Abstract
Mycoplasma suis belongs to the hemotrophic mycoplasma group and causes infectious anemia in pigs. According to the present state of knowledge, this organism adheres to the surface of erythrocytes but does not invade them. We found a novel M. suis isolate that caused severe anemia in pigs with a fatal disease course. Interestingly, only marginal numbers of the bacteria were visible on and between the erythrocytes in acridine orange-stained blood smears for acutely diseased pigs, whereas very high loads of M. suis were detected in the same blood samples by quantitative PCR. These findings indicated that M. suis is capable of invading erythrocytes. By use of fluorescent labeling of M. suis and examination by confocal laser scanning microscopy, as well as scanning and transmission electron microscopy, we proved that the localization of M. suis was intracellular. This organism invades erythrocytes in an endocytosis-like process and is initially surrounded by two membranes, and it was also found floating freely in the cytoplasm. In conclusion, we were able to prove for the first time that a member of the hemotrophic mycoplasma group is able to invade the erythrocytes of its host. Such colonization should protect the bacterial cells from the host's immune response and hamper antibiotic treatment. In addition, an intracellular life cycle may explain the chronic nature of hemotrophic mycoplasma infections and should serve as the foundation for novel strategies in hemotrophic mycoplasma research (e.g., treatment or prophylaxis).
Mycoplasma suis is a member of the family Mycoplasmataceae. This organism belongs to a group of uncultivable highly specialized bacteria which parasitize the surface of erythrocytes of a variety of mammals (34). These species represent a distinct new cluster in the genus Mycoplasma and have been given the trivial name hemotrophic mycoplasmas (HM). Infections with HM are identified clinically by overt life-threatening hemolytic anemia or by subtle chronic anemia characterized by infertility, immune suppression, and greater susceptibility to infections (34). It is noteworthy that organisms that morphologically resemble HM have also been detected in the blood of humans (1, 8, 42, 50).
M. suis causes febrile acute icteroanemia in pigs (IAP), which is accompanied by high numbers of M. suis cells in the blood, as confirmed by microscopy as well as by PCR (18, 21, 34). Clinical symptoms are successfully cured by treatment with tetracycline. Nevertheless, once pigs are infected with M. suis, they remain lifelong carrier animals and therefore are epidemiologically important (19). Chronic M. suis infections result in reproductive disorders in sows, growth retardation in piglets, and increased susceptibility to respiratory and enteric infections in feeder pigs. M. suis occurs worldwide, and chronic IAP, in particular, is of major economic importance (19).
Contrary to the well-established clinical picture of IAP (i.e., high morbidity and low mortality), we recently observed an increased incidence of acute IAP in feeder pigs with a predominantly fatal outcome despite antibiotic therapy. And contrary to the expected high numbers of M. suis cells on and between the erythrocytes in acridine orange-stained blood smears (the established diagnostic feature of acute IAP), only marginal numbers of bacterial cells were observed. This microscopic finding conflicted with the results of quantitative real-time PCR, which detected high numbers of M. suis cells (109 to 1010 cells per ml of blood) in the same blood samples. The striking difference between the microscopy and PCR results raises the issue of putative intracellular localization of a novel M. suis isolate within the erythrocytes.
To date, several Mycoplasma species are known to invade cells (2, 28, 33, 46); for example, M. penetrans invades the epithelial cells of the human urogenital tract (28), and M. genitalium infects human lung fibroblasts (2, 33). M. gallisepticum can invade nonphagocytic cell lines, such as HeLa cells and chicken embryo fibroblasts (49), and is the only known Mycoplasma species that is able to invade erythrocytes (47). The general advantages associated with invasion of eukaryotic cells by bacterial pathogens include protection from the immune system, reduction in the efficacy of antibiotics during treatment, and nutritional benefits. The intraerythrocytic localization of M. suis might provide the organism with a supply of iron, large amounts of which can be found inside the red blood cells (RBCs) in the form of hemin, or other trace metals (47). It is known that hemin can support the growth of invasive bacteria, such as Bartonella quintana (43). Moreover, the persistence of some Bartonella species is directly linked to nonhemolytic erythrocyte parasitism (43). However, no influence of hemin on the growth of mycoplasmas has been demonstrated so far.
