Abstract
Although adhesion to host cells is a critical step in the delivery of cytotoxic Yop proteins by Yersinia pestis, the mechanism has not been defined. To identify adhesins critical for Yop delivery, we initiated two transposon mutagenesis screens using the mariner transposon. To avoid redundant cell binding activities, we initiated the screen with a strain deleted for two known adhesins, pH 6 antigen and the autotransporter, YapC, as well as the Caf1 capsule, which is known to obscure some adhesins. The mutants that emerged contained insertions within the ail (attachment and invasion locus) gene of Y. pestis. A reconstructed mutant with a single deletion in the ail locus (y1324) was severely defective for delivery of Yops to HEp-2 human epithelial cells and significantly defective for delivery of Yops to THP-1 human monocytes. Specifically, the Yop delivery defect was apparent when cell rounding and translocation of an ELK-tagged YopE derivative into host cells were monitored. Although the ail mutant showed only a modest decrease in cell binding capacity in vitro, the KIM5 Δail mutant exhibited a >3,000-fold-increased 50% lethal dose in mice. Mice infected with the Δail mutant also had 1,000-fold fewer bacteria in their spleens, livers, and lungs 3 days after infection than did those infected with the parental strain, KIM5. Thus, the Ail protein is critical for both Y. pestis type III secretion in vitro and infection in mice.
Yersinia pestis, the causative agent of plague, employs a type III secretion system (T3SS) to deliver cytotoxic Yop proteins into host cells (16). Yops have various cell-altering activities, including Rho-GAP activity (YopE) (6, 64), tyrosine phosphatase activity (YopH) (9), serine/threonine kinase activity (YpkA) (23), and protease activity (YopT) (56), and the ability to disrupt MAP kinase signaling pathways via acetylation of MAP kinases (YopJ) (44-46). A sixth Yop, YopM, leads to disruption of the immune system via depletion of NK cells (26). The injection of Yops into host cells by the Ysc T3SS of pathogenic Yersinia species is required for virulence (49). Mutants lacking the Yop-encoding virulence plasmid are avirulent by all routes of entry, as are mutants unable to assemble a functioning T3SS for Yop delivery (49). An essential step for secretion of type III effector proteins is host cell binding. This docking step is required for pathogens to deliver a cargo of cell-altering effector proteins. In the case of enteropathogenic Yersinia species, at least two adhesins are capable of mediating T3SS delivery of Yops, invasin and YadA (53). Both of these proteins are inactive in Y. pestis, due to an insertion element or frameshift mutation, respectively (20, 48, 54, 57). One known Y. pestis adhesin, pH 6 antigen, restores adhesion and T3SS delivery of ExoS when heterologously expressed in a nonadherent mutant of Pseudomonas aeruginosa (60). pH 6 antigen can also fulfill this T3SS docking function in Y. pestis (S. Felek and E. S. Krukonis, unpublished). However, pH 6 antigen is only expressed under certain environmental conditions (>35°C and pH of <6.7) (5), a pH 6 antigen mutant has a modest defect in virulence via the intravenous, subcutaneous, or intranasal routes of infection (14, 32), and the expression of pH 6 antigen in a mouse model of fully virulent pneumonic plague is greatly downregulated (31). Thus, identification of the adhesins required for Y. pestis Yop delivery is still a priority.
Recently, the Y. pestis Ail protein was also shown to mediate cell binding, cell invasion, bacterial autoaggregation, and serum resistance (3, 28). This finding was somewhat surprising, since Y. pestis Ail is nearly identical to Yersinia pseudotuberculosis Ail, which has been reported to confer serum resistance but not play a role in adhesion or invasion of tissue culture cells (67). On the other hand, in Yersinia enterocolitica, Ail mediates attachment and invasion of host cells and confers serum resistance to the bacterium (8, 40, 50). Ail of Y. enterocolitica shows some cell selectivity allowing interaction with HEp-2 and CHO cells, but not MDCK cells (40). Ail is an outer membrane protein of the OmpX family, proposed to have eight membrane-spanning domains and four extracellular loops (22, 63). The role of these loops has been extensively studied in Y. enterocolitica Ail. Mutations in either loop 2 or 3 were found to affect serum resistance, adhesion/invasion, or both activities (39). Furthermore, a peptide derived from a sequence within loop 2 of Y. enterocolitica Ail was able to disrupt invasion of CHO cells (39).
While many researchers have used nonphagocytic cells as targets of Yop secretion to identify adhesin molecules (11, 53), Yop delivery to phagocytic cells, such as macrophages and neutrophils, may be adhesin independent, since the bacteria can be readily engulfed by these host cells (11). During Yop delivery to phagocytic cells, the pore-forming translocon molecules YopB and YopD of the T3SS apparatus may serve as a cholesterol-binding docking complex, by analogy to other homologous T3SSs (25). To identify Y. pestis factors critical for cell binding and Yop delivery to nonphagocytic human cells and potentially to phagocytic human cells, we applied a genetic enrichment strategy. In tissue culture models of infection, we demonstrate that Y. pestis Ail mediates cell attachment and facilitates Yop delivery to a human epithelial cell line (HEp-2) and a human monocyte cell line (THP-1). In mouse infections, Ail is critical for virulence, as reflected by the >3,000-fold increase in the 50% lethal dose (LD50) of the Δail mutant.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Y. pestis strains were cultivated in heart infusion broth (HIB) overnight or on heart infusion agar (HIA) for 48 h at 28°C. Escherichia coli strains were cultured in Luria-Bertani (LB) broth or LB agar at 28°C or 37°C. Antibiotics were used at the following concentrations: streptomycin (Sm), 100 μg/ml; kanamycin (Km), 30 μg/ml; chloramphenicol (Cm), 10 μg/ml; and ampicillin (Amp), 100 μg/ml. Isopropyl-β-d-thiogalactopyranoside (IPTG) was used at a 100 μM concentration. Strain KIM5-3001 (Table 1), an Sm-resistant version of KIM5, is hereafter referred to as KIM5 in the text, for simplicity.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or features | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| AAEC185 | supE44 hsdR17 mcrA mcrB endA1 thi-1 ΔfimB-fimH ΔrecA | 10 |
| SM10(λpir) | thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu Kmr (λpir) | Laboratory collection |
| Y. pestis | ||
| KIM5-3001 | pgm−, Smr (referred to as KIM5 in text) | 32 |
| KIM5-3001 ΔpsaA1 ΔyapC Δcaf1 mutant | pgm−, Smr | This study |
| KIM5-3001 Δail mutant | pgm−, Smr | This study |
| KIM5-3001 pCD1− | pgm−, Smr | 21 |
| Plasmids | ||
| pFD1 | Kmr transposon and Himar1 transposase on an Ampr R6K-based suicide plasmid | 55 |
| pMMB207 | 9.1 kb, Cmr | 43 |
| pMMB207-ail | 9.7 kb, Cmr | This study |
| pIV2 | 3.5-kb Yersinia cloning vector; Kmr | 59 |
| pIV2-ail | 4.1 kb, Kmr | This study |
| pYopE129-Elk | Fusion protein consisting of residues 1 to 129 of YopE fused to the Elk tag; Ampr | 19 |
| pKD46 | 6.3-kb, Ampr, red recombinase expression plasmid | 18 |
| pKD4 | 3.3-kb, Kmr, template plasmid | 18 |
| pCP20 | 9.4-kb, Flp recombinase expression plasmid; Ampr, Cmr | 18 |
Transposon mutagenesis and identification of ail.
We previously observed that the deletion of two known adhesins, pH 6 antigen and YapC, does not abolish the ability of Y. pestis to bind to eukaryotic cells when it is grown at 28°C (21). To find other potential adhesins in Y. pestis, we performed a random transposon mutagenesis by using a Y. pestis KIM5 ΔpsaA ΔyapC Δcaf1 mutant strain. We used a caf1 mutant to exclude the masking effect of capsule during cell binding enrichment steps (33). The strain was conjugated with E. coli SM10(λpir)/pFD1 harboring the mariner transposon (55) and then selected for Sm resistance (Y. pestis) and Km resistance (mariner transposon).
