓ | ND | ND |
| Legionella pneumophila (7, 28, 33) | ↓ | ↓ | ↑ | ↑ | |
| Porphyromonas gingivalis (1) | ND | NS | ↑ | ND | |
| Pseudomonas aeruginosa (73) | ND | NS | ↑ (early) | ND | |
| Streptococcus pneumoniae (21) | ↓ | ↓ | ↑ | ↑ | |
| TLR4 | Acinetobacter baumannii (43) | ↓ | ↓ | ↑ | ↑ |
| Haemophilus influenzae (88) | ↓ | ↓ | ↑ | ND | |
| Klebsiella pneumoniae (13) | ↓ | ND | ↑ | ND | |
| Pseudomonas aeruginosa (73) | ND | ↓ (late) | NS | ND | |
| Streptococcus pneumoniae (22) | ↓ | ↓ | ↑ | ND | |
| TLR5 | Legionella pneumophila (32) | ND | ↓ (early) | NS | ND |
| TLR9 | Klebsiella pneumoniae (9) | ↓ | NS | ↑ | ↑ |
| Legionella pneumophila (10) | ↓ | NS | ↑ | ND | |
| Streptococcus pneumoniae (4) | ↓ | NS | ↑ | ↑ | |
| TLR adaptors | |||||
| MyD88 | Escherichia coli (38, 40) | ↓ | ↓ | ND | ND |
| Haemophilus influenzae (91) | ND | ND | ↓ | ND | |
| Klebsiella pneumoniae (41) | ND | ↓ | ND | ND | |
| Legionella pneumophila (7, 33) | ↓ | ↓ | ↑ | ↑ | |
| Pseudomonas aeruginosa (73, 78, 80) | ↓ | ↓ | ↑ | ↑ | |
| Staphylococcus aureus (78) | ND | ↓ | ND | ND | |
| Streptococcus pneumoniae (4) | ↓ | ↓ | ↑ | ↑ | |
| TIRAP | Klebsiella pneumoniae (41) | ↓ | ↓ | ↑ | ↑ |
| Pseudomonas aeruginosa (41) | ND | ↓ | ↑ | ND | |
| TRIF | Escherichia coli (38, 40) | ↓ | ↓ | ↑ | ↑ |
Phenotype was determined using gene-deficient or mutant mice after intrapulmonary infection. ↓, decreased; ↑, increased; ND, not determined; NS, no significant difference.
Neutrophil influx was determined using bronchoalveolar lavage fluid and/or lung parenchyma.
Bacterial burden was measured as the number of CFU in the lungs.
Bacterial dissemination was measured as the number of CFU in the blood or spleen.
NLRs.
NLRs are cytoplasmic proteins and have three domains that are thought to integrate into different intracellular signaling pathways (51). The human NLRs comprise more than 22 members, such as NOD1, NOD2, NAIP, cryopyrin, and interleukin-converting enzyme-protease-activating factor (Ipaf; NLRC4). Caspase recruitment domains (CARDs) place certain, but not all, NOD proteins in pathways leading to apoptosis. The central nucleotide-binding domain is found in several NLRs and is implicated in signal transduction cascades and in protein-protein interactions. Most importantly, a series of leucine-rich repeats in NOD1 and NOD2 is thought to recognize bacterial cell wall components (76). To date, muramyl dipeptide is the only identified ligand for the NOD2 proteins, whereas NOD1 recognizes meso-diaminopimelic acid-containing stem peptides from gram-negative peptidoglycan, although it is not clear how cytosolic NODs can find their ligands (76, 84). The modular nature of NOD proteins and the presence of the leucine-rich repeats, which are also found in TLRs and are responsible for TLRs' recognition of intracellular pathogens and their products, support the hypothesis that NOD proteins are the intracellular equivalent of the TLR system. Upon ligand binding, NOD1 and NOD2 induce a signaling cascade independent of TLRs to activate NF-κB and mitogen-activated protein (Jun N-terminal protein and p38) kinases via an adaptor protein, RIP2, which is a serine/threonine kinase (76, 84). RICK/RIP2/CARDIAK can bind to a variety of CARD-containing molecules through CARD-CARD interactions (76, 84). Studies have demonstrated that Ipaf or NLRC4 is expressed in myeloid cells and is known to activate caspase-1 within a multiprotein complex termed the “inflammasome” (reviewed in reference 51). This event seems to be important for antibacterial host defense against P. aeruginosa and L. pneumophila. The underlying mechanisms in the activation of NLRC4-mediated caspase-1 have been recently elucidated. The type III secretion systems of Salmonella spp., Shigella spp., and P. aeruginosa and the type IV secretion system of L. pneumophila are required for the activation of the inflammasome (reviewed in reference 51). The role of NLRs in lung bacterial infections has not been well characterized, although cell culture studies have suggested that NLRs play an important role in lung bacterial infections. For example, an investigation has demonstrated, using a bronchial epithelial cell line, BEAS-2B, that NOD2 is essential to induce signaling cascades associated with NF-κB activation against internalized S. pneumoniae (69). Moreover, the cytokine secretion pattern and bacterial killing are impaired in NOD1-deficient cells infected with P. aeruginosa (85), demonstrating a clear role for NOD1 against this respiratory pathogen. Although NOD1 activation selectively induces neutrophil influx in the peritoneums of mice in response to KiE-DAP compounds (KF1B) (51), less is known about the role of NLRs in respiratory bacterial infections.
