Abstract
Enteropathogenic Escherichia coli, enterohemorrhagic E. coli, and Citrobacter rodentium are classified as attaching and effacing pathogens based on their ability to adhere to the intestinal epithelium via actin-filled membranous protrusions (pedestals). Infection of mice with C. rodentium causes a breach of the intestinal epithelial barrier, leading to colitis via a vigorous inflammatory response resulting in diarrhea and a protective antibody response that clears the pathogen. Here we show that interleukin-1 receptor (IL-1R) signaling protects mice following infection with C. rodentium. Upon infection, mice lacking the type I IL-1R exhibit increased mortality together with severe colitis characterized by intramural colonic bleeding and intestinal damage including gangrenous mucosal necrosis, phenotypes also evident in MyD88-deficient mice. However, unlike MyD88−/− mice, IL-1R−/− mice do not exhibit increased pathogen loads in the colon, delays in the recruitment of innate immune cells such as neutrophils, or defects in the capacity to replace damaged enterocytes. Further, we demonstrate that IL-1R−/− mice have an increased predisposition to intestinal damage caused by C. rodentium but not to that caused by chemical irritants, such as dextran sodium sulfate. Together, these data suggest that IL-1R signaling regulates the susceptibility of the intestinal epithelia to damage caused by C. rodentium.
Pathogenic strains of Escherichia coli, including enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli, pose a significant public health risk, especially in developing countries, where these strains contaminate food and water supplies. EPEC causes infantile diarrhea (10), which leads to dehydration, contributing to as many as 1 million infant deaths per year (26). EPEC, enterohemorrhagic E. coli, and the murine pathogen Citrobacter rodentium are classified as attaching and effacing (A/E) pathogens based on the ability of these extracellular bacteria to intimately attach to the intestinal epithelium and flatten absorptive microvilli (effacement). Another hallmark feature of A/E pathogens is their ability to induce actin rearrangements that form membranous protrusions, called “pedestals,” beneath the attached bacteria. Pedestal formation is associated with the development of A/E lesions, breach of the epithelial barrier, and disease (25, 28).
Upon infection, A/E pathogens displace the commensal flora and cause an intestinal pathology that includes damage characterized by cellular necrosis, disruption of the epithelium, and occasionally bleeding (21, 22). Damage induces a localized repair response characterized by hyperplasia (22), which reflects an increased division of stem cells at the base of crypts to replace damaged enterocytes (34). As hyperplasia develops, goblet cells become less evident because they are not replenished as readily as enterocytes. Thus, the apparent loss of goblet cells may be further evidence of repair. Infection with A/E pathogens also induces the recruitment of immune cells and causes edema within the lamina propria. Indeed, in addition to providing protection, immune cell types such as neutrophils may contribute to colitis and epithelial damage, including the formation of crypt abscesses (17). Nevertheless, an effective innate immune response allows the proper recruitment and activation of immune cell types necessary for a robust antibody response both to promote the clearance of A/E pathogens (23) and to reduce the severity of pathology upon reinfection (8).
The detection of pathogens by the innate immune system is accomplished by highly conserved families of receptors, such as Toll-like receptors (TLRs), and their respective downstream signaling cascades. Several lines of evidence suggest that signaling initiated by particular TLRs is important for protective responses to C. rodentium, whereas signaling by other TLRs appears unnecessary and perhaps even deleterious. TLRs recognize and respond to conserved structural motifs associated with microbes, which include proteins (e.g., flagellin), nucleic acids (e.g., unmethylated CpG DNA), and lipids (e.g., lipid A of lipopolysaccharide [LPS]) (18). LPS is abundant on the surface of C. rodentium and is a known ligand for the TLR4 receptor complex. When LPS binds to its receptor, NF-κB becomes derepressed, and as a consequence, proinflammatory cytokines are expressed (9). Notably, infection of TLR4−/− mice with C. rodentium results in a slower, less severe inflammatory response and reduced mortality (19), suggesting that TLR4 signaling exacerbates disease. In contrast, TLR2 signaling appears to be required for protective responses to C. rodentium. Thus, following infection TLR2−/− mice suffer from colonic mucosal ulcerations, bleeding, increased apoptosis, and increased mortality (14). Together, these studies suggest that the activation of TLRs by C. rodentium can cause both protective and deleterious responses.
A variety of innate immune receptors, including TLRs, the type I interleukin-1 receptor (IL-1R), and the IL-18 receptor, utilize the signaling adaptor myeloid differentiation factor 88 (MyD88) to activate NF-κB and produce an array of cytokines and chemokines (1, 18). Following detection of C. rodentium, MyD88 signaling in epithelial and hematopoietic cells provides protection from disseminating infection and mortality. MyD88 signaling facilitates epithelial repair responses (33), initiates recruitment of innate immune cells, limits bacterial load, and controls the amount of intestinal damage (21). We proposed that MyD88 signaling provides protection from C. rodentium by inducing the timely recruitment of neutrophils and thus stemming the growth of bacteria in the colon, which could exacerbate epithelial damage. Thus, by regulating both epithelial damage and repair, MyD88 signaling appears to prevent bacteria from escaping the colon and reduce dissemination to peripheral organs (21). However, because MyD88 signaling contributes to several aspects of the innate immune response, it is difficult to discern whether particular phenotypes result from direct or indirect consequences of TLR signaling or from other receptors that utilize MyD88. Such designations are important to understand how TLRs or other receptors mediate a balanced response to a pathogen that is sufficient for containment and clearance but limits damage due to inflammation.
Here we consider whether a loss of signaling through receptors besides TLRs that also signal via MyD88 might contribute to some of the phenotypes evident in MyD88−/− mice after C. rodentium infection. Using mice deficient in these signaling pathways, we reasoned that it might be possible to distinguish MyD88-dependent phenotypes directly controlled by TLRs or other receptors from secondary consequences of MyD88-dependent signaling. We have focused in particular on IL-1 and IL-18 and their cognate receptors. IL-18 is expressed by macrophages and was first identified as a factor that induces gamma interferon (IFN-γ) (30, 31). IL-1β and the type I IL-1R (CD121a) have been implicated in protection and control against infections caused by Staphylococcus aureus (27). Further, IL-1β and IL-18 have both been shown to be protective against Salmonella enterica serovar Typhimurium (36) and Shigella flexneri (37) infections.
The type I IL-1R associates with an accessory protein and is activated by IL-1α or IL-1β (7), molecules structurally related to IL-18. The type II IL-1R is a decoy receptor, which does not lead to any cellular responses following ligand binding (7). Although IL-1α is constitutively expressed by epithelial cells, the expression of IL-1β or IL-18 precursors is induced by NF-κB in innate immune or epithelial cells, respectively (3, 15). Whereas IL-1α is produced in an active form, IL-1β and IL-18 are expressed as proforms, which, upon proteolytic cleavage, are secreted and bind to their cognate receptors. Ligand-dependent activation of IL-1R and the IL-18 receptor in turn activates NF-κB in a MyD88-dependent fashion. This positive-feedback circuit, in which IL-1β and IL-18 are both produced and signal in a MyD88-dependent fashion, has been hypothesized to amplify the NF-κB response, leading to a localized inflammatory responses (3). Although IL-1β expression is not induced by C. rodentium by 6 days postinfection (p.i.) (13), mRNA levels do increase by 2 weeks p.i. (24), raising the possibility that IL-1R signaling, perhaps activated by IL-1α or the late expression of IL-1β, provides protection.