Mutant pools were then used to serially infect HEp-2 cells in 24-well tissue culture plates six times per day for 3 days (18 rounds of enrichment). For each day of infection, the bacteria were put in the first well at a multiplicity of infection (MOI) of 100 and centrifuged for 5 min at 200 × g to facilitate bacterial contact with the HEp-2 cells; infections were incubated for 1 h at 37°C in 5% CO2. The unbound bacteria from the previous binding step were transferred to the next well, centrifuged, and incubated, as described above. After six rounds of enrichment, unbound bacteria were collected, cultured overnight in HIB at 28°C, and used for more enrichment the next day. We also performed an erythrocyte binding enrichment three times per day for 3 days. Sheep erythrocytes were incubated with mutant pools, incubated for 2 h at 37°C, and centrifuged at 800 rpm in a microcentrifuge for 3 min, and supernatants were mixed with new erythrocytes. After the last enrichment, unbound bacteria were collected and cultured overnight in HIB at 28°C and used for more enrichment the next day.
To identify the gene disrupted by mariner transposition, the insertion region was amplified with primers Mariner-1 (5′-GGCCACGCGTGCACTAGTACNNNNNNNNNNTACNG-3′) and SFMar2 (5′-TACGGTATCGATAAGC), and PCR products were purified (Qiagen, Gaithersburg, MD) and subjected to a second nested PCR by using primers SFMar3 (5′-CACGCGTGCACTAGTAC-3′) and SFMar4 (5′-AATCATTTGAAGGTTGGTAC-3′). Final PCR products were purified and sequenced by the University of Michigan Sequencing Core by using sequencing primer SFMarseq (5′-ACGCCATCTATGTGTCAGAC-3′).
Deletion of ail.
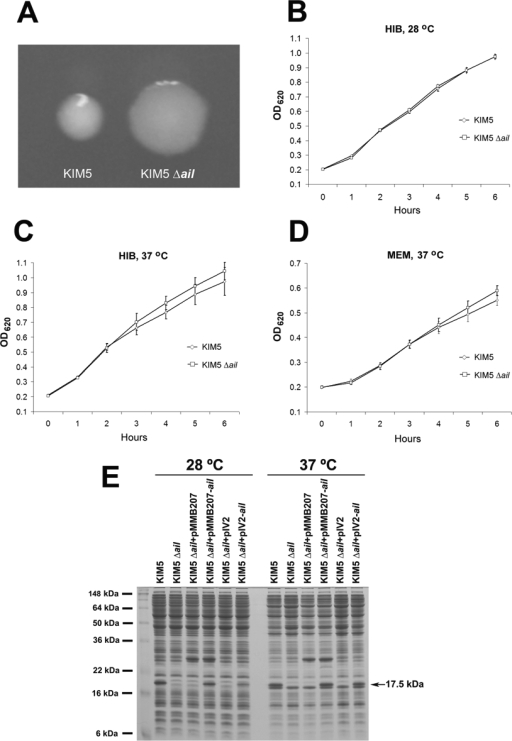
Gene deletion in Y. pestis KIM5 was performed as previously described (18, 21, 68). Briefly, the pKD4 Km resistance cassette was amplified by PCR with primers aildf (5′-ATTTTTCATGTGTCAGATATTTGTTAATATTTGGCTGGCCACTTTAGTCTTGTGTAGGCTGGAGCTGCTTC-3′) and aildr (5′- TCAGCAATTTGAAACCACCATTATGGTGGGTTTTCATGGTTAGGAGG ACGCATATGAATATCCTCCTTAGT-3′). PCR products were purified and digested with DpnI and then transformed into Y. pestis KIM5, which had been previously transformed with pKD46 and induced with arabinose. Km-resistant colonies were selected, and the deletion of ail was confirmed by PCR with primers ailf1 (5′-TCAATGGGGCTATTGATTTCG-3′) and ailr1 (5′-GGTGACTTGCTCGAAATAATG-3′). Replacement of ail with this cassette results in a complete deletion of ail (all 585 bp). The strain was then transformed with pCP20 to resolve out the Km resistance cassette. This resolution results in an 82- to 85-nucleotide FLP recombination target site scar in the place of ail (18). Plasmids were cured from the strains by growing in HIB at 28°C and screening for Amp sensitivity. The Δail mutant grew identically to the starting strain, KIM5, in HIB at 28°C. It grew slightly faster than KIM5 at 37°C in HIB and minimal essential medium (Fig. 1).
FIG. 1.
Colony morphology assessment, growth curve analysis, and complementation of a KIM5 Δail mutant. (A) Colony morphology of the strain KIM5 and a KIM5 Δail mutant grown on HIB agar. KIM5 forms smaller, shinier colonies, while the Δail mutant is flatter, wider, and more opaque and has rougher colony edges. (B to D) Growth curve analysis of KIM5 and the Δail mutant under various growth conditions. (E) Deletion and complementation of ail was confirmed by SDS gel electrophoresis. Y. pestis KIM5 and derivative strains were grown overnight in HIB at 28°C or 37°C. For pMMB207-containing strains, 100 μM IPTG was added to induce ail expression. pIV2 is a plasmid derived from Yersinia enterocolitica that can be stably maintained during in vivo experiments for ail complementation (59). The identity of the 17.5-kDa band absent in the KIM5 Δail mutant was confirmed to be Ail protein by mass spectrometry at the University of Michigan Proteomics Consortium.
Cloning of ail.
The gene encoding Ail was amplified from Y. pestis KIM5 with PCR by using primers ailcf1 (5′-GCGCGGATCCTTGGCTGGCCACTTTAGTCT-3′) and ailcr1 (5′-GCGCCTGCAGGGTTAGGAGGACGTTAGAAC-3′). Amplification primers for cloning were designed to include the ribosomal binding site of ail. PCR products were purified and digested with BamHI and PstI, gel purified, and ligated into pMMB207 (43). The sequences of clones containing the insert were confirmed. To construct pIV2-ail, pMMB207-ail was digested with BamHI and PstI, and the band harboring ail was gel purified and then ligated into the BamHI- and PstI-digested pIV2 plasmid. The KIM5 Δail mutant strain was transformed with pMMB207-ail and pIV2-ail or empty vectors for complementation studies.
SDS-PAGE.
Deletion and complementation of ail were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Briefly, Y. pestis KIM5 and derivative strains were grown overnight in HIB containing Sm at 28°C or 37°C. For pMMB207-containing strains, 100 μM IPTG and Cm were added to HIB while only Km was added to the pIV2-containing strains (no IPTG). Bacteria were pelleted and resuspended in SDS-PAGE sample buffer to normalize for optical densities at 620 nm (OD620s), boiled for 5 min, and then subjected to SDS-PAGE followed by Coomassie blue staining.
Adhesion and invasion assays.
HEp-2 and THP-1 cells were cultured in 24-well tissue culture plates until reaching 100% and 60% confluence, respectively. THP-1 cells were activated with 10 pg/ml of phorbol 12-myristate 13-acetate for 3 days to stimulate attachment to 24-well plates. Y. pestis KIM5 or E. coli derivatives were cultured overnight at 28°C (100 μM IPTG and Cm was added for the strains containing pMMB207). Tissue culture cells were washed twice with 1 ml of serum-free minimal essential medium (for HEp-2 cells) or RPMI 1640 (for THP-1 cells) and infected with the bacterial strains at an MOI of 100 (Y. pestis) or 500 (E. coli) bacteria per cell. For THP-1 cells, the cells were pretreated with 5 μg/ml cytochalasin D for 45 min prior to infection to inhibit phagocytosis. This blocked phagocytosis and decreased cellular uptake by 100-fold (49% decreased to 0.47%) as assessed by a gentamicin protection assay. For the adhesion assay, plates were incubated for 2 h at 37°C in 5% CO2. Cells were then washed, and cell-associated bacteria were liberated by the addition of sterile H2O containing 0.1% Triton X-100 for 15 min. Serial dilutions of samples were plated onto HIA or LB agar for CFU analysis. In parallel wells, the entire population of bacteria was harvested and enumerated by dilution and CFU analysis to determine the total number of bacteria per well. Percent adhesion was calculated by dividing bound CFU by total bacteria in the well at the end of 2 hours of incubation and then multiplying by 100. Presented data are from three experiments performed in duplicate. Statistical analysis was performed using the Student t test (n = 6).