It is interesting that hemotrophic mycoplasmas have a tendency to establish chronic infections that often are not apparent clinically (34). The long persistence of M. suis (and other HM) might be linked to their ability to invade the cytoplasm of host cells. Dallo and Basemann (6) assumed that the chronic nature of mycoplasma infections (M. pneumoniae, M. penetrans, M. genitalium) can be explained by a subpopulation of mycoplasmas that enter mammalian cells, where they can persist in a latent or nongrowth maintenance form. Such biological latency would circumvent the killing action of antimicrobials. The usual establishment of chronic disease in M. gallisepticum-infected birds and the limited effects of antibiotic treatments (44) have also been linked to the ability of this agent to enter nonphagocytic host cells (35, 49).
In order to confirm our hypothesis that M. suis invades blood cells, splenectomized pigs were experimentally infected with a novel M. suis isolate. The clinical course was monitored, and blood samples were taken during the experiments. Furthermore, we used fluorescent labeling of M. suis coupled to confocal laser scanning microscopy (CLSM), as well as scanning electron microscopy (SEM) and transmission electron microscopy (TEM), in order to investigate the intracellular life cycle of M. suis.
MATERIALS AND METHODS
Bacterial isolate and experimental infection.
M. suis isolate 08/07 originated from a representative pig in a stock of feeder animals that had severe IAP infections with high mortality. The pig used suffered from acute IAP. A splenectomized pig model was used to maintain the new M. suis isolate, as described elsewhere (16). Briefly, 5-week-old pigs (n = 10) were screened to make sure that they were M. suis negative by quantitative PCR and M. suis immunoblotting as described previously (18, 20). Pigs were splenectomized by using the method of Heinritzi (16). For infection, 2 ml of the infected animal's blood was inoculated intramuscularly. Clinical symptoms, feeding behavior, and body temperature were monitored at least daily for each pig. Blood samples were taken prior to infection (day 0) and then weekly until necropsy, as well as when there were clinical attacks. Clinical diagnosis of acute IAP was confirmed by quantitative PCR and M. suis immunoblotting, as well as by microscopic detection of M. suis cells in blood smears stained with acridine orange. Briefly, blood smears obtained using anticoagulated blood were fixed in ethanol for 1 min and air dried. Staining was done by using an acridine orange solution (0.25 μg/ml) for 1 h in the dark. The slides were rinsed with water and air dried. M. suis cells on and between the erythrocytes were detected using UV light excitation with a fluorescence microscope. When there were acute clinical attacks, pigs were treated with tetracycline (40 mg/kg of body weight) and glucose (35 g glucose/liter of drinking water) to prevent death caused by M. suis 08/07-induced hypoglycemia.
Hematological investigations.
The erythrocyte counts, hemoglobin concentrations, and hematocrit levels of acutely diseased pigs infected with M. suis 08/07 were determined using blood that was anticoagulated by EDTA and an automatic blood cell counter (Celltek; Bayer Diagnostics, Munich, Germany). Serum was obtained by centrifugation of a serum monovette (Hettich Rotixa/AP; Hettich, Tuttlingen, Germany). Glucose and bilirubin levels were determined in the laboratory of the farm animal clinic of Ludwig Maximilians University of Munich by using a selective, discrete, multiple-analysis system (Hitachi 911; Roche, Mannheim, Germany). The values obtained were compared to data obtained from classical M. suis infections (17). Reference values (erythrocyte counts, hematocrit levels, hemoglobin concentrations) for healthy pigs were obtained from previously published data of Kraft and coworkers (25). Reference ranges for bilirubin and blood glucose levels were obtained from the data of Kixmoeller et al. (24).