Here we demonstrate that IL-1R signaling provides protection from increased mortality and pathology upon infection with C. rodentium, whereas IL-18 signaling does not appear to participate. Notably, mice lacking the type I IL-1R provide a means to separate the complex and interrelated phenotypes associated with inflammation. Thus, the increased pathology evident in the absence of IL-1R signaling appears to result from an increased susceptibility to tissue damage by C. rodentium and not from dysregulated immune or repair responses.
MATERIALS AND METHODS
Mouse strains and breeding.
IL-1R−/−, IL-18−/−, and wild-type control C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were kept in sterile housing, and care was provided in accordance with protocols approved by the Institutional Animal Care and Use Committee of Emory University.
In vivo infections.
C. rodentium was prepared by overnight culturing (12 to 16 h) at 37°C in Luria-Bertani broth (LB; Becton Dickinson, Franklin Lakes, NJ), without shaking. Cultures were harvested by centrifugation and resuspended in 20% sucrose distilled water. For infections of mice, drinking water was replaced with C. rodentium suspension overnight. The volume of the suspension was measured before and after administration, and the number of bacteria in the inoculum was calculated following retrospective plating. The average dosage was 1.5 × 108 CFU/mouse. Survival of infected mice and changes in body weight were monitored daily. Mice losing more than 15% of their original weight were euthanized. Clearance of C. rodentium was assessed by measuring the number of colonies in fecal samples.
Histology and pathology scoring.
For histology studies, colons were removed from uninfected or infected mice, fixed in 10% formalin, and embedded in paraffin. Sections (5 μm) were cut and stained with hematoxylin and eosin (H&E) by the Histology Core Laboratory in the Department of Pathology at Emory University. The degree of intestinal pathology was assessed under the microscope by one of us (M.A.S.). The samples were coded and the observations made in a blinded fashion to avoid bias in the evaluation process. Samples were scored for degree (from 0 to 3) of gangrenous mucosal necrosis (GMN), bleeding, raggedness of epithelium, hyperplasia, loss of goblet cells, edema, and neutrophil infiltration, where a score of 0 represents no signs of pathology, a score of 1 is a mild-pathology measurement, a score of 2 is a moderate-pathology measurement, and a score of 3 is a severe-pathology measurement. Crypt heights were measured by micrometry using a Zeiss 200M microscope, a 20× numerical aperture (NA) 1.4 lens, and Slidebook software (Intelligent Imaging Innovations, Denver, CO). Well-oriented crypts were used for measurements. Two measurements were made on each image, and one image was taken per colon. The number of samples refers to the number of colons measured rather than the number of measurements.
In vitro bacterial growth assays and Western blot analyses.
To determine whether blood affects C. rodentium growth, we employed methods similar to those of Dann et al. (6). Briefly, blood was obtained from wild-type mice via cardiac puncture, and serum was separated from cells by centrifugation at 10,000 rpm for 10 min. After removal of serum, equal volumes of red blood cell (RBC) lysis buffer (Sigma) were applied to the pelleted blood cells for 10 min, and the samples were again centrifuged. This RBC lysate was then removed and used for growth assays. For growth assays, overnight cultures of C. rodentium were diluted 1:1,000 into LB alone, LB plus mixed amino acids (1 mg/ml; Teknova, Hollister, CA), LB plus serum (3.5 mg/ml), or LB plus RBC lysate (3.5 mg/ml) and grown for 2, 4, 6, or 8 h standing at 37°C. At each time point, the optical density at 600 nm (OD600) was measured and the number of bacteria per milliliter was determined by serial dilution and plating on MacConkey agar. For each medium condition, a medium sample lacking the bacterial inoculum was used as the blank. An assessment of virulence factor levels by Western analysis with anti-Tir polyclonal antibody was carried out essentially as described previously (40), with the following modification. Tir was enriched by first boiling supernatants for 10 minutes at 94°C and then precipitating with 10% trichloroacetic acid.
In vivo permeability assays.
In vivo permeability assays to assess intestinal barrier function were performed using fluorescein isothiocyanate (FITC)-labeled dextran, as described previously (12). Briefly, food was withdrawn for 4 h from 8- to 10-week-old wild-type or IL-1R−/− mice, which were left uninfected or had been infected for 7 days. Mice were then gavaged with a permeability tracer (60 mg/100 g body weight of FITC-labeled dextran, molecular weight 4,000; Sigma-Aldrich). Serum was collected retroorbitally, blood cells were removed by centrifugation, and the fluorescence intensity of each sample was measured with a fluorimeter (excitation, 492 nm; emission, 525 nm; FLUOstar Galaxy 2300; BMG Labtech, Durham, NC). FITC-dextran concentrations were determined from standard curves generated by serial dilution of FITC-dextran. Permeability was calculated by linear regression of sample fluorescence (Excel 5.0; Microsoft).
ELISA.
For cytokine detection, sandwich enzyme-linked immunosorbent assay (ELISA) kits were used according to the specifications of the manufacturer to measure levels of IL-6 (Becton Dickinson), keratinocyte chemokine (Biosource, Camarillo, CA), IFN-γ (eBioscience, San Diego, CA), IL-4 (eBioscience), and IL-10 (Biosource) in colon supernatant, which was derived by culturing colons in Dulbecco's modified Eagle's medium with antibiotics for 48 h before the supernatant was removed.
TUNEL assay staining and scoring.
To assess the level of apoptosis in colonic tissue, sections were cut from the same paraffin blocks described above and stained using an ApopTag fluorescein in situ apoptosis detection kit (S7110; Millipore, Billerica, MA) according to the manufacturer's instructions. Briefly, the tissue was digested with TdT enzyme and stained with digoxigenin conjugated to fluorescein and 4′,6-diamindino-2-phenylindole. The intensity of fluorescein staining was assessed from digital images using a Nikon Eclipse 80i microscope, a 205 NA 0.75 objective, and Spot software (Diagnostic Instruments, Sterling Heights, MI). Longitudinal sections, which displayed the entire lengths of crypts, were used for measurements. Samples were coded and observations made in a blinded fashion to avoid bias in the evaluation process. Samples were given a score of 0, 1, 2, or 3, where a score of 0 represents minimal terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining, a score of 1 is a few apoptotic cells per field view, a score of 2 is one apoptotic cell per crypt, and a score of 3 is multiple apoptotic cells per crypt. The numbers of apoptotic cells were counted in 10 crypts, and the scoring scheme used was identical to that described by Gibson et al. (14).
Colony counts of C. rodentium.