Invasion assays were performed similarly except that at the end of 2 hours of bacterial binding, cells were washed once with phosphate-buffered saline (PBS) to remove unbound bacteria and minimal essential medium containing 7.5 μg/ml gentamicin was added for 1 hour at 37°C in 5% CO2 to kill extracellular bacteria. Cells were then washed twice with PBS and lysed and plated as described for the adhesion assay.
Cytotoxicity assay.
HEp-2 cells were cultivated until they reached about 50% confluence in 24-well tissue culture plates. Y. pestis KIM5 and derivative strains were cultured in HIB overnight at 28°C. Cultures were diluted to an OD620 of 0.15 in HIB and incubated for 3 to 4 more hours at 28°C. Plates were washed two times with 2 ml of serum-free tissue culture medium, and bacteria were added to each well at an MOI of 10. IPTG (100 μM) was added to tissue culture medium to induce ail expression. Plates were incubated at 37°C in 5% CO2. Rounding was observed, and pictures were taken under a phase-contrast microscope at 0, 2, 4, and 8 h.
Yop delivery assay.
KIM5 derivatives carrying a YopE-Elk-encoding plasmid (pYopE129-Elk) (19) were cultivated overnight in HIB at 28°C, diluted to an OD620 of 0.15 in HIB, and incubated for 3 to 4 more hours at 28°C. HEp-2 or THP-1 cells were cultured in six-well tissue culture plates until they reached 100% or 60% confluence, respectively. Each well was washed two times with serum-free tissue culture medium, and bacteria were added at an MOI of 10 in the presence of 100 μM IPTG (to induce yopE-ELK and ail expression). The bacteria were allowed to interact and translocate Yops for 2 to 8 h. Cells were then directly lysed in 100 μl of 1.5× Laemmli sample buffer containing protease inhibitor cocktail (Sigma P-8340) and phosphatase inhibitor cocktail (Sigma P-2850), combined with bacteria from the tissue culture medium supernatant, and boiled for 5 min. Extracts (7.5 μl) were run for Western blot analysis and probed with anti-ELK or anti-phospho-ELK antibodies (Cell Signaling Technology, Danvers, MA). Cells deleted for yopB served as a negative control for Yop delivery, since YopB is part of the T3SS translocation pore and is required for Yop delivery.
Mouse experiments.
To find the median lethal dose (LD50), 6- to 8-week-old female Swiss Webster mice (Harlan Sprague-Dawley, Indianapolis, IN) were inoculated intravenously (i.v.) via tail vein injection with 5- to 10-fold-increased concentrations of each strain. Each bacterial strain group consisted of three different doses and 10 mice in each dose. Mice were observed daily for 16 days for survival. For the pCD1-negative KIM5 avirulent mutant, only five mice were infected with a single dose of 106 bacteria. Experiments were performed in accordance with the University of Michigan UCUCA guidelines. Bacteria for mouse inoculation were grown overnight at 28°C in HIB medium.
For tissue analysis experiments, 10 mice were inoculated with about 100 bacteria i.v. (10 LD50s) and euthanized with CO2 at day 1, 2, 3, or 7. Spleen, liver, and lung tissues were removed and weighed, and samples were divided into two parts. One part was homogenized, and serial dilutions were plated for CFU. The other part was fixed in formalin for histology. Data presented are from two experiments (20 mice per strain per time point). Histological sections and staining were done at the University of Michigan Dental School Core Histology Laboratory.
Statistical analysis.
Adhesion data, invasion data, and spleen weights of the mice were analyzed with Student's t test. Tissue colonization levels were analyzed using a two-tailed two-sample Wilcoxon rank-sum (Mann-Whitney) test.
RESULTS
Transposon mutagenesis and enrichment identify Ail as a dominant adhesin of Y. pestis.
To identify novel bacterial proteins that equip Y. pestis to bind host cells, we initiated our enrichment strategy with a KIM5 strain lacking two previously described adhesins of Y. pestis—pH 6 antigen (ΔpsaA) (67) and the putative autotransporter YapC (ΔyapC) (21). This strain maintains wild-type levels of adhesion. Thus, this mutant screen was designed to identify redundant adhesins of Y. pestis. Since Y. pestis can become nonadherent due to mutations resulting in constitutive expression of the Caf1 capsule (Krukonis and Felek, unpublished observations), the caf1 locus was also deleted from our KIM5 ΔpsaA ΔyapC starting strain. Three pools of ∼1,000,000 Y. pestis mutants (∼3,000,000 total mutants) with random mariner transposon insertions were generated (55). The pools were subjected to two independent enrichment strategies. In the first, bacterial cells were allowed to bind HEp-2 cells for 1 h, and then nonadherent bacteria were removed and used to infect a fresh well of HEp-2 cells for 1 h. This panning procedure was repeated for six rounds/day for 3 days (18 rounds of enrichment).
The other enrichment strategy took advantage of the fact that Y. pestis can agglutinate sheep red blood cells when grown at 28°C; at 37°C, this activity is blocked by expression of the Caf1 capsule (Krukonis and Felek, unpublished observations). Bacterial cells were incubated with sheep erythrocytes for 2 h at 37°C in PBS (our strain for mutagenesis was a Δcaf1 mutant), and then the erythrocytes were pelleted gently in a microcentrifuge. Nonadherent Y. pestis bacteria were then mixed with fresh red blood cells, and the enrichment procedure was repeated three times/day for 3 days (nine rounds of enrichment).
After either enrichment, we noticed that a vast majority of KIM5 mutant derivatives had an altered colony morphology (larger, more opaque, rough colony edges) (Fig. 1A). Genomic sequencing of three of these mutants identified the locus of the mariner insertion as the ail gene of Y. pestis (20). Ail has been defined previously in pathogenic Yersinia species as mediating cell attachment and invasion as well as serum resistance (3, 28, 40, 67). Homologues of Ail in other genera have also been referred to as an opacity factor, since deletion of the locus results in an altered colony morphology phenotype (7). One other sequenced mutant that emerged from this screen (with a normal colony morphology) had an insertion in the gene encoding the regulatory protein DeoR. We have not characterized this mutant further.
To assess the specific importance of ail in our parental strain, we next constructed a targeted single-deletion ail mutant of Y. pestis KIM5. SDS-PAGE and Coomassie blue staining confirmed that the Ail protein was lacking in the reconstructed Δail mutant and that it could be complemented by ail expressed from the broad-host-range plasmid pMMB207 (Fig. 1E) (43). Targeted ail mutants also had an altered colony morphology. Despite the altered colony morphology, KIM5 and the Δail mutant had similar growth kinetics in HIB and tissue culture medium (Fig. 1B to D).
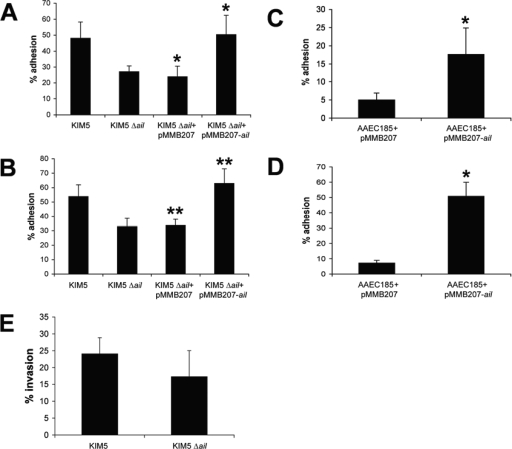
The ail mutant showed an approximately twofold reduction in cell binding to HEp-2 and THP-1 cells (Fig. 2A and B, respectively) compared to the parental strain, KIM5. THP-1 cell adhesion assays were performed in the presence of 5 μg/ml cytochalasin D to inhibit phagocytosis. A freshly reconstructed Δcaf1 ΔpsaA ΔyapC Δail mutant also showed a (35%) defect in adhesion relative to the Δcaf1 ΔpsaA ΔyapC starting strain for mutagenesis (data not shown). We interpret this to mean that while Ail may be a dominant adhesin in our KIM5 ΔpsaA ΔyapC Δcaf1 mutant (allowing it to be enriched to the exclusion of other adhesin mutants), additional redundant adhesins must also be present. Expression of Ail in the heterologous host, E. coli AAEC185 (fim negative), also facilitated binding to both HEp-2 and THP-1 cells (Fig. 2C and D, respectively).