Quantitative real-time PCR.
M. suis DNA was quantified using the LightCycler 2.0 system (Roche Diagnostics, Rotkreuz, Switzerland), as described previously (20). Primers msg1-Fw (5′-ACAACTAATGCACTAGCTCCTATC-3′) and msg1-Rv (5′-GCTCCTGTAGTTGTAGGAA TAATTGA-3′), as well as probes 5′-TTCACGCTTTCACTTCTGACCAAAGAC-fluorescein-3′ and 5′-LCRed-640-CAAGACTCTCCTCACTCTGACCTAAGAAGAGC-3′, were purchased from Metabion (Martinsried, Germany). Real-time PCR was performed using a LightCycler Fast Start DNA MasterPLUS hybridization probe kit (Roche Diagnostics) with 0.5 μM of each primer, as well as 0.2 μM of each probe. For quantification, M. suis genomic DNA was extracted from experimentally infected pigs and quantified as described previously (20).
Antibody reagents.
Polyclonal monospecific antisera for the detection of M. suis by immunostaining were obtained by immunization of rabbits with recombinant HspA1, as described elsewhere (21). HspA1 is a surface protein of M. suis (21).
CLSM.
For staining of the erythrocyte surface and differential staining of intracellular and extracellular M. suis, a modified version of the double-immunofluorescence (DIF) method adapted for use with erythrocytes (47) was used, with the following modifications. Peripheral anticoagulated blood samples (40 μl) were diluted in 2.5 ml phosphate-buffered saline (PBS) containing 10 mM glucose and 0.1% bovine serum albumin (BSA). Cells were fixed in 4% PBS-buffered paraformaldehyde (Sigma-Aldrich, Steinheim, Germany) containing 0.01% glutaraldehyde (grade I; Sigma-Aldrich, Buchs, Switzerland) and seeded onto poly-l-lysine-coated glass slides (SuperFrost; Menzel, Braunschweig, Germany). Nonreacted aldehydes were blocked with 0.1 M glycine (Carl Roth, Karlsruhe, Germany) in PBS for 20 min. Nonspecific binding of antibodies was reduced by incubation of samples in blocking buffer (3% BSA in PBS) for 30 min. The erythrocyte surface and extracellular M. suis cells were stained with purified mouse anti-pig CD235a (glycophorin A) monoclonal antibody (1:100; PharMingen, BD Biosciences, Europe) and rabbit monospecific anti-HspA1 serum (1:100) for 1 h, followed by tetramethyl rhodamine isocyanate (TRITC)-conjugated goat anti-mouse immunoglobulin G (IgG) (1:100, Sigma) and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (Sigma), respectively, for 1 h. The cells were permeabilized with 0.1% Triton X-100 (Sigma) in PBS for 10 s and incubated in blocking buffer for 30 min. Intracellular M. suis cells were detected after incubation with rabbit monospecific anti-HspA1 serum for 1 h and Alexa Fluor 405-labeled goat anti-rabbit IgG (Molecular Probes, Basel, Switzerland) for 1 h. The staining procedure resulted in Alexa Fluor 405-labeled intracellular bacteria (blue) and FITC- and Alexa Fluor 405-labeled extracellular bacteria (blue and green). Confocal microscopy was performed with a Leica SP2 confocal microscope (Leica Microsystems, Mannheim, Germany).
Colocalization measurement.
Colocalization of two labels indicates that they are located close enough to each other in the sample that they cannot be resolved optically. The colocalization of two labels was measured using the “Colocalization” module of Imaris 5.0.2 (64-bit version; Bitplane AG, Switzerland) (www.bitplane.com). When this application was used, extracellular M. suis cells appeared to be white since they were double labeled with both secondary fluorescent conjugates, as described above. Intracellular bacteria were stained with a single stain and were blue.
TEM.