Tissue samples of colon, liver, spleen, or mesenteric lymph node weighing ∼0.1 to 0.3 g were homogenized at low speed with a Tissuemizer (Fisher Scientific, Pittsburgh, PA) in 1 ml of phosphate-buffered saline. The lysates were plated on MacConkey agar plates at various dilutions, and C. rodentium colonies were recognized as pink with a white rim after incubation for 20 h at 37°C, as previously described (41). Random colonies were confirmed as C. rodentium by PCR with Tir-specific primers (41).
Manual neutrophil counts.
Crypts were observed in the slides stained with H&E as described above by use of a 63× NA 1.4 lens on a Zeiss 200M microscope. Neutrophils were identified by their distinctive nuclear morphology and counted in 10 crypts per colon. The mean number of neutrophils per crypt was calculated for each colon observed. The number of samples refers to the number of colons measured.
Neutrophil killing assays.
C. rodentium bacteria were cultured overnight in LB and serially diluted in phosphate-buffered saline. To isolate neutrophils, bone marrow was removed from C57BL/6 or IL-1R−/− mice, and RBCs were lysed. As previously noted, 22 to 30% of cells were positive for GR-1 by use of this method (21). Bacteria (5 × 105) were added to 2.5 × 105 cells in 350 ml of RPMI 1640 medium without antibiotics. After 1, 2, or 3 h, the bacterium-cell culture was diluted 1:10 in water for 10 min to lyse neutrophils and serial dilutions were plated on MacConkey agar to determine the number of bacterial colonies remaining. Assays were conducted in triplicate.
DSS administration.
For dextran sodium sulfate (DSS) treatment, drinking water was replaced with 2% DSS dissolved in sterile distilled water for 7 days followed by 7 days of regular drinking water. The volume of the suspension was measured before and after administration to ensure that mice in different cages drank the same amounts of water. Survival of treatment mice and changes in body weight were monitored daily.
Statistical analysis.
For mortality curves, a two-sided Fisher exact test determined the statistical significance of the data. For all other experiments, the level of statistical significance was determined by a Mann-Whitney rank sum test. Results were considered significant if the P value was <0.05.
RESULTS
IL-18 is not required to protect against infection with C. rodentium.
IL-18 acts via MyD88 to induce IFN-γ, which has previously been shown to participate in the immune response to C. rodentium (39). To determine whether IL-18 itself plays a role in the immune response to C. rodentium, we infected mice deficient in IL-18 (IL-18−/− mice). IL-18−/− mice survived infection with C. rodentium to nearly the same extent as wild-type mice (Fig. 1A). Pathology scores for IL-18−/− mice were only marginally higher than those for wild-type mice and were not statistically significant (P > 0.05) (Fig. 1B). The main determinant of the change was a slight increase in neutrophil recruitment (Fig. 1C). Notably, IL-18−/− mice also suffered from intermittent bleeding in the cecum by 7 days p.i. (data not shown). In all other respects, including bacterial load, the profiles of IL-18−/− and wild-type mice were nearly identical (Fig. 1D). Notably, increased immune cell infiltration, in particular that of neutrophils, alone does not exacerbate damage or facilitate repair responses. Together, these data indicate that IL-18 plays a limited role in the response to C. rodentium infection.
FIG. 1.
IL-18 is not necessary for survival, although it is associated with slightly reduced colonic pathology. (A) Survival curves of 6- to 10-week-old IL-18−/− mice (n = 6) and their C57BL/6 controls (n = 12) infected with 1.5 × 108 CFU of C. rodentium. (B) Pathology scores were assessed in colonic tissue from uninfected C57BL/6 or IL-18−/− mice or from infected C57BL/6 or IL-18−/− mice 3 or 7 days p.i. The numbers of mice for each group ranged from 8 to 12 for C57BL/6 mice and 5 to 7 for IL-18−/− mice. Uninf, uninfected. (C) Pathology scores for day 7 p.i. are shown in each category assessed, including bleeding, ragged epithelium (Epi.), GMN, hyperplasia, goblet cell loss, edema, and polymorphonuclear leukocyte infiltration (PMN Infil.), for C57BL/6 (n = 12) and IL-18−/− (n = 7) mice. (D) Various tissues were harvested aseptically from C57BL/6 (n = 12) and IL-18−/− (n = 7) mice 7 days p.i. C. rodentium CFU were quantitated as described in Materials and Methods. MLN, mesenteric lymph nodes. Error bars represent the standard deviations.
IL-1R signaling protects mice following infection with C. rodentium.
To determine whether IL-1R signaling participates in the immune response to C. rodentium, we assessed morbidity and mortality in mice deficient in the type I IL-1R (IL-1R−/− mice). Following infection, 78.8% of IL-1R−/− mice died between 1 and 2 weeks p.i. (Fig. 2A). In contrast, the control strain, C57BL/6 mice, all survived (Fig. 2A). Interestingly, the IL-1R−/− mice that survived the infection cleared the pathogen by 4 weeks p.i., a rate similar to that observed for wild-type mice (data not shown). Therefore, IL-1R signaling is required to protect mice from mortality.
FIG. 2.
IL-1R signaling is necessary for survival and prevention of severe pathology following infection with C. rodentium. (A) Survival curves of 6- to 10-week-old IL-1R−/− mice (n = 9) and their C57BL/6 controls (n = 12) infected with 1.5 × 108 CFU of C. rodentium. (B) Colons from IL-1R−/− and C57BL/6 mice 7 days p.i. (C) Pathology scores for colonic tissues from uninfected (Uninf) C57BL/6 or IL-1R−/− mice or from infected C57BL/6 or IL-1R−/− mice were assessed 3 or 7 days p.i. The numbers of mice ranged from 8 to 12 for C57BL/6 mice and from 8 to 13 IL-1R−/− mice for each bar (*, statistical significance in comparison to uninfected C57BL/6 samples, P < 0.01; **, statistical significance in comparison to uninfected IL-1R−/− samples, P < 0.001; #, statistical significance between C57BL/6 and IL-1R−/− samples, P < 0.001). Error bars represent the standard deviations. (D and E) Colon supernatants from uninfected C57BL/6 mice or C57BL/6 mice 3 or 7 days p.i. were assessed for IL-6 (n = 9 to 12) and IFN-γ (n = 12 or 13) levels by ELISA. The difference between levels of cytokine production in uninfected mice and mice 3 days p.i. was significant at P < 0.02 for IL-6 and P < 0.01 for IFN-γ. Colon supernatants from uninfected IL-1R−/− mice, IL-1R−/− mice 3 days p.i., and IL-1R−/− mice 7 days p.i. were assessed also for IL-6 (n = 7 to 15) and IFN-γ (n = 7 to 13) levels by ELISA. No statistically significant difference was evident. Horizontal bars indicate sample means.
IL-1R−/− mice suffer from increased colonic pathology.