FIG. 2.
Adhesion and invasion of Y. pestis derivatives or E. coli expressing ail to HEp-2 or THP-1 cells. HEp-2 (A, C, and E) and THP-1 (B and D) cells were infected with Y. pestis derivatives (A and B) or E. coli AAEC185 derivatives (C and D). Percent adhesion was calculated by dividing cell-associated CFU by the number of all bacteria in the well (bound plus unbound) and multiplying by 100. Data are from three independent experiments performed in duplicate (n = 6). *, P < 0.0005; **, P < 0.00005. HEp-2 cell invasion (E) was assessed after the addition of gentamicin to kill extracellular bacteria for 1 h (P = 0.1; n = 6).
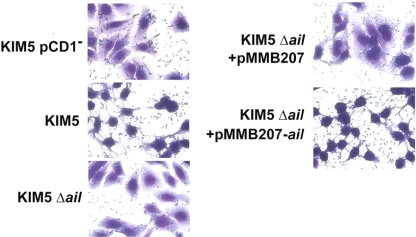
Some of the HEp-2 cell-associated bacteria were likely internalized in this assay, since Y. pestis is known to invade tissue culture cells (17, 28). However, based on our analysis of KIM5 invasion of HEp-2 cells, about 60% of adhered bacteria remain extracellular (compare Fig. 2A and Fig. 2E). Deletion of ail results in only a modest decrease in invasion frequency (P = 0.1) (Fig. 2E). Giemsa staining of infected cells also indicated that many of the bacteria remain at the cell periphery, and some even appear to be associated with the extracellular matrix (Fig. 3).
FIG. 3.
Giemsa staining of KIM5, the KIM5 Δail mutant, and the complemented mutant binding to HEp-2 cells. The shrunken cytoplasm in KIM5-infected cells is indicative of Yop-mediated cytotoxicity. KIM5 lacking the Yop-encoding virulence plasmid, pCD1, does not mediate cytotoxicity.
Ail is important for Yop delivery to host cells.
One of the critical steps in Y. pestis pathogenesis is the delivery of Yop proteins. In the absence of Yops, all pathogenic Yersinia species are completely avirulent (49). We next assessed the role of Ail in Yop delivery to both phagocytic and nonphagocytic cells by using human monocytes (THP-1 cells) and human epithelial cells (HEp-2 cells). Yop delivery was measured by assessing delivery and phosphorylation of ELK-tagged YopE from the KIM5 Δail mutant, compared to the parental strain, KIM5. ELK is an epitope tag from the eukaryotic transcription factor Elk-1 that is normally phosphorylated by MAP kinase (37) or stress-activated protein kinase (SAPK)/Jun N-terminal protein kinase (JNK) (15).
Upon infection of HEp-2 cells with KIM5, phosphorylated ELK-YopE could be detected within 2 hours. However, for the KIM5 Δail mutant, no Yop delivery was detected even by 8 hours (Fig. 4A). In THP-1 human monocytes, phospho-ELK-YopE was detected upon KIM5 infection by 2 hours, but not detected until hour 4 in the Δail mutant (Fig. 4B). YopE-ELK delivery to THP-1 cells by the Δail mutant at 4 hours was less efficient than that by KIM5 (Fig. 4B). Complementation of the Δail mutant with Ail expressed from an inducible plasmid demonstrated that expression of Ail alone could restore Yop delivery to the Δail mutant (Fig. 4A and B). Expression of the entire pool of YopE-ELK (phosphorylated and unphosphorylated) within bacterial cells and tissue culture cells was detected using an anti-ELK antibody (Fig. 4). Deletion of yopB (a component of the Yop translocation pore) completely prevented the ability of YopE-ELK to be translocated and phosphorylated within host cells (Fig. 4). Thus, the yopB mutant serves as a negative control for YopE-ELK delivery. These data indicate that Ail is an important adhesin for mediating Yop delivery in Y. pestis. These studies also demonstrate that Ail plays a role in Yop delivery to phagocytic cells, the primary target of Yops early in infection.
FIG. 4.
The Δail mutant is delayed for delivery of ELK-tagged YopE to the cytoplasm of host cells. HEp-2 (A) or THP-1 (B) cells were infected with KIM5 derivatives carrying a YopE-Elk-encoding plasmid (pYopE129-Elk) (19) at an MOI of 10 in the presence of 100 μM IPTG (to induce YopE-ELK and Ail expression). The bacteria were allowed to interact and translocate Yops for 2 to 8 h. Western blots were probed with anti-ELK (ELK) or anti-phospho-ELK (P-Elk) antibodies. Cells lacking yopB served as a negative control for Yop delivery, since they lack a critical component of the T3SS translocation pore required for Yop delivery.
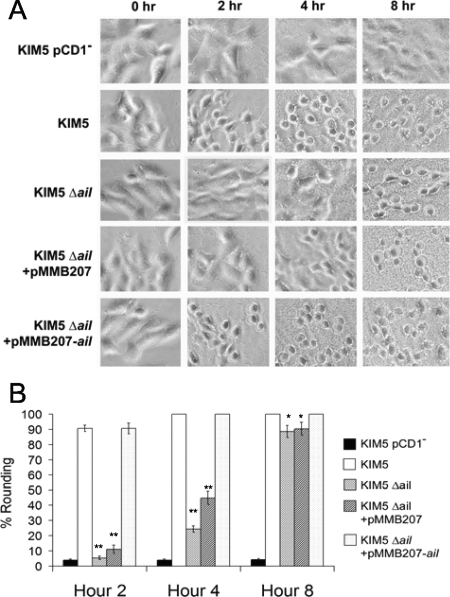
The delay in Yop delivery to HEp-2 cells was also confirmed by monitoring Yop-mediated cytotoxicity as indicated by cell rounding (Fig. 5). Counting of 500 cells per infecting strain indicated that at hour 2, 90% of KIM5-infected cells had rounded, yet only 5 to 10% of the Δail mutant-infected cells were rounded (Fig. 5B). By hour 4, some rounding was detected in HEp-2 cells infected with the Δail mutant (25 to 45%), and by hour 8, about 85% of cells infected with the Δail mutant had undergone cell rounding (100% of KIM5-infected cells were rounded at hour 8) (Fig. 5B). This indicates that the Δail mutant is not completely attenuated for Yop delivery. Rounding of THP-1 cells was not evaluated, since they are quite round normally. Further rounding due to Yop delivery was difficult to assess.
FIG. 5.
Deletion of ail delays cytotoxicity. (A) HEp-2 cells were infected with Y. pestis KIM5 derivatives at an MOI of 10. IPTG (100 μM) was added to the tissue culture medium to induce ail expression. Plates were incubated at 37°C in 5% CO2. Rounding was observed and pictures were taken under a phase-contrast microscope at hours 0, 2, 4, and 8. Cell rounding was also quantified by counting 500 cells per sample at each time point (B). *, P < 0.001; **, P < 5 × 10−9.
Lack of Yop-mediated cytotoxicity was also seen in Giemsa-stained HEp-2 cells 2 hours after infection by the KIM5 Δail mutant, as indicated by the failure of the mutant to cause cytoplasmic shrinkage (Fig. 3).
Ail plays a major role in Y. pestis virulence.