Transcription factors.
Transcription factors are proteins that bind to promoter regions and induce the expression of genes. Although several transcription factors have been identified, NF-κB is the most studied factor (34). In resting cells, NF-κB is present as an inactive, IκB-bound complex in the cytosol. Upon stimulation, IκB is degraded, allowing active NF-κB to enter the nucleus, and activates the expression of several immune response genes. Numerous signals can activate NF-κB (34). The MyD88-dependent cascade in TLR signaling leads to the expression of proinflammatory genes, including those for cytokines/chemokines and adhesion molecules, through the early activation of NF-κB, consisting of p65 and p50 (2-3). On the other hand, the MyD88-independent cascade leads to the expression of inflammatory genes through the late activation of NF-κB (2, 3). Recent studies have demonstrated that NOD-dependent signaling cascades can also activate NF-κB (76, 84). Furthermore, several neutrophil chemokine genes, including those for keratinocyte-derived chemokine (KC), macrophage inflammatory protein 2 (MIP-2), and CXC ligand 5 (CXCL5), contain NF-κB binding sites in their 5′ untranslated regions (70). Moreover, the transcription factors are important for neutrophil influx in the lungs. For example, it has been documented that intratracheal LPS administration results in the nuclear translocation of NF-κB in the lungs and subsequent neutrophil influx in the lungs (59). Extended observations using RelA and tumor necrosis factor receptor 1 (TNF-R1) or RelA and TNF-α double-gene-deficient mice lead to the attenuation of neutrophil accumulation in the lungs in response to LPS challenge or viable E. coli challenge compared with the neutrophil accumulation of either TNF-R1 or TNF-α single-gene-deficient mice, demonstrating that NF-κB is an important mediator of neutrophil recruitment into the lungs (5). A further advancement in our understanding of transcriptional factors is the finding that RelA deficiency (in the TNF-R1 knockout background) resulted in the attenuation of neutrophil influx during E. coli (58) and pneumococcal (42, 72) pneumonia.
Neutrophil chemokines.
The secretion of chemokines, which are small protein molecules that induce the chemotactic migration of neutrophils, is the first critical step of infiltration (47). Chemokines are categorized into four groups based on their compositions of a cysteine motif positioned near the N terminus: C, CC, CXC, and CX3C. The CXC family of chemokines has four cysteine residues at the N terminus, the first two of which are separated by a nonconserved amino acid. This group is further categorized by the presence or absence of an ELR (glutamic acid-leucine-arginine) motif immediately before the CXC sequence. Importantly, ELR-positive (ELR+) CXC chemokines exert potent neutrophil chemotactic and activating properties both in vitro and in vivo (8). Seven members of this class have been identified so far in humans: IL-8; neutrophil-activating peptide 2; growth-related oncogenes α, ß, and γ; epithelium-derived neutrophil-activating peptide 78 (ENA-78/human CXCL5); and granulocyte chemotactic protein 2 (GCP-2).
Compelling evidence exists to support an important role for ELR+ CXC chemokines in the lung's antibacterial host defense. Human ELR+ CXC chemokines, most notably IL-8, have been shown to be present in increased quantities in patients with pulmonary bacterial infection (8). Additionally, expression of murine ELR+ chemokines, such as KC (the functional murine homolog of IL-8), MIP-2, CXCL5/LIX (LPS-induced CXC chemokine), and lungkine, is upregulated in the murine lung in response to intratracheal bacterial challenge (8). Inhibition of selected ELR+ chemokines, such as KC and MIP-2, impair neutrophil influx into the lung in response to bacteria and/or their products (12, 27, 31). In addition, it has been demonstrated that the influx of neutrophils via the production of a neutrophil chemokine, such as KC, can be modulated by TNF-R1 and IL-R1 during E. coli pneumonia (58). However, lungkine does not appear to induce neutrophil influx into the lung parenchyma (16). In our initial studies, we observed the upregulation of a neutrophil chemokine, CXCL5, in murine lungs in response to LPS (39, 77). CXCL5 upregulation was also observed in the lungs in response to the gram-negative pathogens L. pneumophila, P. aeruginosa, K. pneumoniae, and Bordetella bronchiseptica (13, 82, 83, 90).