Blood samples were diluted in PBS containing 10 mM glucose and 0.1% BSA and fixed in a 1% glutaraldehyde solution. Cells were washed consecutively in PBS and 0.05 M cacodylate buffer and suspended in the latter buffer. Following postfixation in a mixture of 1% osmium tetroxide (Fluka Chemie, Buchs, Switzerland) and 1.5% potassium ferrocyanide (Fluka), cells were washed once in 0.05 M cacodylate buffer. Samples were embedded in molten 2.5% agar (Difco Laboratories, Detroit, MI) for dehydration using increasing concentrations of ethanol (70 to 100%) and propylene oxide (Fluka Chemie). During further processing samples were embedded in Epon 812 (Fluka Chemie) and sectioned. Grids with ultrathin sections were contrasted consecutively with 4% uranyl acetate (Fluka Chemie) and lead citrate as described elsewhere (41) and were analyzed with a Philips CM100 transmission electron microscope.
SEM.
Blood samples were diluted in PBS containing 10 mM glucose and 0.1% BSA and fixed in a 1% glutaraldehyde solution. Cells were settled on carbon-coated coverslips (Assistant, Sondheim, Germany) using a Cytospin 2 (Shandon, Dako-Diagnostica, Zug, Switzerland) centrifuge, followed by dehydration using increasing concentrations of acetone and critical point drying (BAL-TEC CPD 030 critical point dryer; Balzers, Liechtenstein). Finally, the samples were sputter coated with 3 nm of gold using the BAL-TEC MED 020 coating system and analyzed with a Zeiss Supra 50 VP scanning electron microscope.
Array tube hybridization.
M. suis genomic DNA was extracted from experimentally infected pigs and quantified, as previously described (20). To exclude the possibility that there were bacterial contaminants in the blood, the 16S rRNA gene was amplified using universal primers and sequenced (26). The sequences obtained were compared with data bank entries.
Genomic DNA (10 to 100 ng) was labeled by using a random primed polymerization reaction and Sequenase (version 2.0; USB Corporation, Cleveland, OH) as described previously (39). The protocol used for the labeling reaction was a modified protocol (modified by the DeRisi Laboratory, University of California, San Francisco, in June 2001) based on the method of Bohlander and coworkers (4). All reactions were carried out using an Eppendorf Mastercycler gradient (Vaudaux-Eppendorf, Schönenbuch, Switzerland). The DNA microarray used to detect 90 antibiotic resistance genes of gram-positive bacteria (39) was kindly provided by Vincent Perreten, Institute of Veterinary Bacteriology, University of Bern. DNA hybridization was carried out with a Thermomixer comfort instrument (Eppendorf AG, Hamburg, Germany), and the peroxidase staining procedure and online detection were performed using an atr01 array tube reader (Clondiag, Jena, Germany) as described by Perreten and coworkers (39). The data were analyzed and processed using the Iconoclust software (Clondiag).
RESULTS
Clinical manifestations and bacteriology of M. suis novel isolate 08/07.
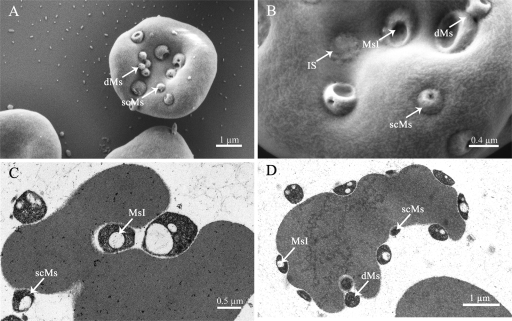
For blood samples taken from experimental pigs on day 3 postinfection (p.i.), no M. suis cells were found in acridine orange-stained blood smears examined microscopically (Fig. 1A). In SEM, only single erythrocytes were marked by sporadically attached M. suis 08/07 cells (Fig. 1B). These microscopic findings corresponded both to the lack of symptoms in the animals and to the PCR detection of low numbers of M. suis cells (approximately 103 bacterial cells per ml of blood). With the clinical onset of acute IAP on day 7 p.i., numerous M. suis particles were found on and between the erythrocytes in acridine orange-stained blood smears, as well as by SEM (Fig. 1C and D). Two different morphological forms of M. suis were detected: smaller coccoid cells ranging from 0.2 to 0.4 μm in diameter and large rounded flattened cells up to 1 μm in diameter (Fig. 2). In SEM, larger forms had one or two conspicuous invaginations in the cell membrane (Fig. 2A and B). In TEM, these invaginations appeared to be vacuole-like structures (Fig. 2C and D). Smaller coccoid forms often appeared as a cluster of cells and might have resulted from cell division on the erythrocyte surface (Fig. 2A). In this stage of severe bacteremia, the animals suffered from febrile anemia, hypoglycemia, and bilirubinemia, as shown in Table 1. Quantification by real-time PCR revealed a bacterial load of 1010 to 1011 M. suis cells per ml of blood.