The increased mortality seen with IL-1R−/− mice is associated with increased macroscopic and microscopic intestinal pathologies. Colons of most IL-1R−/− mice infected with C. rodentium exhibited severe intramural colonic bleeding by 7 days p.i. (Fig. 2B), reminiscent of that seen in colons of infected MyD88−/− mice (21). This bleeding was not evident in colons from uninfected wild-type or IL-1R−/− animals (data not shown) or in colons from infected wild-type animals (Fig. 2B). Further, at 7 days p.i., the overall pathology score of colonic tissue from IL-1R−/− mice was significantly higher that that seen with wild-type colonic tissue (Fig. 2C). However, prior to that time point little pathology was evident in the colonic tissue from either wild-type or IL-1R−/− mice (Fig. 2C). Together, these data suggest that the increased pathology following C. rodentium infection of IL-1R−/− mice may contribute to increased mortality.
IL-1R signaling contributes to cytokine induction.
Following infection with C. rodentium, mice deficient in IL-6 are more likely to suffer from mucosal ulceration (6) and mice lacking IFN-γ suffer higher morbidity (39). Moreover, IL-6 is important for protection from intestinal injury induced by chemical irritants, such as DSS (35). To determine whether the increased pathology evident in IL-1R−/− mice correlated with altered cytokine production, we next assessed levels of the TH1 cytokines IL-6 and IFN-γ in colons of infected animals. Notably, by 3 days p.i., the levels of IL-6 and IFN-γ in colon supernatants from wild-type animals increased by ∼2.5-fold and 3.2-fold, respectively, upon infection (Fig. 2D and E). In contrast, no such increases were detectable in colonic tissue from IL-1R−/− mice (Fig. 2D and E), indicating that IL-1R signaling was required to induce IL-6 and IFN-γ in response to C. rodentium. TH1 cytokines, such as IFN-γ and IL-6, suppress TH2 cytokines. However, despite the lack of induction of IFN-γ, the TH2 cytokines IL-4 and IL-10 showed no induction in IL-1R−/− mice (data not shown). Notably, this pattern of IL-6 induction is similar to that seen in MyD88−/− mice in response to C. rodentium (21) and to the chemical irritant DSS (35). Together, these data indicate that IL-1R signaling regulates the production of IL-6 and IFN-γ.
These data raised the possibility that the limited induction of IL-6 or IFN-γ in the absence of IL-1R signaling might increase the susceptibility of the epithelium to damage by C. rodentium. To test this possibility, we next assessed the contribution to the overall pathology of specific determinants. The overall pathology score is comprised of (category 1) measurements of damage, which includes bleeding, epithelial injury or “raggedness” (including loss of epithelial cells), and GMN; (category 2) measurements of repair response, which includes the presence of hyperplasia and decreased evidence of goblet cells; and (category 3) measurements of immune cell recruitment, including neutrophil infiltration and edema. Notably, the infiltration of neutrophils into the crypts and the accumulation of fluid and immune cells in the lamina propria (edema) comprise an inflammatory response or colitis. However, here we seek to define possible causes of damage as inflammatory or bacterial. As such, we distinguish measures of damage (pathology category 1) from possible causes, which include changes in repair response (pathology category 2), immune cell infiltration (pathology category 3), or bacterial load. We next set out to more rigorously characterize elements within each of these categories so as to determine which contributed most to the overall pathology.
IL-1R−/− mice have increased levels of colonic damage and barrier permeability.
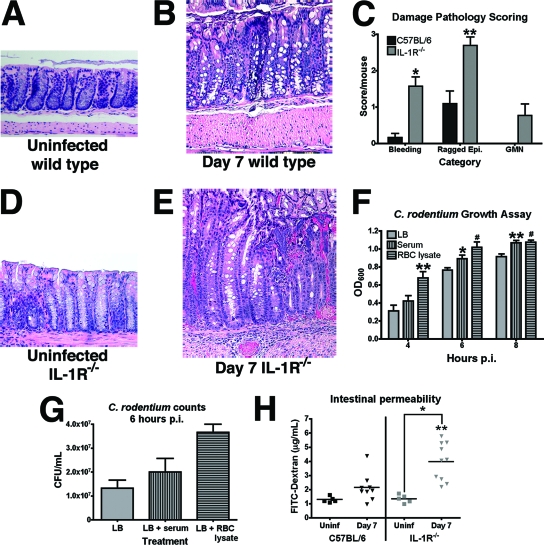
To characterize the histological pathology associated with C. rodentium infection in IL-1R−/− mice, longitudinal sections of colonic tissue were stained with H&E and observed under a microscope for evidence of intestinal damage. An inspection revealed that both IL-1R−/− and wild-type mice suffer from microscopic bleeding between crypts and epithelial injury by 7 days p.i. (compare Fig. 3A, B, C, and E). However, the incidence of bleeding and the extent of injury to the epithelium (raggedness) were significantly higher in IL-1R−/− mice than in their wild-type counterparts (P < 0.001 and P < 0.01, respectively) (Fig. 3C). In addition, colonic tissue from IL-1R−/− mice also displayed pathology consistent with GMN, a pathological feature characterized by foci of mucosal necrosis associated with large colonies of bacteria (Fig. 3E). GMN was not evident in colonic tissue from uninfected mice of either strain (Fig. 3A and D) or in colons of wild-type mice infected with C. rodentium (Fig. 3B).
FIG. 3.
IL-1R signaling is required to prevent increased colonic damage and specifically GMN following infection with C. rodentium. (A) H&E staining of colonic tissue from an uninfected C57BL/6 mouse. (B) H&E staining of colonic tissue from a C57BL/6 mouse 7 days p.i. (C) Pathology scores of the damage measurements, including bleeding, ragged epithelium (Epi.), and GMN, were assessed in colonic tissue from C57BL/6 (n = 12) or IL-1R−/− (n = 13) mice 7 days p.i. (*, statistical significance in comparison to C57BL/6 samples, P < 0.001; **, statistical significance in comparison to C57BL/6 samples, P < 0.01). (D) H&E staining of uninfected colonic tissue from an IL-1R−/− mouse. (E) H&E staining of IL-1R−/− colonic tissue 7 days p.i. Magnification, ×400 for all H&E images. (F) C. rodentium was grown as standing cultures for up to 8 h in LB without supplement or in LB supplemented with 3.5 mg/ml serum or with 3.5 mg/ml RBC lysate, and OD600 was measured. OD600 differences were evident in cultures supplemented with RBC lysate by 4 h p.i. and with serum by 6 h p.i. Each sample (n = 6) was blanked to an uninfected sample of the media (*, statistical significance in comparison to LB samples, P < 0.05; **, statistical significance in comparison to LB samples, P < 0.02; #, statistical significance in comparison to LB samples, P < 0.01). (G) C. rodentium was incubated in LB without supplement or supplemented with 3.5 mg/ml serum or with 3.5 mg/ml RBC lysate for 6 h, and the number of bacteria in cultures was determined as described in Materials and Methods. Data presented are from a representative experiment. The experiment was repeated twice with three replicates each. Error bars represent the standard deviations. (H) Intestinal permeability was measured by the ability of FITC-dextran to migrate from the intestine to serum in C57BL/6 or IL-1R−/− mice that were left uninfected (Uninf) or infected with C. rodentium. Serum was harvested 7 days p.i. Infected IL-1R−/− mice displayed a significant increase in intestinal permeability compared to that for uninfected controls, and no changes in permeability were evident in infected or uninfected C57BL/6 mice (*, statistical significance in comparison to uninfected levels, P < 0.01; **, statistical significance in comparison to wild-type levels at 7 days p.i., P < 0.01).