ELR+ chemokines are produced by numerous types of lung cells, including myeloid and nonmyeloid cells. The role of myeloid cells in the production of neutrophil chemokines in response to bacterial lung inflammation is well established (6, 35, 66). In addition, alveolar endothelial cells are also important for neutrophil recruitment in the lungs in response to LPS challenge (17). Furthermore, recent studies have demonstrated the role of non-bone marrow-derived cells, including respiratory epithelial cells, in regulating neutrophil influx in patients with lung inflammation induced by bacteria and/or their products (18, 79). Neutrophil chemokines such as KC and MIP-2 are produced primarily by myeloid cells, whereas lungkine is produced primarily by bronchial/airway epithelial cells (12, 16, 27, 31). However, the cells which express CXCL5 and the signaling cascades required to produce CXCL5 were not defined until recently. We have demonstrated that alveolar epithelial type II (AEII) cells in mice exposed to LPS produce CXCL5 via the TLR4-dependent, MyD88-mediated pathway (37, 39). These results suggest that alveolar epithelial type II cells sense bacteria and contribute to the influx of neutrophils via the production of CXCL5.
CXC chemokines induce a signaling cascade via CXCRs on the surfaces of myeloid and nonmyeloid (resident) cells. Five CXCRs have been identified to date (CXCR1 to -5). CXCR1 and CXCR2 are the receptors for ELR+ CXC chemokines (15, 45, 74), whereas CXCR3 is the receptor for ELR-negative CXC chemokines (15, 45, 74). Human IL-8 binds to both CXCR1 and CXCR2 (15, 45, 74). Although it was widely believed that CXCR2 is the only functional receptor to induce downstream signaling upon ELR+ CXC chemokine binding in mice, a recent investigation demonstrated that murine CXCR1 is also functional (23). All known murine neutrophil chemoattractants, including KC, MIP-2, CXCL5, and lungkine, bind to murine CXCR2 (15, 45, 74). Furthermore, in vivo studies using either blocking antibodies or gene-disrupted mice have demonstrated that CXCR2 is important for neutrophil-associated lung inflammation induced by Pseudomonas aeruginosa, Nocardia asteroides, or L. pneumophila (63, 82, 86).
Adhesion molecules.
The recruitment of neutrophils into the lungs from the bloodstream involves (i) transient attachment to endothelial cells (tethering), (ii) firm attachment to the endothelium, and (iii) eventual migration into the extravascular space. Studies have obtained evidence that selectins and β2 integrins (CD11/CD18), as well as other cell adhesion molecules (vascular cell adhesion molecules [VCAMs] and intracellular cell adhesion molecules [ICAMs]), play an important role in neutrophil trafficking (54). Neutrophil recruitment to the lungs in response to S. pneumoniae infection was not attenuated in mice lacking P- or E-selectin, suggesting that S. pneumoniae-induced neutrophil migration involves molecules other than P- or E-selectin (60). Earlier studies have reported that CD18-deficient neutrophils have a reduced capacity to emigrate to the lungs in response to intratracheal E. coli LPS or P. aeruginosa challenge (57, 62), whereas CD18-deficient neutrophils do not have a reduced ability to migrate to the lungs during S. pneumoniae pneumonia (64), demonstrating that CD18 plays a pathogen-specific role in eliciting neutrophil influx in the lungs. Additional investigations suggest that very late antigen 4 (VLA-4) plays a small role in CD18-independent neutrophil emigration to the lungs despite the fact that most of the CD18-independent neutrophil migration induced by pneumococci in the lungs occurs through VLA-4-independent cascades (81). The roles of other cell adhesion molecules in pneumonia in animals were delineated primarily using blocking antibodies. Evidence obtained from those studies demonstrate that ICAM-1 is important for neutrophil recruitment to the lungs in response to K. pneumoniae (67). In this regard, we observed reduced VCAM-1 and ICAM-1 upregulation in the lungs of TIRAP gene-disrupted mice compared with levels of upregulation in controls, and this reduced upregulation correlates well with reduced neutrophil influx in the lungs of TIRAP gene-disrupted mice against E. coli challenge (38).