FIG. 1.
Acridine orange-stained blood smears from pigs infected with M. suis 08/07 (A, C, and E) and corresponding SEM images (B, D, and F). On day 3 p.i. no mycoplasmas were detected in acridine orange-stained (A) blood smears, whereas in SEM single M. suis cells were visible (B). During acute clinical attack on day 7 p.i. numerous M. suis cells were identified on the erythrocytes by fluorescence microscopy (C) and SEM (D). Both methods revealed a significant reduction in the number of M. suis cells on the surface of RBCs on day 11 p.i. (E and F). Ms, M. suis. (A, C, and E) Magnification, ×1,000. (B, D, and F) Bar = 2 μm.
FIG. 2.
SEM (A and B) and TEM (C and D) micrographs of M. suis 08/07-infected RBCs. The different morphological forms and features of M. suis 08/07 are shown. dMs, dividing M. suis; scMs, small coccoid M. suis; MsI, M. suis invagination; IS, invasion scar.
TABLE 1.
Hematological investigation of pigs infected either with the “classical” isolate or with M. suis 08/07
| Reference range or isolate | Erythrocyte count (1012 cells/liter)
|
Hemoglobin concn (mmol/liter)
|
Hematocrit concn (liter/liter)
|
Glucose concn (mmol/liter)
|
Bilirubin concn (μmol/liter)
|
M. suis load (cells/ml blood)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference range | Mean (SD) | Reference range | Mean (SD) | Reference range | Mean (SD) | Reference range | Mean (SD) | Reference range | Mean (SD) | Reference range | Mean (range) | |
| Reference range | 5.80-8.10a | 6.70-9.18a | 0.33-0.45a | 3.80-6.70b | <4.3b | 0 | ||||||
| “Classical” isolate | 2.58 (0.288)c | 3.81 (0.313)c | 0.21 (0.025)c | 2.40 (0.93)c | 9.06 (4.81)c | 1.5 × 106 (3.50 × 104 to 7.90 × 108)d,e | ||||||
| M. suis 08/07f | 1.76 (0.582) | 2.98 (0.857) | 0.12 (0.053) | 0.60 (1.523) | 24.50 (10.709) | 1.8 × 1011 (1.73 × 109 to 8.85 × 1012)e | ||||||
Reference range according to Kraft and coworkers (25).
Reference range according to Kixmoeller and coworkers (24).
Value for pigs infected with a classical M. suis isolate according to Heinritzi (17) (n = 42).
Value according to Hoelzle and coworkers (20).
The standard deviation was not determined.
Data from this study (n = 10).
Due to the life-threatening effect of the IAP attack, tetracycline therapy and glucose infusion over a 5-day period were inevitable. The animals, however, did not respond to the therapy, and the severe clinical IAP continued. To examine the cause of this therapy failure, we performed a microarray-based detection assay used to screen for antibiotic resistance genes in gram-positive bacteria. However, hybridization analysis of M. suis 08/07 genomic DNA with the microarray revealed no common tetracycline resistance genes.