Since the KIM5 Δail mutant was strongly defective for Yop delivery in vitro, we predicted that it might be strongly attenuated for mouse virulence. We next assessed the role of Ail in a mouse infection model. Since KIM5 derivatives are pigmentation negative (pgm−) and cannot acquire iron readily in peripheral tissues due to lack of yersiniabactin (4, 13) encoded within the 102-kb pgm high-pathogenicity island (12), we used the i.v. route of infection. Three groups of 10 Swiss Webster mice were infected with 5- or 10-fold increasing doses of bacteria, and the LD50 was determined and compared with either that of the KIM5 parental strain or that of a Yop-negative plasmid-cured derivative of KIM5 (pCD1−). Whereas the LD50 of our wild-type KIM5 strain was calculated to be 10 or 7 bacteria in two experiments (Table 2) (52), the KIM5 Δail mutant had an LD50 of 167,000 or 23,800 organisms in two experiments, a 16,000- or 3,400-fold increase in the LD50, respectively. This strong attenuation of the KIM5 Δail mutant is consistent with a poor ability to deliver Yop proteins during animal infection. The lack of virulence of the Δail mutant could also be partially complemented in vivo by expressing ail from the Y. enterocolitica-derived plasmid pIV2 (Table 2), which is stably maintained in Yersinia strains for many generations without antibiotic selection (59), indicating that the virulence defect in this strain is due to the lack of Ail protein. We suspect that the incomplete complementation of the Δail mutant is due to poor expression of ail from pIV2-ail at 28°C (Fig. 1E).
TABLE 2.
Virulence of KIM5 Δail mutant in micea
| Experiment | Strain | LD50 | Fold attenuation | Fold complementation |
|---|---|---|---|---|
| 1 | KIM5 | 10 | 1 | NT |
| KIM5 Δail mutant | 166,400 | 16,640 | NT | |
| pCD1− KIM5 mutant | >1,000,000 | >100,000 | NT | |
| 2 | KIM5 | 7 | 1 | NA |
| pIV2-containing KIM5 Δail mutant | 23,800 | 3,400 | 1 | |
| pIV2-ail-containing KIM5 Δail mutant | 860 | 123 | 27.6 | |
| pCD1− KIM5 mutant | >1,000,000 | >100,000 | NA |
The LD50 was calculated according to Reed and Muench (52). NT, not tested; NA, not applicable.
KIM5 colonizes host tissue to much higher levels than does the KIM5 Δail mutant.
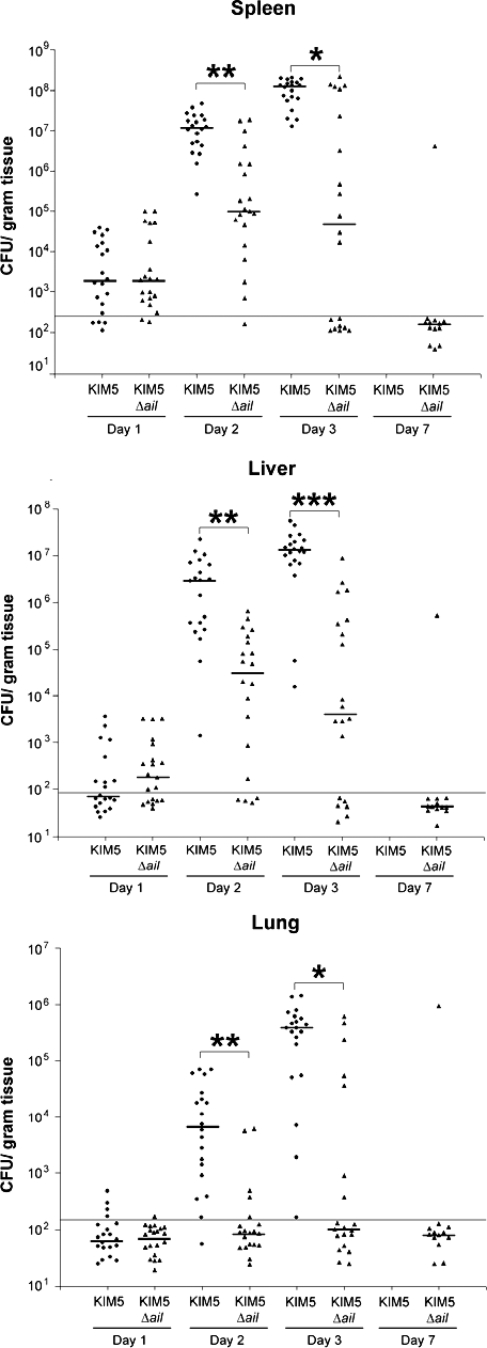
Due to the decrease in virulence of the KIM5 Δail mutant, we analyzed the number of bacteria in various mouse organs to assess colonization and/or clearance of the infection. Mice were infected with 100 organisms of either KIM5 or the Δail mutant (∼10 LD50s for the KIM5 parental strain). After 1, 2, 3, and 7 days, mice were euthanized, and bacteria were harvested from their spleens, livers, and lungs. Ten mice were used per strain at each time point, and the experiment was repeated once to give 20 mice per time point. At day 1, infection with the Δail mutant showed similar numbers of bacteria in the spleen (a blood-filtering organ) (Fig. 6). By day 2, infection with the Δail mutant showed 100-fold fewer bacteria/gram tissue in the spleen, liver, and lungs (P value of <0.00005 for all three tissues) (Fig. 6). Finally, by day 3, the CFU of Δail mutant was decreased by >1,000-fold in spleen, liver, and lung compared to that of the parental strain, KIM5 (Fig. 6). In fact, by day 3, some mice infected with the Δail mutant had now cleared the infection from the spleen (Fig. 6). Mice infected with KIM5 typically begin to die on days 3 to 5, and all succumb to infection when infected with 10 LD50s. However, we could follow survivors of infection with the KIM5 Δail mutant in this experiment. Almost all such mice (all but one of the survivors) cleared the infection from all three tissues by day 7 (Fig. 6). These data indicate that although early in infection (day 1), the KIM5 Δail mutant is present in the spleen at levels similar to those of the parental strain, KIM5, over the course of a weeklong infection, the Δail mutant is gradually cleared from the host, while KIM5 is able to replicate to high numbers and kill the host.
FIG. 6.
The Δail mutant has reduced levels of tissue colonization compared to the wild-type strain, KIM5. Twenty Swiss Webster mice were inoculated with ∼100 bacteria i.v. and euthanized at days 1, 2, 3, and 7 (if they survived). Spleen, liver, and lung tissues were removed, weighed, and homogenized. The horizontal line at about 150 CFU/gram of tissue indicates the limit of detection in this assay. The median level of colonization for each strain in each tissue is indicated with a horizontal bar. Colonization results were statistically analyzed using a two-tailed, two-sample Wilcoxon rank-sum (Mann-Whitney) test. *, P < 0.0005; **, P < 0.00005; ***, P = 0.000001.
Histology from day 3 of the infection revealed more immune infiltration and tissue damage/degradative debris during infection with the Δail mutant than during infection with KIM5, indicating that the Δail mutant induced a more robust immune response (Fig. 7). This is predicted if the delivery of immune-suppressing Yops is defective. Host tissues lacking pathology in the presence of the KIM5 Δail mutant had cleared the infection, based on low CFU numbers from the same tissue sample (Fig. 7, panels D, F, J, and L), or the infection had not yet established high bacterial numbers in that tissue (Fig. 7, panel G). While many Δail mutant infections had been cleared (Fig. 7, panels D and F [spleen] and J and L [liver]), those that had not yet been cleared promoted robust immune infiltration that would lead to eventual clearance of the infection and showed large areas of debris (Fig. 7, panel E [spleen] and K [liver]). Spleens isolated from Δail mutant-infected mice were enlarged by about 25% over 3 days, while spleen sizes in KIM5-infected mice decreased (Fig. 8). Again, this indicates a more dramatic immune response against infection by the Δail mutant, resulting in immune cell infiltration in the spleen.
FIG. 7.
Histopathology upon Y. pestis infection in the spleen and liver. Swiss Webster mice were inoculated with ∼100 bacteria i.v. and euthanized at day 3. Spleen and liver tissues were fixed in formalin and stained with hematoxylin-eosin. Sections from three infected mice in each group were prepared (three panels per bacterial strain [e.g., KIM5 spleen infection in panels A to C]). Images were taken at a magnification of ×400. Some areas of pathology are indicated in boxed areas. Those boxed areas are enlarged in the bottom row of panels. Recovered CFU/gram in the same mouse tissue are provided within the white boxes.
FIG. 8.