PERSPECTIVE
Neutrophil-mediated innate immune responses against bacteria in the lungs determine the outcome of infection; an insufficient neutrophil recruitment can lead to life-threatening infection despite the fact that an extreme accumulation of neutrophils can result in excessive lung injury associated with inflammation. Therefore, the ideal therapeutic approach for targeting neutrophils is to attenuate their destructive potential while maintaining their critical role in antibacterial defense. Since the identification of neutrophil influx is a major pathological hallmark of lower respiratory tract bacterial infections, our understanding regarding the molecular and cellular mechanisms responsible for neutrophil recruitment, priming, and activation in the lungs has been substantially increased in recent years using animal models. Despite limitations with using mice, the availability of transgenic mice and ample reagents makes the murine model more attractive than other animal models. A better understanding of the signal transduction pathways initiated by the binding of microbial products to TLRs and NLRs and the culmination of our knowledge of the precise mechanisms underlying neutrophil-mediated lung damage may enable well-focused strategies to attenuate excessive neutrophil accumulation in the lungs. Investigating the complex interactions between pathogens and lung cells and the biology of proinflammatory mediators may lead to the development of novel therapeutic strategies to modulate neutrophil-mediated lung damage. This is extremely important in view of the emergence of antibiotic-resistant microbes, which pose a threat to human health and constitute an economic burden for the health care system. The challenge for the next decade, therefore, is to develop approaches to keep neutrophils in the lung for defensive functions while modulating their undesirable effects leading to inflammatory lung damage.
Acknowledgments
We are indebted to Scott Worthen for his support, guidance, and contribution to the work presented in this article. We thank Rachel Zemans, Ken Malcolm, and Gayathriy Balamayooran for the critical reading of the manuscript. We extend an apology to any of the investigators whose hard work on acute lower respiratory tract bacterial infections was either inadvertently overlooked or not included because of space limitations.
This work was supported by a research grant from the American Lung Association (grant RG-22442-N to S.J.), a Scientist Award from the Flight Attendant Medical Research Institute (grant YCSA-062466 to S.J.), and an R01 grant from the NIH (grant HL-091958 to S.J.). Ann Craig was supported by an NIH-sponsored Summer Research Scholar Program grant to LSU.
Editor: J. B. Kaper
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Ajishengallis, G., M. Wang, G. J. Bagby, and S. Nelson. 2008. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J. Immunol. 1814141-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signaling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. [DOI] [PubMed] [Google Scholar]
- 4.Albiger, B., S. Dahlberg, A. Sandgren, F. Wartha, K. Beiter, H. Katsuragi, S. Akira, S. Normark, and B. Henriques-Normark. 2007. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell. Microbiol. 9633-644. [DOI] [PubMed] [Google Scholar]
- 5.Alcamo, E., J. P. Mizgerd, B. H. Horwitz, R. Bronson, A. A. Beg, M. Scott, C. M. Doerschuk, R. O. Hynes, and D. Baltimore. 2001. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-kappa B in leukocyte recruitment. J. Immunol. 1671592-1600. [DOI] [PubMed] [Google Scholar]
- 6.Andonegui, G., C. S. Bonder, F. Green, S. C. Mullaly, L. Zbytnuik, E. Raharjo, and P. Kubes. 2003. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J. Clin. Investig. 1111011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archer, K. A., and C. R. Roy. 2006. MyD88-dependent responses involving Toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires' disease. Infect. Immun. 743325-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baggiolinim, M., B. Dewaldm, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv. Immunol. 5597-179. [PubMed] [Google Scholar]
- 9.Bhan, U., N. W. Lukacs, J. J. Osterholzer, M. W. Newstead, X. Zeng, T. A. Moore, T. R. McMillan, A. M. Krieg, S. Akira, and T. J. Standiford. 2007. TLR9 is required for protective innate immunity in Gram-negative bacterial pneumonia: role of dendritic cells. J. Immunol. 1793937-3946. [DOI] [PubMed] [Google Scholar]