Especially remarkable on day 11 p.i. were the different sizes (anisocytosis) of the erythrocytes and the formation of echinocytes, as observed by fluorescent examination. Compared to blood samples taken on day 7 p.i., blood samples taken from diseased animals on day 11 p.i. showed a clear reduction in the number of visible M. suis cells in acridine orange-stained smears, as well as in SEM (Fig. 1E and F). As determined by real-time PCR, the high bacterial loads (1010 to 1011 M. suis cells per ml of blood) persisted. The striking discrepancy between the microscopy results on the one hand and the PCR results on the other hand suggests that M. suis 08/07 might enter the erythrocytes after day 7 p.i., thus escaping detection by surface scanning microscopy.
Localization of M. suis within porcine erythrocytes.
In order to investigate whether M. suis 08/07 is able to invade porcine erythrocytes, blood samples from experimental pigs were analyzed in detail by using SEM, TEM, and CLSM.
The interaction of M. suis and erythrocytes is marked by the formation of invaginations of variable depth in the erythrocyte membrane (Fig. 2A to D and 3A). In addition to superficially attached M. suis cells, the agents were also found in shallow pits. It is noteworthy that deep invaginations in which M. suis was surrounded almost completely by the erythrocyte membrane (Fig. 2C and D and 3A) occurred sporadically. In addition, in the M. suis-containing sections with deep invaginations, as shown by TEM, there were vacuoles in which the organism was entirely enclosed and therefore located in the RBC cytoplasm (Fig. 3A and D). M. suis cells in deep invaginations were covered by membrane material (Fig. 2B and 3B and C), leading to the formation of invasion scars (Fig. 2B).
FIG. 3.
Invasion of porcine erythrocytes by M. suis 08/07 shown by SEM (B) and TEM (A and C to F). During invasion M. suis 08/07 is located in deep invaginations of the RBC membrane (A). As invasion progresses, the erythrocyte membrane conforms to the shape of the bacterial cells (A and C), and newly formed membrane material covers the surface of the bacteria (B and C). As a result, invading mycoplasmas are located within an intraerythrocytic vacuole (D). Intracellular forms that are free in the RBC cytoplasm (E) have a shape and size similar to the shape and size of extracellular attached M. suis cells (F). exMs, extracellular M. suis; inMs, intracellular M. suis; IS, invasion scar; Ms, M. suis; MsV, M. suis vacuole; RBCC, RBC cytoplasm.
We also detected intracellular mycoplasma cells which were not surrounded by the vacuole membrane (Fig. 3E), indicating that these bacterial cells were free in the cytoplasm. These free intracellular M. suis cells were approximately 300 to 500 nm in diameter, like extracellular attached M. suis cells (Fig. 3F), and they had a similar morphology.
The intracellular localization of M. suis was confirmed by a DIF technique (Fig. 4). Using this method, extra- and intracellular mycoplasmas were distinguished by consecutive incubation of infected erythrocytes with M. suis-specific antisera and different fluorescently labeled secondary antibodies before and after chemical permeabilization of the erythrocyte membrane. Bacteria with both green and blue marks were extracellular bacteria, whereas bacteria that stained only blue were intracellular (Fig. 4.). In superimposed images (Fig. 4D) green and blue fluorescence signals appeared to be light blue. By using this method we clearly showed that M. suis invaded the porcine erythrocytes. Uninfected porcine erythrocytes that were stained in parallel and served as negative controls did not exhibit any fluorescence signals (data not shown). Figure 5 shows erythrocytes that had a green label (FITC) due to glycophorin A. We used the Imaris Colocalization software to analyze whole stacks of confocal sections of erythrocytes that were double labeled for extra- and intracellular M. suis cells. When this method was used, extracellular M. suis cells stained red and blue with TRITC and Alexa Fluor 405 appeared to be white, while intracellular M. suis cells were blue. This method allowed definite discrimination of double-stained (extracellular) and single-stained mycoplasmas, confirming the intraerythrocytic localization of M. suis.