Spleen weights of Y. pestis-infected animals. Twenty Swiss Webster mice were inoculated with ∼100 bacteria i.v. and euthanized at day 1, 2, 3, or 7. Spleens were removed and weighed. Increased spleen weight is an indication of increased immune infiltration in mice infected with the KIM5 Δail mutant. Decreased spleen weight is an indication of cell death within the spleen in a KIM5 infection. Statistical comparisons are between spleen weights on day 1 versus those on day 3 for KIM5 or for the KIM5 Δail mutant. *, P < 5 × 10−5 by the Student t test.
DISCUSSION
By coupling random mutagenesis with a phenotypic enrichment strategy, we determined that the Ail adhesin is critical for Y. pestis to deliver Yop proteins to host cells. The defect in Yop delivery to the phagocytic cell line, THP-1, is dramatic, yet less prolonged than the defect in Yop delivery to nonphagocytic HEp-2 cells (Fig. 4).
While the YopE-ELK phosphorylation assay indicates very little Yop delivery to HEp-2 cells by the KIM5 Δail mutant even after 8 hours of infection (Fig. 4), the Yop-mediated cell-rounding assay shows that some Yop delivery is occurring as early as 4 hours after infection with the Δail mutant and that quite a bit of Yop delivery is occurring by 8 hours postinfection (Fig. 5). Several explanations may address this discrepancy. The YopE129-ELK fusion protein may be translocated less efficiently than wild-type Yops. The fact that several Yops can mediate cell rounding by disrupting the actin cytoskeleton (16) may allow more rapid cell rounding than detection of a single translocated molecule. The phosphorylated YopE-ELK molecule may also be harder to detect than the activity of multiple Yops. In the former case, one must have sufficient translocation and phosphorylation to detect the protein by Western blotting. In the latter case, one detects the enzymatic activity of the Yops. Thus, for cytotoxicity, less Yop delivery may be required to give a positive signal. Whatever the reason for the difference in detection, it is clear that the KIM5 Δail mutant is delayed for Yop delivery and is severely attenuated for virulence (Table 2). It remains possible that the measurement of YopE-ELK phosphorylation is not a direct indicator of Yop delivery and that the Δail mutation may affect phosphorylation of YopE-ELK after translocation rather than translocation itself. Given the role of Ail as a cellular adhesin and the fact that MAP kinase pathways (the kinase that phosphorylates Elk-1) are known to be operating in cells affected by Yops (45), we prefer the explanation that Ail mediates efficient docking to the host cell membrane and Yop delivery.
Ail of pathogenic Yersinia sp. strains has been shown to mediate adhesion, invasion, autoaggregation, and serum resistance (3, 8, 28, 40, 50, 67). Although ail mutants are not sensitive to mouse serum (3, 65), it remains to be determined which of the other activities of Ail are critical for pathogenesis in mice. Our data indicate that one critical activity of Ail is to facilitate efficient Yop delivery to targeted host cells during infection. We propose that loss of Ail results in a defect in Yop delivery and in dramatically reduced virulence in vivo.
We note that the Δail mutant is not as defective for virulence (103- to 104-fold increase in LD50) as a Y. pestis strain lacking the pCD1 Yop-containing virulence plasmid (∼107-fold increase in LD50) (Felek and Krukonis, unpublished). We are currently investigating whether other Y. pestis surface molecules may play a role specifically in delivering Yops to phagocytic cells. Such a surface component may provide a residual level of Yop delivery in the absence of Ail.
In a recent study, Bartra et al. characterized an independently generated KIM5 Δail mutant. In their studies, using retro-orbital inoculation, they found no defect in virulence in Swiss Webster mice for the Δail mutant (3). We recently obtained this strain and tested it in our tail-vein injection Swiss Webster mouse model and found it to be attenuated (∼3,000-fold increase in LD50) similarly to our KIM5 Δail mutant (data not shown). It is unclear whether the alternative route of retro-orbital inoculation did not reveal the attenuating effect of the Δail mutation or whether the differences in our results are due to the way the bacteria were grown prior to inoculation. Under conditions of overnight growth at 28°C in HIB prior to tail-vein inoculation, both our KIM5 Δail strain and the KIM5 Δail strain of Bartra et al. were strongly attenuated. If Barta et al. grew their strains in a way to induce other adhesins that are redundant to Ail, they may not have observed the virulence defects of the ail mutant. We do find that although the calculated LD50 of the KIM5 Δail mutant is >3,000-fold higher than that of KIM5, at low-dose inoculations of 100 organisms, the Δail mutant can sometimes kill a few mice, even when the LD50 is calculated to be 28,000 organisms. We take this to mean that during infection, other adhesins can be induced in vivo. If those adhesins are induced early enough, we hypothesize that the attenuating defect of the Δail mutation is compensated for by the newly expressed adhesin.
Studies on the Y. pseudotuberculosis Ail protein suggested that it had no adhesive or invasive activity (67). Given that there are only two amino acid substitutions between Y. pseudotuberculosis and Y. pestis Ail proteins, we did not anticipate identifying Ail as a Y. pestis adhesin. In addition, it had been reported previously that Y. pestis ail in some strains is inactivated by an insertion element (61). Thus, we were surprised to identify Ail as the predominant adhesin in our transposon screen. Y. pestis Ail has also recently been shown to act as an adhesin by another group (28). We are currently investigating the nature of the different activities of Y. pseudotuberculosis Ail and Y. pestis Ail. The two amino acid differences in Ail from Y. pestis and Ail from Y. pseudotuberculosis are predicted to reside within surface-exposed loops 1 and 3 (3).
Another adhesin of Y. pestis is plasminogen activator Pla. Pla has been shown to mediate adhesion to the extracellular matrix (30, 34) and to cells (27) and also to mediate cellular invasion (17, 29). Much of the adhesion of KIM5 to HEp-2 cells is to the cell periphery or even the extracellular matrix surrounding the cells, especially in a KIM5 Δail mutant (Fig. 3). This suggests that Pla may also function as an adhesin in KIM5. We find that a KIM5 Δail Δpla mutant shows a drastic (85%) reduction in binding to HEp-2 cells and a similar decrease in cellular invasion (95% reduction) (S. Felek, unpublished results). We hypothesize that Pla was not identified in our transposon screen because it is encoded on the pPCP1 plasmid. Thus, there are multiple copies per cell. Our identification of Ail as an adhesin of Y. pestis is in agreement with the recent findings of Kolodziejek et al. (28), although the adhesion defects in our KIM5 Δail mutant are less dramatic than the defect they observed with their KIM6+ Δail mutant. Kolodziejek et al. also found a dramatic decrease in invasion with their KIM6+ Δail mutant. We feel that they may have observed such strong adhesion and invasion phenotypes because their KIM6+ strain lacks Pla. In fact, their strain list indicates that their KIM6+ strain lacks the pPCP1 plasmid.
Given the loss of virulence of the Δail mutant (Table 2), Ail may contribute to multiple steps of pathogenesis—as an adhesin during colonization, to deliver Yops to macrophages and neutrophils (the first line of defense against Y. pestis) and to exacerbate Yop-mediated tissue damage and necrosis of nonphagocytic cells (as represented by cytotoxicity on HEp-2 cells) (Fig. 3 and 5).