Previous reports indicate that the growth rate of C. rodentium increases upon extended exposure to blood components (6). These data raised the possibility that the increased bleeding evident in IL-1R−/− animals (Fig. 3C) might cause local increases in bacterial growth associated with GMN. To test this possibility, we measured the growth rates of C. rodentium in vitro upon exposure to serum or RBC lysate. Upon addition of either serum or RBC lysate, C. rodentium growth was enhanced, as measured by OD600 (Fig. 3F) and quantification of bacterial numbers (Fig. 3G). Specifically, addition of RBC lysate increased the OD600 by 2.1-fold within 4 h (Fig. 3F), in agreement with previous reports showing increases of similar magnitude after 24 h (6). Notably, we could not detect a concomitant increase in secretion of the virulence factor Tir in cultures grown under these conditions (data not shown). Together, these data suggest that the increased bleeding in the colonic tissue of IL-1R−/− mice facilitates localized increases in bacterial density and thus the development of GMN.
To determine whether the increased damage resulted from the decreased integrity of the epithelial barrier, we measured the permeability of the gastrointestinal tract to FITC-dextran in wild-type and IL-1R−/− mice. As shown in Fig. 3H, significantly more FITC-dextran was evident in serum samples from IL-1R−/− mice 7 days p.i. than in samples from uninfected IL-1R−/− mice or infected wild-type mice (P < 0.01). Moreover, in wild-type mice no difference in serum FITC-dextran levels between infected and uninfected mice was evident, suggesting that the intestinal inflammation caused by C. rodentium infection of wild-type mice does not significantly alter intestinal barrier permeability. However, because orally administered FITC-dextran may be absorbed in regions of the gastrointestinal tract not affected by C. rodentium, we cannot rule out the possibility that background levels may be too high to detect subtle differences between uninfected and infected wild-type animals. Together, these data suggest that the increased morbidity and mortality evident in IL-1R−/− mice may at least in part be due to increased epithelial damage and permeability.
Severe colonic pathology in IL-1R−/− mice is not caused by increased colonic bacterial load or by delayed neutrophil infiltration.
To determine whether the increased damage in IL-1R−/− colonic tissue was caused by an increased bacterial load, C. rodentium colonization was measured in several organs. Despite visually evident local increases in bacterial density in GMN (Fig. 3E), no differences in overall colonization were evident between the colons taken from wild-type and IL-1R−/− mice 7 days p.i. (Fig. 4A). In contrast, levels of bacteria were 2 to 3 logs higher in colons of MyD88−/− mice at this time (21). However, we did find significant increases (0.5 to 1 log) in colonization of the liver and the spleen 7 days p.i. (Fig. 4A). Together, these data suggest that increases in bacterial load in the colon cannot account for the increased colonic damage seen with IL-1R−/− mice.
FIG. 4.
IL-1R−/− mice have C. rodentium loads and neutrophil responses equivalent to those seen in C57BL/6 mice. (A) Various tissues were harvested aseptically from C57BL/6 (n = 12) and IL-1R−/− (n = 9) mice 7 days p.i. C. rodentium CFU were quantitated as described in Materials and Methods. MLN, mesenteric lymph nodes. (B) Pathology scores for recruitment measurements, including edema and polymorphonuclear leukocyte infiltration (PMN Infil.), were assessed in colonic tissue from C57BL/6 (n = 12) or IL-1R−/− (n = 13) mice 7 days p.i. (*, statistical significance in comparison to C57BL/6 samples, P < 0.01). (C) Manual neutrophil counts in crypts from C57BL/6 (n = 5 to 15) and IL-1R−/− (n = 7 to 15) colons (*, statistical significance in comparison to uninfected [uninf] C57BL/6 samples, P < 0.01; **, statistical significance in comparison to uninfected IL-1R−/− samples, P < 0.02; #, statistical significance in comparison to uninfected samples, P < 0.001). (D) CFU (5 × 105) of C. rodentium incubated with neutrophils (2.5 × 105) from C57BL/6 or IL-1R−/− mice were killed with equal efficacies. Results are from a representative experiment repeated three times in triplicate. Error bars represent the standard deviations.
Interestingly, the increased load of C. rodentium in only livers and spleens has previously been associated with a lack of neutrophils following depletion by treatment with a neutralizing anti-GR-1 antibody in wild-type mice (21). When colonic tissue from IL-1R−/− mice or wild-type mice was scored for neutrophil infiltration and edema, an increase in both parameters was evident in colons from IL-1R−/− mice (P < 0.01 for both parameters) (Fig. 4B). Because pathology scoring of neutrophil infiltration is a rather imprecise measure and is subject to inaccuracy, we next quantitated the recruitment of neutrophils by performing manual counts of neutrophils per crypt (Fig. 4C). These data suggest that there was no significant difference in neutrophil recruitment to the colon between IL-1R−/− and wild-type animals at any point during the infection (Fig. 4C). Further, we could find no difference in the capacities of neutrophils taken from IL-1R−/− and wild-type animals to kill C. rodentium in vitro (Fig. 4D), suggesting that the neutrophils from IL-1R−/− mice have no intrinsic defects. Together, these data suggest that IL-1R signaling regulates the susceptibility of the epithelia to damage upon infection with C. rodentium, although damage cannot be attributed to the increased bacterial load in the colon or to the effects of neutrophil recruitment. However, the extensive damage to the colonic epithelia of IL-1R−/− mice likely contributes to the dissemination of bacteria to the spleen and liver.
Increased colonic damage in IL-1R−/− mice is not due to changes in enterocyte apoptosis or replacement.
To determine whether the increased damage in colonic tissue from IL-1R−/− mice was caused by an increase in apoptosis of epithelial cells, TUNEL assays were performed. TUNEL staining revealed no difference in the staining of apoptotic cells in the colons of IL-1R−/− mice compared to that in the colons of wild-type mice at any point during the infection (data not shown). Together, these data suggest that, as for MyD88−/− mice (21), the increased damage in the colonic tissue of infected IL-1R−/− mice results from necrosis rather than apoptosis.