- 10.Bhan, U., G. Trujillo, K. Lyn-Kew, M. W. Newstead, X. Zeng, C. M. Hogaboam, A. M. Krieg, and T. J. Standiford. 2008. Toll-like receptor 9 regulates the lung macrophage phenotype and host immunity in murine pneumonia caused by Legionella pneumophila. Infect. Immun. 762895-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishai, W. R. 2005. Clinical significance of pneumococcal resistance and factors influencing outcomes. Treat. Respir. Med. 4(Suppl. 1)19-23. [DOI] [PubMed] [Google Scholar]
- 12.Bozic, C. R., L. F. Kolakowski, N. P. Gerard, C. Garcia-Rodriguez, C. von Uexkull-Guldenband, M. J. Conklyn, R. Breslow, H. J. Showell, and C. Gerard. 1995. Expression and biologic characterization of the murine chemokine KC. J. Immunol. 1546048-6057. [PubMed] [Google Scholar]
- 13.Branger, J., S. Knapp, S. Weijer, J. C. Leemans, J. M. Pater, P. Speelman, S. Florquin, and T. van der Poll. 2004. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 72788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carty, M., R. Goodbody, M. Schröder, J. Stack, P. N. Moynagh, and A. G. Bowie. 2006. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat. Immunol. 71074-1081. [DOI] [PubMed] [Google Scholar]
- 15.Cascieri, M. A., and M. S. Springer. 2000. The chemokine/chemokine-receptor family: potential and progress for therapeutic intervention. Curr. Opin. Chem. Biol. 4420-427. [DOI] [PubMed] [Google Scholar]
- 16.Chen, S. C., B. Mehrad, J. C. Deng, G. Vassileva, D. J. Manfra, D. N. Cook, M. T. Wiekowski, A. Zlotnik, T. J. Standiford, and S. A. Lira. 2001. Impaired pulmonary host defense in mice lacking expression of the CXC chemokine lungkine. J. Immunol. 1663362-3368. [DOI] [PubMed] [Google Scholar]
- 17.Chen, S. M., D. S. Cheng, B. J. Williams, T. P. Sherrill, W. Han, M. Chont, L. Saint-Jean, J. W. Christman, R. T. Sadikot, F. E. Yull, and T. S. Blackwell. 2008. The nuclear factor kappa-B pathway in airway epithelium regulates neutrophil recruitment and host defence following Pseudomonas aeruginosa infection. Clin. Exp. Immunol. 153420-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng, D. S., W. Han, S. M. Chen, T. P. Sherrill, M. Chont, G. Y. Park, J. R. Sheller, V. V. Polosukhin, J. W. Christman, F. E. Yull, and T. S. Blackwell. 2007. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J. Immunol. 1786504-6513. [DOI] [PubMed] [Google Scholar]
- 19.Combes, A., C. E. Luyt, J. Y. Fagon, M. Wollf, J. L. Trouillet, C. Gibert, and J. Chastre. 2004. Impact of methicillin resistance on outcome of Staphylococcus aureus ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 170786-792. [DOI] [PubMed] [Google Scholar]
- 20.Cronkite, E. P., and T. M. Fliedner. 1964. Granulopoiesis. N. Engl. J. Med. 2701403-1408. [DOI] [PubMed] [Google Scholar]
- 21.Dessing, M. C., S. Florquin, J. C. Paton, and T. van der Poll. 2008. Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci. Cell. Microbiol. 10237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dessing, M. C., M. Schouten, C. Draing, M. Levi, S. von Aulock, and T. van der Pol. 2008. Role played by Toll-like receptors 2 and 4 in lipoteichoic acid-induced lung inflammation and coagulation. J. Infect. Dis. 197245-252. [DOI] [PubMed] [Google Scholar]
- 23.Fan, X., A. C. Patera, A. Pong-Kennedy, G. Deno, W. Gonsiorek, D. J. Manfra, G. Vassileva, M. Zeng, C. Jackson, I. Sullivan, W. Sharif-Rodriguez, G. Opdenakker, J. Van Damme, J. A. Hedrick, D. Lundell, S. A. Lira, and R. W. Hipkin. 2007. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J. Biol. Chem. 28211658-11666. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara, A. M. 2005. New fluoroquinolones in lower respiratory tract infections and emerging patterns of pneumococcal resistance. Infection 33106-114. [DOI] [PubMed] [Google Scholar]
- 25.Fliedner, T. M., E. P. Cronkite, S. A. Killman, and V. P. Bond. 1964. Granulocytopoesis. II. Emergence and pattern of labeling of neutrophilic granulocytes in humans. Blood 24683-700. [PubMed] [Google Scholar]
- 26.Fliedner, T. M., E. P. Cronkite, and J. S. Robertson. 1964. Granulocytopoesis. I. Senescence and random loss of neutrophilic granulocytes in human beings. Blood 24402-414. [PubMed] [Google Scholar]
- 27.Frevert, C. W., S. Huang, H. Danaee, J. D. Paulauskis, and L. Kobzik. 1995. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J. Immunol. 154335-344. [PubMed] [Google Scholar]
- 28.Fuse, E. T., K. Tateda, Y. Kikuchi, T. Matsumoto, F. Gondaira, A. Azuma, S. Kudoh, T. J. Standiford, and K. Yamaguchi. 2007. Role of Toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model. J. Med. Microbiol. 56305-312. [DOI] [PubMed] [Google Scholar]