Histological and colonization studies indicate that infection by the KIM5 Δail mutant leads to greater immune cell infiltration and eventual clearance of the infection (Fig. 6 and 7). Previous studies with specific yop mutant strains of KIM5 also demonstrated clearance of the yop mutants following initial colonization, as well as distinct histopathology (58). In the spleen, a robust immune response against the ail mutant is also indicated by splenomegaly (increased spleen weight) (Fig. 8). It has been shown in a number of studies in vitro that YopJ plays a role in suppressing the immune response of host cells to Yersinia infections and can direct infected macrophages to undergo apoptosis (41, 45, 47). Not only do we see increased spleen weight in mice infected by a KIM5 Δail mutant, but also, spleens from the KIM5 (ail+) strain-infected mice undergo a decrease in spleen weight, perhaps due to apoptosis of splenocytes (Fig. 8). Thus, we feel that the inability of the Δail mutant to efficiently deliver Yops may lead to a more robust immune response to the infection. This is in contrast to some recent findings in the related pathogen Y. pseudotuberculosis. In a recent study, it was shown that yopE and yopH mutants of Y. pseudotuberculosis induce a less dramatic immune response than wild-type Y. pseudotuberculosis during an intragastric infection (36). In fact, the yop mutants were more readily cleared when coinoculated with the wild-type strain, due to increased immune stimulation by the wild-type strain (36). This clearing effect on yop mutants could even be mediated by inoculating the host with wild-type Y. pseudotuberculosis strains intraperitoneally (i.p.) while challenging the mice with the yop mutants intragastrically (36). While these results suggest that wild-type Y. pseudotuberculosis strains can induce inflammation when injected i.p., we would point out that we use a different species of Yersinia, Y. pestis, and a different route of inoculation (i.v.). Furthermore the lack of inflammation by yop mutants seen in the study by Logsdon and Mecsas occurs at the site of the intestine (36). If the yopE and yopH mutants were delivered to deeper tissues, they would likely be rapidly cleared, as has been demonstrated previously for other yop mutants (58). i.p. inoculation of mice with a yopE or yopH mutant might also have stimulated inflammation which would lead to the clearing of yopE and yopH mutants colonizing the intestine. Previous studies have revealed a delayed progression of yopE or yopH mutant infections to deeper tissues following intragastric inoculation (35). Thus, we feel that our study and the study by Logsdon and Mecsas have some distinctions that result in differential effects on inflammation and clearance of yop mutants (or in our case, an ail mutant unable to deliver Yops). It should also be noted that a recent study on the progression of Y. pestis and Y. pseudotuberculosis infections after intradermal injection indicated that by day 2, there were distinct differences in the way these two pathogens interact with the innate immune system, with Y. pseudotuberculosis stimulating a much more organized polymorphonuclear leukocyte response (24). Differences in the way specific Yersinia species are dealt with by the immune system may reflect distinctions in lipopolysaccharide-mediated immune stimulation (42, 51).
When Y. pestis is grown at 28°C, Ail is the predominant adhesin for Yop delivery (Fig. 3 to 5). In Y. pseudotuberculosis, it has been demonstrated that the nature of the adhesin and the specific signaling cascade that it triggers in host cells are critical in determining the efficiency of Yop delivery (38). It has been proposed that integrin-mediated signaling in host cells following Y. pseudotuberculosis invasin-integrin interaction results in a series of events leading to efficient YopB/YopD pore formation in host cells and subsequent T3SS-mediated Yop delivery (1, 2, 38, 62, 66). We hypothesize that since Y. pestis lacks invasin, Ail may mediate a receptor interaction that initiates a similar series of events leading to pore formation and Yop delivery. Further characterization of the role of Ail in Y. pestis Yop delivery may lead to the identification of steps in plague pathogenesis that are vulnerable to pharmaceutical intervention.
Acknowledgments
This work was supported by grants to E.S.K. from the University of Michigan Biomedical Research Council (BMRC), the University of Michigan Rackham Graduate School, and the University of Michigan Office of the Vice President for Research.
Susan Murray at the University of Michigan School of Public Health, Department of Biostatistics, assisted with statistical analysis of tissue colonization. Many thanks to Edward Krukonis for reading histology sections for pathology. Finally, we thank Michele Swanson and Victor DiRita for critical reading of the manuscript.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 8 December 2008.
REFERENCES
- 1.Alrutz, M. A., and R. R. Isberg. 1998. Involvement of focal adhesion kinase in invasin-mediated uptake. Proc. Natl. Acad. Sci. USA 9513658-13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alrutz, M. A., A. Srivastava, K. W. Wong, C. D'Souza-Schorey, M. Tang, L. E. Ch'Ng, S. B. Snapper, and R. R. Isberg. 2001. Efficient uptake of Yersinia pseudotuberculosis via integrin receptors involves a Rac1-Arp 2/3 pathway that bypasses N-WASP function. Mol. Microbiol. 42689-703. [DOI] [PubMed] [Google Scholar]
- 3.Bartra, S. S., K. L. Styer, D. M. O'Bryant, M. L. Nilles, B. J. Hinnebusch, A. Aballay, and G. V. Plano. 2008. Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein. Infect. Immun. 76612-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearden, S., J. Fetherston, and R. Perry. 1997. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect. Immun. 651659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Efraim, S., M. Aronson, and L. Bichowsky-Slomnicki. 1961. New antigenic component of Pasteurella pestis formed under specified conditions of pH and temperature. J. Bacteriol. 81704-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37515-527. [DOI] [PubMed] [Google Scholar]
- 7.Blake, M. S., C. M. MacDonald, and K. P. Klugman. 1989. Colony morphology of piliated Neisseria meningitidis. J. Exp. Med. 1701727-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bliska, J., and S. Falkow. 1992. Bacterial resistance to complement killing mediated by the Ail protein of Yersinia enterocolitica. Proc. Natl. Acad. Sci. USA 893561-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bliska, J. B., K. L. Guan, J. E. Dixon, and S. Falkow. 1991. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. USA 881187-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blomfield, I. C., M. S. McClain, J. A. Princ, P. J. Calie, and B. I. Eisenstein. 1991. Type 1 fimbriation and fimE mutants in Escherichia coli K-12. J. Bacteriol. 1735298-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd, A. P., N. Grosdent, S. Totemeyer, C. Geuijen, S. Bleves, M. Iriarte, I. Lambermont, J.-N. Octave, and G. R. Cornelis. 2000. Yersinia enterocolitica can deliver Yop proteins into a wide range of cell types: development of a delivery system for heterologous proteins. Eur. J. Cell Biol. 79659-671. [DOI] [PubMed] [Google Scholar]
- 12.Buchrieser, C., C. Rusniok, L. Frangeul, E. Couve, A. Billault, F. Kunst, E. Carniel, and P. Glaser. 1999. The 102-kilobase pgm locus of Yersinia pestis: sequence analysis and comparison of selected regions among different Yersinia pestis and Yersinia pseudotuberculosis strains. Infect. Immun. 674851-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burrows, T. W., and S. Jackson. 1956. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br. J. Exp. Pathol. 37577-583. [PMC free article] [PubMed] [Google Scholar]
- 14.Cathelyn, J. S., S. D. Crosby, W. W. Lathem, W. E. Goldman, and V. L. Miller. 2006. RovA, a global regulator of Yersinia pestis, specifically required for bubonic plague. Proc. Natl. Acad. Sci. USA 10313514-13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavigelli, M., F. Dolfi, F. X. Claret, and M. Karin. 1995. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 145957-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M.-P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 621315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowan, C., H. Jones, Y. Kaya, R. Perry, and S. Straley. 2000. Invasion of epithelial cells by Yersinia pestis: evidence for a Y. pestis-specific invasin. Infect. Immun. 684523-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day, J. B., F. Ferracci, and G. V. Plano. 2003. Translocation of YopE and YopN into eukaryotic cells by Yersinia pestis yopN, tyeA, sycN, yscB and lcrG deletion mutants measured using a phosphorylatable peptide tag and phosphospecific antibodies. Mol. Microbiol. 47807-823. [DOI] [PubMed] [Google Scholar]
- 20.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 1844601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felek, S., M. B. Lawrenz, and E. S. Krukonis. 2008. The Yersinia pestis autotransporter YapC mediates host cell binding, autoaggregation and biofilm formation. Microbiology 1541802-1812. [DOI] [PubMed] [Google Scholar]