To measure the capacity of IL-1R−/− mice to repair their increased colonic damage, the degrees of hyperplasia and goblet cell loss were measured. Hyperplasia occurs when stem cells present at the base of intestinal crypts differentiate into enterocytes, divide, and migrate along the crypts to the site of damage (34). Such hyperplasia, measured as an increase in crypt length, is also a hallmark feature of A/E pathogen infections (4, 22) and is indicative of restoration of the damaged intestinal epithelium. In this process, stem cells are no longer available to differentiate into other cell types, such as goblet cells; thus, reduced evidence of goblet cells is an indirect measure of hyperplasia. When colonic tissue was scored for the presence of hyperplasia, we could detect no differences between the two strains of mice (Fig. 5A). Likewise, there was no significant difference in loss of colonic goblet cells between wild-type and IL-1R−/− mice (data not shown). To directly quantify the extent of hyperplasia, crypt lengths were measured in colonic tissues from both strains of mice. We observed that 7 days p.i., crypts of both wild-type and IL-1R−/− mice were nearly twice as long as those found in uninfected animals (Fig. 5B). Moreover, we found no evidence for a proportional increase of crypt lengths in colonic tissue from IL-1R−/− despite the increased level of damage. Together, these data suggest that IL-1R signaling is not required either for sensing C. rodentium or for the development of hyperplasia initiated upon damage to the epithelia.
FIG. 5.
IL-1R−/− and C57BL/6 mice have equal levels of hyperplasia following infection with C. rodentium. (A) The pathology scores were assessed for the degrees of hyperplasia in colonic tissue taken from IL-1R−/− (n = 13) and C57BL/6 (n = 12) mice. (B) Crypt lengths of colonic tissue from uninfected (uninf) C57BL/6 or IL-1R−/− mice or from C57BL/6 or IL-1R−/− mice 3 or 7 days p.i. were measured (*, statistical significance in comparison to uninfected samples, P < 0.001). Error bars represent the standard deviations.
DSS increases colitis in IL-1R−/− mice.
Our data suggest that, in the absence of IL-1R signaling, mice are more susceptible to damage caused by C. rodentium infection but without a concomitant increase in bacterial load, reduction in enterocyte replacement capacity, or reduced neutrophil recruitment. We hypothesized that hyperplasia and recruitment of immune cells would also be evident in colonic tissue from IL-1R−/− mice in response to chemical injury. Intestinal damage induced by DSS has been reported to induce a repair mechanism that replenishes epithelial cells and restores the integrity of the intestinal barrier in a TLR4- and MyD88-dependent manner (11, 35). Following oral administration of DSS, IL-1R−/− mice suffered from significantly increased levels of pathology after 14 days (Fig. 6A), though, as with wild-type mice, the pathology was not as severe as that induced upon infection with C. rodentium (Fig. 2C). Pathology scores indicative of hyperplasia and infiltration in colonic tissue from IL-1R−/− mice were equal to or higher than those for wild-type animals (Fig. 6B). Accordingly, following DSS treatment, crypt lengths in both strains significantly increased, indicating that aspects of a repair process were unperturbed in these two strains (Fig. 6C). However, by 14 days posttreatment, the crypt lengths seen in IL-1R−/− colonic tissue were significantly longer than those seen in tissue from wild-type mice (P < 0.001) (Fig. 6C), accounting for much of the increased overall pathology score (Fig. 6B). Interestingly, C. rodentium, but not DSS treatment, induced elevated epithelial injury and bleeding in IL-1R−/− mice (Fig. 6B), indicating that the increased susceptibility to damage in IL-1R−/− mice is a specific rather than a generalized phenotype. Together, these data suggest that IL-1R does not mediate the development of hyperplasia or infiltration of immune cells in response to damage induced by either pathogens or chemicals.
FIG. 6.
IL-1R−/− mice suffer from increased pathology, particularly hyperplasia, following DSS administration. (A) Pathology scores were assessed in colonic tissue from untreated C57BL/6 or IL-1R−/− mice or from treated C57BL/6 or IL-1R−/− mice 7 or 14 days posttreatment. The numbers ranged from 6 to 10 C57BL/6 mice and 9 to 12 IL-1R−/− mice for each bar (*, statistical significance in comparison to samples from C57BL/6 mice 14 days posttreatment, P < 0.01). (B) Pathology scores for each category were assessed in colonic tissue from treated C57BL/6 or IL-1R−/− mice 14 days posttreatment. Epi., epithelium; PMN Infil., polymorphonuclear leukocyte infiltration. (C) Crypt lengths of colonic tissue from untreated C57BL/6 or IL-1R−/− mice or from C57BL/6 or IL-1R−/− mice 7 or 14 days posttreatment were measured (*, statistical significance in comparison to uninfected samples, P < 0.01; **, statistical significance in comparison to uninfected samples, P < 0.001; #, statistical significance between C57BL/6 and IL-1R−/− samples, P < 0.02; ^, statistical significance between C57BL/6 and IL-1R−/− samples, P < 0.01). Error bars represent the standard deviations.
DISCUSSION
Use of innate immune knockout mice to assign causes of pathological phenotypes.
MyD88−/− mice exhibit a wide variety of phenotypes upon infection with C. rodentium, including mortality, delayed neutrophil recruitment, higher bacterial loads, and severe colonic pathology (21). It is problematic to assign these diverse phenotypes to specific causes for two reasons. First, MyD88 is used to mediate the signaling from many TLRs and other innate immune receptors activated upon C. rodentium infection (1). Second, the phenotypes and the signaling pathways that regulate them may be interrelated and covariant. Notably, phenotypes displayed in animals lacking particular TLRs are more restricted than those seen in animals lacking adaptors such as MyD88. Thus, TLR2−/− animals display increased mortality and pathology (14) but do not have the concomitant increases in pathogen load or delayed neutrophil infiltration seen in MyD88−/− mice (13, 21), suggesting that MyD88 mediates signaling through other receptors during C. rodentium infection.
In this paper, we have extended this comparative approach by using mice deficient in cytokine receptor signaling, which, like TLR2−/− mice, have relatively restricted phenotypes. The advance made here is that separation of the overall pathology score into components has distinguished the contributions of various pathologies to disease. IL-1 plays a protective role in host defense against many pathogens, including Listeria monocytogenes (5), S. aureus (27), S. enterica serovar Typhimurium (36), and S. flexneri (37). Our data suggest that IL-1R signaling likewise mediates protection against C. rodentium. Unlike the multifaceted phenotype seen in MyD88−/− mice, the defect in IL-1R−/− mice is limited to intestinal damage and mortality. Thus, the knockout mice allow us to assess how IL-1R signaling contributes to protection from such damage and more importantly how damage alone, without attendant effects on bacterial load, immune cell infiltration, and enterocyte replacement, contributes to disease. Our data with IL-18−/− mice (Fig. 1C) suggest that increased neutrophil infiltration per se in response to C. rodentium is not sufficient to cause increased epithelial damage and is consistent with a protective role for this cell type.