- 29.Gallin, J. I. 1984. Neutrophil specific granules: a fuse that ignites the inflammatory response. Clin. Res. 32320-328. [PubMed] [Google Scholar]
- 30.Garvy, B. A., and A. G. Harmsen. 1996. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation 20499-512. [DOI] [PubMed] [Google Scholar]
- 31.Greenberger, M. J., R. M. Strieter, S. L. Kunkel, J. M. Danforth, L. L. Laichalk, D. C. McGillicuddy, and T. J. Standiford. 1996. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J. Infect. Dis. 173159-165. [DOI] [PubMed] [Google Scholar]
- 32.Hawn, T. R., W. R. Berrington, I. A. Smith, S. Uematsu, S. Akira, A. Aderem, K. D. Smith, and S. J. Skerrett. 2007. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J. Immunol. 1796981-6987. [DOI] [PubMed] [Google Scholar]
- 33.Hawn, T. R., K. D. Smith, A. Aderem, and S. J. Skerrett. 2006. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J. Infect. Dis. 1931693-1702. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann, A., and D. Baltimore. 2006. Circuitry of nuclear factor kappaB signaling. Immunol. Rev. 210171-186. [DOI] [PubMed] [Google Scholar]
- 35.Hollingsworth, J. W., B. J. Chen, D. M. Brass, K. Berman, M. D. Gunn, D. N. Cook, and D. A. Schwartz. 2005. The critical role of hematopoietic cells in lipopolysaccharide-induced airway inflammation. Am. J. Respir. Crit. Care Med. 171806-813. [DOI] [PubMed] [Google Scholar]
- 36.Jeyaseelan, S., H. W. Chu, S. K. Young, M. W. Freeman, and G. S. Worthen. 2005. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infect. Immun. 731754-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeyaseelan, S., H. W. Chu, S. K. Young, and G. S. Worthen. 2004. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect. Immun. 727247-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeyaseelan, S., R. Manzer, S. K. Young, M. Yamamot, S. Akira, R. J. Mason, and G. S. Worthen. 2005. Toll-IL-1 receptor domain-containing adaptor protein is critical for early lung immune responses against Escherichia coli lipopolysaccharide and viable Escherichia coli. J. Immunol. 1757484-7495. [DOI] [PubMed] [Google Scholar]
- 39.Jeyaseelan, S., R. Manzer, S. K. Young, M. Yamamoto, S. Akira, R. J. Mason, and G. S. Worthen. 2005. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am. J. Respir. Cell Mol. Biol. 32531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeyaseelan, S., S. K. Young, M. B. Fessler, Y. Liu, K. C. Malcolm, M. Yamamoto, S. Akira, and G. S. Worthen. 2007. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J. Immunol. 1783153-3160. [DOI] [PubMed] [Google Scholar]
- 41.Jeyaseelan, S., S. K. Young, M. Yamamoto, P. G. Arndt, S. Akira, J. K. Kolls, and G. S. Worthen. 2006. Toll/IL-1R domain-containing adaptor protein (TIRAP) is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J. Immunol. 177538-547. [DOI] [PubMed] [Google Scholar]
- 42.Jones, M. R., B. T. Simms, M. M. Lupa, M. S. Kogan, and J. P. Mizgerd. 2005. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J. Immunol. 1757530-7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knapp, S., C. W. Wieland, S. Florquin, R. Pantophlet, L. Dijkshoorn, N. Tshimbalanga, S. Akira, and T. van der Poll. 2006. Differential roles of CD14 and toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am. J. Respir. Crit. Care Med. 173122-129. [DOI] [PubMed] [Google Scholar]
- 44.Krause, P. J., M. D. Todd, W. W. Hancock, W. T. Pastuszak, E. G. Maderazo, D. H. Hild, and C. M. Kosciol. 1990. The role of cellular maturation in neutrophil heterogeneity. Blood 761639-1646. [PubMed] [Google Scholar]
- 45.Lee, J., G. Calcalano, K. Toy, M. W. Moore, and W. I. Wood. 1995. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 1552158-2164. [PubMed] [Google Scholar]
- 46.Lettinga, K. D., S. Florquin, P. Speelman, R. van Ketel, T. van der Poll, and A. Verbon. 2002. Toll-like receptor 4 is not involved in host defense against pulmonary Legionella pneumophila infection in a mouse model. J. Infect. Dis. 186570-573. [DOI] [PubMed] [Google Scholar]
- 47.Lukacs, N. W., C. Hogaboam, E. Campbell, and S. L. Kunkel. 1999. Chemokines: function, regulation and alteration of inflammatory responses. Chem. Immunol. 72102-120. [DOI] [PubMed] [Google Scholar]
- 48.Lynch, J. P., III., and F. J. Martinez. 1998. Community-acquired pneumonia. Curr. Opin. Pulm. Med. 4162-172. [PubMed] [Google Scholar]