- 22.Fernández, C., C. Hilty, S. Bonjour, K. Adeishvili, K. Pervushin, and K. Wuthrich. 2001. Solution NMR studies of the integral membrane proteins OmpX and OmpA from Escherichia coli. FEBS Lett. 504173-178. [DOI] [PubMed] [Google Scholar]
- 23.Galyov, E. E., S. Hakansson, A. Forsberg, and H. Wolf-Watz. 1993. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature 361730-732. [DOI] [PubMed] [Google Scholar]
- 24.Guinet, F., P. Ave, L. Jones, M. Huerre, and E. Carniel. 2008. Defective innate cell response and lymph node infiltration specify Yersinia pestis infection. PLoS ONE 3e1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayward, R. D., R. J. Cain, E. J. McGhie, N. Phillips, M. J. Garner, and V. Koronakis. 2005. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells. Mol. Microbiol. 56590-603. [DOI] [PubMed] [Google Scholar]
- 26.Kerschen, E. J., D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2004. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 724589-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kienle, Z., L. Emody, C. Svanborg, and P. O'Toole. 1992. Adhesive properties conferred by the plasminogen activator of Yersinia pestis. J. Gen. Microbiol. 1381679-1687. [DOI] [PubMed] [Google Scholar]
- 28.Kolodziejek, A. M., D. J. Sinclair, K. S. Seo, D. R. Schnider, C. F. Deobald, H. N. Rohde, A. K. Viall, S. S. Minnich, C. J. Hovde, S. A. Minnich, and G. A. Bohach. 2007. Phenotypic characterization of OmpX, an Ail homologue of Yersinia pestis KIM. Microbiology 1532941-2951. [DOI] [PubMed] [Google Scholar]
- 29.Lähteenmäki, K., M. Kukkonen, and K. T. Korhonen. 2001. The Pla surface protease/adhesin of Yersinia pestis mediates bacterial invasion into human endothelial cells. FEBS Lett. 50469-72. [DOI] [PubMed] [Google Scholar]
- 30.Lähteenmäki, K., R. Virkola, A. Saren, L. Emody, and T. K. Korhonen. 1998. Expression of plasminogen activator Pla of Yersinia pestis enhances bacterial attachment to the mammalian extracellular matrix. Infect. Immun. 665755-5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lathem, W. W., S. D. Crosby, V. L. Miller, and W. E. Goldman. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc. Natl. Acad. Sci. USA 10217786-17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindler, L., M. Klempner, and S. Straley. 1990. Yersinia pestis pH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect. Immun. 582569-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, F., H. Chen, E. M. Galvan, M. A. Lasaro, and D. M. Schifferli. 2006. Effects of Psa and F1 on the adhesive and invasive interactions of Yersinia pestis with human respiratory tract epithelial cells. Infect. Immun. 745636-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobo, L. A. 2006. Adhesive properties of the purified plasminogen activator Pla of Yersinia pestis. FEMS Microbiol. Lett. 262158-162. [DOI] [PubMed] [Google Scholar]
- 35.Logsdon, L. K., and J. Mecsas. 2003. Requirement of the Yersinia pseudotuberculosis effectors YopH and YopE in colonization and persistence in intestinal and lymph tissues. Infect. Immun. 714595-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logsdon, L. K., and J. Mecsas. 2006. The proinflammatory response induced by wild-type Yersinia pseudotuberculosis infection inhibits survival of yop mutants in the gastrointestinal tract and Peyer's patches. Infect. Immun. 741516-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marais, R., J. Wynne, and R. Treisman. 1993. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell 73381-393. [DOI] [PubMed] [Google Scholar]
- 38.Mejía, E., J. B. Bliska, and G. I. Viboud. 2008. Yersinia controls type III effector delivery into host cells by modulating Rho activity. PLoS Pathog. 414-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, V. L., K. B. Beer, G. Heusipp, B. M. Young, and M. R. Wachtel. 2001. Identification of regions of Ail required for the invasion and serum resistance phenotypes. Mol. Microbiol. 411053-1062. [DOI] [PubMed] [Google Scholar]
- 40.Miller, V. L., and S. Falkow. 1988. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect. Immun. 561242-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 1882127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montminy, S. W., N. Khan, S. McGrath, M. J. Walkowicz, F. Sharp, J. E. Conlon, K. Fukase, S. Kusumoto, C. Sweet, K. Miyake, S. Akira, R. J. Cotter, J. D. Goguen, and E. Lien. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 71066-1073. [DOI] [PubMed] [Google Scholar]
- 43.Morales, V. M., A. Backman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 9739-47. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee, S., G. Keitany, Y. Li, Y. Wang, H. L. Ball, E. J. Goldsmith, and K. Orth. 2006. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 3121211-1214. [DOI] [PubMed] [Google Scholar]
- 45.Orth, K., L. E. Palmer, Z. Q. Bao, S. Stewart, A. E. Rudolph, J. B. Bliska, and J. E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 2851920-1923. [DOI] [PubMed] [Google Scholar]
- 46.Orth, K., Z. Xu, M. B. Mudgett, Z. Q. Bao, L. E. Palmer, J. B. Bliska, W. F. Mangel, B. Staskawicz, and J. E. Dixon. 2000. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science 2901594-1597. [DOI] [PubMed] [Google Scholar]
- 47.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27953-965. [DOI] [PubMed] [Google Scholar]
- 48.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. G. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. F. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413523-527. [DOI] [PubMed] [Google Scholar]
- 49.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis-etiologic agent of plague. Clin. Microbiol. Rev. 1035-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pierson, D., and S. Falkow. 1993. The ail gene of Yersinia enterocolitica has a role in the ability of the organism to survive serum killing. Infect. Immun. 611846-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rebeil, R., R. K. Ernst, B. B. Gowen, S. I. Miller, and B. J. Hinnebusch. 2004. Variation in lipid A structure in the pathogenic yersiniae. Mol. Microbiol. 521363-1373. [DOI] [PubMed] [Google Scholar]
- 52.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 53.Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol. 4657-667. [DOI] [PubMed] [Google Scholar]
- 54.Rosqvist, R., M. Skurnik, and H. Wolf-Watz. 1988. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature 334522-525. [DOI] [PubMed] [Google Scholar]
- 55.Rubin, E. J., B. J. Akerley, V. N. Novik, D. J. Lampe, R. N. Husson, and J. J. Mekalanos. 1999. In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria. Proc. Natl. Acad. Sci. USA 961645-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao, F., and J. E. Dixon. 2003. YopT is a cysteine protease cleaving Rho family GTPases. Adv. Exp. Med. Biol. 52979-84. [DOI] [PubMed] [Google Scholar]
- 57.Simonet, M., B. Riot, N. Fortineau, and P. Berche. 1996. Invasin production by Yersinia pestis is abolished by insertion of an IS200-like element within the inv gene. Infect. Immun. 64375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Straley, S., and M. Cibull. 1989. Differential clearance and host-pathogen interactions of YopE− and YopK− YopL− Yersinia pestis in BALB/c mice. Infect. Immun. 571200-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strauch, E., I. Voigt, H. Broll, and B. Appel. 2000. Use of a plasmid of Yersinia enterocolitica biogroup 1A strain for the construction of cloning vectors. J. Biotechnol. 7963-72. [DOI] [PubMed] [Google Scholar]
- 60.Sundin, C., M. C. Wolfgang, S. Lory, A. Forsberg, and E. Frithz-Lindsten. 2002. Type IV pili are not specifically required for contact dependent translocation of exoenzymes by Pseudomonas aeruginosa. Microb. Pathog. 33265-277. [DOI] [PubMed] [Google Scholar]
- 61.Torosian, S., and R. Zsigray. 1996. The ail locus of Yersinia pestis EV76-51F is disrupted by IS285 insertions. Abstr. 96th Gen. Meet. Am. Soc. Microbiol., abstr. B-213.
- 62.Viboud, G. I., and J. B. Bliska. 2001. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 205373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vogt, J., and G. E. Schulz. 1999. The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Structure 71301-1309. [DOI] [PubMed] [Google Scholar]
- 64.Von Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36737-748. [DOI] [PubMed] [Google Scholar]
- 65.Wachtel, M., and V. Miller. 1995. In vitro and in vivo characterization of an ail mutant of Yersinia enterocolitica. Infect. Immun. 632541-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong, K.-W., and R. R. Isberg. 2005. Yersinia pseudotuberculosis spatially controls activation and misregulation of host cell Rac1. PLoS Pathog. 1e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, Y., J. Merriam, J. Mueller, and R. Isberg. 1996. The psa locus is responsible for thermoinducible binding of Yersinia pseudotuberculosis to cultured cells. Infect. Immun. 642483-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu, D., H. M. Ellis, E.-C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 975978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]