Previously, we demonstrated that GMN is present following C. rodentium infection of MyD88−/− mice (21). We hypothesized that this pathology was the result of several interdependent phenotypes, the most important of which appeared to be an increased pathogen load in the colon. However, here we demonstrate that, given equivalent degrees of colonization in the colon (Fig. 4A), IL-1R−/− mice exhibit GMN as well as severe manifestations of damage, such as bleeding, in comparison to wild-type mice (Fig. 3). In this respect, the phenotype of IL-1R−/− mice infected with C. rodentium is very similar to that seen in TLR2−/− mice, which also suffer from lethal colitis without an attendant increase in overall pathogen load (14). Unlike the necrotic intestinal damage seen in IL-1R−/− mice, the intestinal damage seen in TLR2−/− mice is associated with an increased level of epithelial apoptosis (14). Because both TLR2 signaling and IL-1R signaling appear necessary during infection with C. rodentium and both require MyD88 as a signaling mediator, the severity of the MyD88−/− phenotype compared to that for either of the receptor knockout mice suggests that protection from C. rodentium derives from the combination of signaling by multiple innate immune receptors.
Intestinal damage and neutrophil recruitment regulate pathogen dissemination.
Whereas MyD88−/− animals have many defects that contribute to their inability to control bacterial growth (21), the data presented here suggest that IL-1R−/− mice are more susceptible to colonic damage and local increases in bacterial growth associated with GMN, though not in the colon as a whole (Fig. 4A). Notably, the increased damage in IL-1R−/− mice, evident as epithelial injury, bleeding, and GMN following C. rodentium infection, was not recapitulated upon administration of DSS (compare Fig. 3C and 6B), indicating that these mice are more susceptible only to damage induced by C. rodentium. A salient feature of C. rodentium infections but not DSS treatment was bleeding between crypts. Importantly, the incidence of bleeding is significantly increased in colonic tissue from IL-1R−/− and MyD88−/− animals compared to the incidence in tissue of their wild-type counterparts (Fig. 3C). We hypothesize that the increased bleeding in IL-1R−/− and MyD88−/− mice results in a higher local density of bacteria, which may exacerbate damage to the epithelia, leading to the formation of GMN. Such pathology may also disrupt the epithelial barrier and thus provide a portal through which the bacteria can disseminate to distal tissues.
A defect in epithelial repair is another factor that might contribute to GMN and dissemination. However, GMN is evident despite wild-type levels of enterocyte replacement (Fig. 2B and 5B), suggesting that a lack of repair does not contribute to GMN formation. Notably, IL-1R−/− mice exhibit hyperplasia equivalent to that seen in colons of wild-type animals, despite a greater need for enterocyte replacement. These data suggest that the development of hyperplasia may already be at a maximal level and that increases may be limited by factors such as the rate at which stem cells differentiate and migrate to replenish damaged enterocytes.
Our previous data with wild-type mice depleted of neutrophils suggest that this cell type also plays a crucial role in limiting pathogen dissemination (21). The resolution to this apparent paradox is that intestinal damage together with neutrophil infiltration may contribute to dissemination. Thus, the bacterial loads in livers and spleens of MyD88−/− mice, which have both increased colonic damage and decreased neutrophil infiltration, are 2 to 4 logs higher than for wild-type mice (21). In contrast, increases of only 1 log or less are evident in livers and spleens of IL-1R−/− mice, which have increased damage but intact neutrophil infiltration (Fig. 4A).
IL-1R signaling and protection from pathogen-induced damage.
Previous reports indicate that, unlike many other pathogens, C. rodentium induces peak production of IL-1β 2 weeks p.i. (24), when most IL-1R−/− mice have succumbed. These data, together with our results, suggest that IL-1R signaling may instead be mediated by IL-1α. IL-1α is constitutively produced by epithelial cells (29) and reportedly enhances antibacterial activities of the innate immune system against L. monocytogenes (5). Further, LPS, which is present on the surface of C. rodentium, induces IL-1α expression in a MyD88-dependent manner in vitro (16). Previous work also suggests that the activation of MyD88 results in the increased production of cytokines, such as IL-6 (18). IL-6 leads to increased protective acute-phase responses elicited following tissue damage or infection (20) and appears to play a critical role in protecting mice from mucosal ulceration during infection with C. rodentium (6). Our data suggest that IL-1R−/− mice fail to induce IL-6 or IFN-γ above uninfected levels, whereas wild-type mice exhibit marked increases (Fig. 2D and E). However, unlike the IL-1R−/− mice, most of the IL-6−/− mice survive the infection (6), so IL-6 alone cannot completely account for the increased susceptibility to C. rodentium-induced damage in IL-1R−/− mice. Further, previous studies using animals deficient in the IFN-γ receptor demonstrate that this cytokine is necessary to prevent increased morbidity (39). Together, these studies indicate that lower induction of both IL-6 and IFN-γ, as in IL-1R−/− animals, results in severe morbidity.
IL-1R signaling in chemical- and pathogen-induced colitis.
While it has been demonstrated previously that MyD88 mediates protection against both C. rodentium- and DSS-induced intestinal damage (13, 21, 35), our data suggest that the lack of IL-1R signaling increases the susceptibility to intestinal damage induced only by an A/E pathogen. These data may have important therapeutic implications. Previous results suggest that DSS induces in mice colitis that closely resembles that seen in human patients with inflammatory bowel disease (IBD) (32). Based on evidence showing that IL-1R signaling induces proinflammatory cytokines, it has been suggested that therapeutics directed against IL-1R signaling might prove beneficial for IBD patients (2). However, our data indicate that such therapeutics may cause increased susceptibility to pathogenic strains of E. coli, which have been isolated from patients with IBD (38). Further, our data suggest that key features of DSS-induced colitis, including neutrophil infiltration, which contributes to inflammation, and hyperplasia, which contributes to tumor progression, are unperturbed in the absence of IL-1R signaling. Together, these data suggest that therapeutics directed against IL-1R signaling may not be particularly efficacious and indeed may even prove detrimental.
Acknowledgments
We thank Ifor Williams and Andrew Gewirtz for helpful discussions and reading the manuscript and Yutao Yan and Guillaume Dalmasso for help with FITC-dextran permeability assays.