- 49.Marsh, J. C., D. R. Boggs, G. E. Cartwright, and M. M. Wintrobe. 1967. Neutrophil kinetics in acute infection. J. Clin. Investig. 461943-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin, T. R., and C. W. Frevert. 2005. Innate immunity in the lungs. Proc. Am. Thorac. Soc. 2403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masumoto, J., K. Yang, S. Varambally, M. Hasegawa, S. A. Tomlins, S. Qiu, Y. Fujimoto, A. Kawasaki, S. J. Foster, Y. Horie, T. W. Mak, G. Núñez, A. M. Chinnaiyan, K. Fukase, and N. Inohara. 2006. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J. Exp. Med. 203203-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. A. Janeway. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2253-258. [DOI] [PubMed] [Google Scholar]
- 53.Minino, A. M., and B. L. Smith. 2001. Deaths: preliminary data for 2000. Natl. Vital Stat. Rep. 491-40. [PubMed] [Google Scholar]
- 54.Mizgerd, J. P. 2002. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin. Immunol. 14123-132. [DOI] [PubMed] [Google Scholar]
- 55.Mizgerd, J. P. 2006. Lung infection: a public health priority. PLoS Med. 3e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizgerd, J. P. 2008. Acute lower respiratory tract infection. N. Engl. J. Med. 358716-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizgerd, J. P., B. H. Horwitz, H. C. Quillen, M. L. Scott, and C. M. Doerschuk. 1999. Effects of CD18 deficiency on the emigration of murine neutrophils during pneumonia. J. Immunol. 163995-999. [PubMed] [Google Scholar]
- 58.Mizgerd, J. P., M. M. Lupa, M. S. Kogan, H. B. Warren, L. Kobzik, and G. P. Topulos. 2003. Nuclear factor-kappaB p50 limits inflammation and prevents lung injury during Escherichia coli pneumonia. Am. J. Respir. Crit. Care Med. 168810-817. [DOI] [PubMed] [Google Scholar]
- 59.Mizgerd, J. P., M. M. Lupa, and M. S. Spieker. 2004. NF-kappaB p50 facilitates neutrophil accumulation during LPS-induced pulmonary inflammation. BMC Immunol. 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizgerd, J. P., B. B. Meek, G. J. Kutkoski, D. C. Bullard, A. L. Beaudet, and C. M. Doerschuk. 1996. Selectins and neutrophil traffic: margination and Streptococcus pneumoniae-induced emigration in murine lungs. J. Exp. Med. 184639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizgerd, J. P., and S. J. Skerrett. 2008. Animal models of human pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 294L387-L398. [DOI] [PubMed] [Google Scholar]
- 62.Mizgerd, J. P., M. R. Spieker, and C. M. Doerschuk. 2001. Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J. Immunol. 1664042-4048. [DOI] [PubMed] [Google Scholar]
- 63.Moore, T. A., M. W. Newstead, R. M. Strieter, B. Mehrad, B. L. Beaman, and T. J. Standiford. 2000. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. J. Immunol. 164908-915. [DOI] [PubMed] [Google Scholar]
- 64.Moreland, J. G., G. Bailey, W. M. Nauseef, and J. P. Weiss. 2004. Organism-specific neutrophil-endothelial cell interactions in response to Escherichia coli, Streptococcus pneumoniae, and Staphylococcus aureus. J. Immunol. 172426-432. [DOI] [PubMed] [Google Scholar]
- 65.Nelson, S., and W. R. Summer. 1998. Innate immunity, cytokines, and pulmonary host defense. Infect. Dis. Clin. N. Am. 12555-567. [DOI] [PubMed] [Google Scholar]
- 66.Noulin, N., V. F. J. Quesniaux, S. Schnyder-Candrian, B. Schnyder, I. Maillet, T. Robert, B. B. Vargaftig, B. Ryffel, and I. Couillin. 2005. Both hematopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J. Immunol. 1756861-6869. [DOI] [PubMed] [Google Scholar]
- 67.O'Brien, A. D., T. J. Standiford, K. A. Bucknell, S. E. Wilcoxen, and R. Paine III. 1999. Role of alveolar epithelial cell intercellular adhesion molecule-1 in host defense against Klebsiella pneumoniae. Am. J. Physiol. 276L961-L970. [DOI] [PubMed] [Google Scholar]
- 68.O'Neill, L. A., and A. G. Bowie. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7353-364. [DOI] [PubMed] [Google Scholar]
- 69.Opitz, B., A. Püschel, B. Schmeck, A. C. Hocke, S. Rosseau, S. Hammerschmidt, R. R. Schumann, N. Suttorp, and S. Hippenstiel. 2004. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J. Biol. Chem. 27936426-36432. [DOI] [PubMed] [Google Scholar]
- 70.Pahl, H. L. 1999. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 186853-6866. [DOI] [PubMed] [Google Scholar]
- 71.Prince, A. S., J. P. Mizgerd, J. Wiener-Kronish, and J. Bhattacharya. 2006. Cell signaling underlying the pathophysiology of pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 291L297-L300. [DOI] [PubMed] [Google Scholar]