This work was supported by a grant from NIAID (R01AI056067) to D.K.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9143-150. [DOI] [PubMed] [Google Scholar]
- 2.Arai, Y., H. Takanashi, H. Kitagawa, and I. Okayasu. 1998. Involvement of interleukin-1 in the development of ulcerative colitis induced by dextran sulfate sodium in mice. Cytokine 10890-896. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, P. J., and M. Karin. 1997. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 3361066-1071. [DOI] [PubMed] [Google Scholar]
- 4.Borenshtein, D., M. E. McBee, and D. B. Schauer. 2008. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr. Opin. Gastroenterol. 2432-37. [DOI] [PubMed] [Google Scholar]
- 5.Czuprynski, C. J., and J. F. Brown. 1987. Recombinant murine interleukin-1α enhancement of nonspecific antibacterial resistance. Infect. Immun. 552061-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dann, S. M., M. E. Spehlmann, D. C. Hammond, M. Iimura, K. Hase, L. J. Choi, E. Hanson, and L. Eckmann. 2008. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 1806816-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinarello, C. A. 1996. Biologic basis for interleukin-1 in disease. Blood 872095-2147. [PubMed] [Google Scholar]
- 8.Donnenberg, M. S., C. O. Tacket, G. Losonsky, G. Frankel, J. P. Nataro, G. Dougan, and M. M. Levine. 1998. Effect of prior experimental human enteropathogenic Escherichia coli infection on illness following homologous and heterologous rechallenge. Infect. Immun. 6652-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald, K. A., D. C. Rowe, and D. T. Golenbock. 2004. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 61361-1367. [DOI] [PubMed] [Google Scholar]
- 10.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30911-921. [DOI] [PubMed] [Google Scholar]
- 11.Fukata, M., K. S. Michelsen, R. Eri, L. S. Thomas, B. Hu, K. Lukasek, C. C. Nast, J. Lechago, R. Xu, Y. Naiki, A. Soliman, M. Arditi, and M. T. Abreu. 2005. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 288G1055-G1065. [DOI] [PubMed] [Google Scholar]
- 12.Furuta, G. T., J. R. Turner, C. T. Taylor, R. M. Hershberg, K. Comerford, S. Narravula, D. K. Podolsky, and S. P. Colgan. 2001. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 1931027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, D. L., C. Ma, K. S. Bergstrom, J. T. Huang, C. Man, and B. A. Vallance. 2008. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10618-631. [DOI] [PubMed] [Google Scholar]
- 14.Gibson, D. L., C. Ma, C. M. Rosenberger, K. S. Bergstrom, Y. Valdez, J. T. Huang, M. A. Khan, and B. A. Vallance. 2008. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10388-403. [DOI] [PubMed] [Google Scholar]
- 15.Grandjean-Laquerriere, A., F. Antonicelli, S. C. Gangloff, M. Guenounou, and R. Le Naour. 2007. UVB-induced IL-18 production in human keratinocyte cell line NCTC 2544 through NF-kappaB activation. Cytokine 3776-83. [DOI] [PubMed] [Google Scholar]
- 16.Hawn, T. R., A. Ozinsky, D. M. Underhill, F. S. Buckner, S. Akira, and A. Aderem. 2002. Leishmania major activates IL-1 alpha expression in macrophages through a MyD88-dependent pathway. Microbes Infect. 4763-771. [DOI] [PubMed] [Google Scholar]
- 17.Higgins, L. M., G. Frankel, G. Douce, G. Dougan, and T. T. MacDonald. 1999. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 673031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaisho, T., and S. Akira. 2004. Pleiotropic function of Toll-like receptors. Microbes Infect. 61388-1394. [DOI] [PubMed] [Google Scholar]
- 19.Khan, M. A., C. Ma, L. A. Knodler, Y. Valdez, C. M. Rosenberger, W. Deng, B. B. Finlay, and B. A. Vallance. 2006. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect. Immun. 742522-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopf, M., H. Baumann, G. Freer, M. Freudenberg, M. Lamers, T. Kishimoto, R. Zinkernagel, H. Bluethmann, and G. Kohler. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368339-342. [DOI] [PubMed] [Google Scholar]
- 21.Lebeis, S. L., B. Bommarius, C. A. Parkos, M. A. Sherman, and D. Kalman. 2007. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 179566-577. [DOI] [PubMed] [Google Scholar]
- 22.Luperchio, S. A., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3333-340. [DOI] [PubMed] [Google Scholar]
- 23.Maaser, C., M. P. Housley, M. Iimura, J. R. Smith, B. A. Vallance, B. B. Finlay, J. R. Schreiber, N. M. Varki, M. F. Kagnoff, and L. Eckmann. 2004. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 723315-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBee, M. E., P. Z. Zheng, A. B. Rogers, J. G. Fox, and D. B. Schauer. 2008. Modulation of acute diarrheal illness by persistent bacterial infection. Infect. Immun. 764851-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23399-407. [DOI] [PubMed] [Google Scholar]
- 26.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, L. S., E. M. Pietras, L. H. Uricchio, K. Hirano, S. Rao, H. Lin, R. M. O'Connell, Y. Iwakura, A. L. Cheung, G. Cheng, and R. L. Modlin. 2007. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J. Immunol. 1796933-6942. [DOI] [PubMed] [Google Scholar]
- 28.Mundy, R., T. T. MacDonald, G. Dougan, G. Frankel, and S. Wiles. 2005. Citrobacter rodentium of mice and man. Cell. Microbiol. 71697-1706. [DOI] [PubMed] [Google Scholar]
- 29.Murphy, J. E., C. Robert, and T. S. Kupper. 2000. Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J. Investig. Dermatol. 114602-608. [DOI] [PubMed] [Google Scholar]
- 30.Okamura, H., K. Nagata, T. Komatsu, T. Tanimoto, Y. Nukata, F. Tanabe, K. Akita, K. Torigoe, T. Okura, S. Fukuda, et al. 1995. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect. Immun. 633966-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura, H., H. Tsutsui, S. Kashiwamura, T. Yoshimoto, and K. Nakanishi. 1998. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv. Immunol. 70281-312. [DOI] [PubMed] [Google Scholar]
- 32.Okayasu, I., S. Hatakeyama, M. Yamada, T. Ohkusa, Y. Inagaki, and R. Nakaya. 1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98694-702. [DOI] [PubMed] [Google Scholar]
- 33.Pull, S. L., J. M. Doherty, J. C. Mills, J. I. Gordon, and T. S. Stappenbeck. 2005. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA 10299-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radtke, F., and H. Clevers. 2005. Self-renewal and cancer of the gut: two sides of a coin. Science 3071904-1909. [DOI] [PubMed] [Google Scholar]
- 35.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118229-241. [DOI] [PubMed] [Google Scholar]
- 36.Raupach, B., S. K. Peuschel, D. M. Monack, and A. Zychlinsky. 2006. Caspase-1-mediated activation of interleukin-1β (IL-1β) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect. Immun. 744922-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12581-590. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki, M., S. V. Sitaraman, B. A. Babbin, P. Gerner-Smidt, E. M. Ribot, N. Garrett, J. A. Alpern, A. Akyildiz, A. L. Theiss, A. Nusrat, and J. M. Klapproth. 2007. Invasive Escherichia coli are a feature of Crohn's disease. Lab. Investig. 871042-1054. [DOI] [PubMed] [Google Scholar]
- 39.Simmons, C. P., N. S. Goncalves, M. Ghaem-Maghami, M. Bajaj-Elliott, S. Clare, B. Neves, G. Frankel, G. Dougan, and T. T. MacDonald. 2002. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J. Immunol. 1681804-1812. [DOI] [PubMed] [Google Scholar]
- 40.Swimm, A. I., and D. Kalman. 2008. Cytosolic extract induces Tir translocation and pedestals in EPEC-infected red blood cells. PLoS Pathog. 4e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei, O. L., A. Hilliard, D. Kalman, and M. Sherman. 2005. Mast cells limit systemic bacterial dissemination but not colitis in response to Citrobacter rodentium. Infect. Immun. 731978-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]