- 72.Quinton, L. J., M. R. Jones, B. T. Simms, M. S. Kogan, B. E. Robson, S. J. Skerrett, and J. P. Mizgerd. 2007. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J. Immunol. 1781896-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramphal, R., V. Balloy, M. Huerre, M. Si-Tahar, and M. Chignard. 2005. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J. Immunol. 1753927-3934. [DOI] [PubMed] [Google Scholar]
- 74.Sallusto, F., C. R. MacKay, and A. Lanzavecchia. 2000. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18593-620. [DOI] [PubMed] [Google Scholar]
- 75.Schurr, J. R., E. Young, P. Byrne, C. Steele, J. E. Shellito, and J. K. Kolls. 2005. Central role of Toll-like receptor 4 signaling and host defense in experimental pneumonia caused by gram-negative bacteria. Infect. Immun. 73532-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaw, M. H., T. Reimer, Y. G. Kim, and G. Nuñez. 2008. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr. Opin. Immunol. 20377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaw, M. J. 2005. Ventilator-associated pneumonia. Curr. Opin. Pulm. Med. 11236-241. [DOI] [PubMed] [Google Scholar]
- 78.Skerrett, S. J., H. D. Liggitt, A. M. Hajjar, and C. B. Wilson. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 1723377-3381. [DOI] [PubMed] [Google Scholar]
- 79.Skerrett, S. J., H. D. Liggitt, A. M. Hajjar, R. K. Ernst, S. I. Miller, and C. B. Wilson. 2004. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am. J. Physiol. Lung Cell. Mol. Physiol. 287L143-L152. [DOI] [PubMed] [Google Scholar]
- 80.Skerrett, S. J., C. B. Wilson, H. D. Liggitt, and A. M. Hajjar. 2007. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. J. Physiol. Lung Cell. Mol. Physiol. 292312-322. [DOI] [PubMed] [Google Scholar]
- 81.Tasaka, S., S. E. Richer, J. P. Mizgerd, and C. M. Doerschuk. 2008. Very late antigen-4 in CD18-independent neutrophil emigration during acute bacterial pneumonia in mice. Am. J. Respir. Crit. Care Med. 16653-60. [DOI] [PubMed] [Google Scholar]
- 82.Tateda, K., T. A. Moore, J. C. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 1663355-3361. [DOI] [PubMed] [Google Scholar]
- 83.Tateda, K., T. A. Moore, M. W. Newstead, W. C. Tsai, X. Zeng, J. C. Deng, G. Chen, R. Reddy, K. Yamaguchi, and T. J. Standiford. 2001. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect. Immun. 692017-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ting, J. P., S. B. Willingham, and D. T. Bergstralh. 2008. NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 8372-379. [DOI] [PubMed] [Google Scholar]
- 85.Travassos, L. H., L. A. Carneiro, S. E. Girardin, I. G. Boneca, R. Lemos, M. T. Bozza, R. C. Domingues, A. J. Coyle, J. Bertin, D. J. Philpott, and M. C. Plotkowski. 2005. Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J. Biol. Chem. 28036714-36718. [DOI] [PubMed] [Google Scholar]
- 86.Tsai, W. C., R. M. Strieter, B. Mehrad, M. W. Newstead, X. Zeng, and T. J. Standiford. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 684289-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang, Q., C. M. Doerschuk, and J. P. Mizgerd. 2004. Neutrophils in innate immunity. Semin. Respir. Crit. Care Med. 2533-41. [DOI] [PubMed] [Google Scholar]
- 88.Wang, X., C. Moser, J. P. Louboutin, E. S. Lysenko, D. J. Weiner, J. N. Weiser, and J. M. Wilson. 2002. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenza infection in mouse lung. J. Immunol. 168810-815. [DOI] [PubMed] [Google Scholar]
- 89.Weiss, D. S., B. Raupach, K. Takeda, S. Akira, and A. Zychlinsky. 2004. Toll-like receptors are temporally involved in host defense. J. Immunol. 1724463-4469. [DOI] [PubMed] [Google Scholar]
- 90.Widney, D. P., Y. Hu, A. K. Foreman-Wykert, K. C. Bui, T. T. Nguyen, B. Lu, C. Gerard, J. F. Miller, and J. B. Smith. 2005. CXCR3 and its ligands participate in the host response to Bordetella bronchiseptica infection of the mouse respiratory tract but are not required for clearance of bacteria from the lung. Infect. Immun. 73485-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wieland, C. W., S. Florquin, N. A. Maris, K. Hoebe, B. Beutler, K. Takeda, S. Akira, and T. van der Poll. 2005. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable Haemophilus influenzae from the mouse lung. J. Immunol. 1756042-6049. [DOI] [PubMed] [Google Scholar]
- 92.Zhang, P., W. R. Summer, G. J. Bagby, and S. Nelson. 2000. Innate immunity and pulmonary host defense. Immunol. Rev. 17339-51. [DOI] [PubMed] [Google Scholar]