FIG. 4.
CSLM of porcine RBCs infected with M. suis 08/07 after DIF staining. The same area of a confocal microscopic image was analyzed to visualize extracellular M. suis labeled with FITC (green fluorescence) (B) and extra- and intracellular M. suis labeled with Alexa Fluor 405 (blue fluorescence) (C). Erythrocytes had a red label due to glycophorin A (A). (D) Superimposition of the green and blue fluorescence images resulted in white fluorescence, indicating extracellular M. suis cells, while the blue fluorescence indicates intraerythrocytic mycoplasma cells.
FIG. 5.
RBC from an experimental pig infected with M. suis 08/07 after DIF staining: six focal scans from a stack from the top (A) to the bottom (F). Erythrocytes have a green label due to glycophorin A. As determined with a colocalization tool (Imaris), extracellular M. suis cells stained with TRITC and Alexa Fluor 405 appear to be white, while intracellular M. suis cells are blue (Alexa Fluor 405).
DISCUSSION
Our clinical data for experimentally infected splenectomized pigs revealed that M. suis 08/07 is more virulent than “classical” M. suis isolates. All infected pigs developed severe life-threatening hemolytic anemia (significant lower erythrocyte counts and hemoglobin and hematocrit levels), which was not affected by the antibiotic therapy. Despite the fact that as determined by SEM the number of extraerythrocytic attached M. suis cells on day 11 was less than the number on day 7, the bacterial load (1010 to 1011 M. suis cells per ml of blood) was the same. These findings strongly indicated that there was intraerythrocyte localization of M. suis. The HM have previously been described as exclusively epicellular organisms (18, 40, 42), which was the basis for their initial designation as Eperythrozoon suis before they were reclassified in the genus Mycoplasma (37, 38, 45).
In order to define the localization of bacteria inside eukaryotic cells, the two approaches generally used are (i) the gentamicin invasion assay (9, 23, 49), which is a semiquantitative method for comparison of the invasion frequencies of different mycoplasma populations, and (ii) the DIF labeling technique (47, 49) coupled to CLSM, which provides simple and accurate differentiation between intracellular and extracellular mycoplasmas. As the gentamicin invasion assay cannot be used for examination of uncultivable bacteria, such as M. suis, for verification of an intracellular life cycle we had to rely on the DIF assay combined with CLSM, as well as on SEM and TEM. By using these methods we showed for the first time that a member of the HM group is able to invade erythrocytes. The reproducibility of the DIF assay was proved by the results of several repeated experiments performed with the blood of M. suis 08/07-infected pigs, and the data left no doubt concerning the intracellular localization of this bacterium.
Given the fact that acridine orange is a cell-permeable nucleic acid stain, one would expect intraerythrocytic M. suis to be detected by this technique. However, against the intense autofluorescence background of erythrocytes after chemical fixation with ethanol and staining with acridine orange, it is virtually impossible to differentiate the restrained light emission of intracellular M. suis.
Erythrocytes are markedly nonendocytic cells (15) and therefore are suitable hosts for studying intracellular parasitism and potential alternative pathways. In our case, entry of M. suis into the RBCs seemed to begin with invagination of the erythrocyte membrane. As invasion progressed, the depression in the erythrocyte deepened and conformed to the shape of the pathogen. Upon entry, the orifice of the invaginated erythrocyte membrane apparently fused. As a result, M. suis was found in intracellular vacuoles within the erythrocytes.
A similar endocytosis-like mechanism of erythrocyte invasion has been described for Plasmodium falciparum in malaria (14) and for Bartonella bacilliformis, which is responsible for human Oroya fever (3). The finding that M. suis was located within vacuole-like structures indicates that the bacteria must initiate and mediate the process leading to their uptake by the RBCs. The formation of deep invaginations during entry is therefore aided either by mechanical forces generated by the adhering bacteria or by cellular factors secreted by M. suis. In B. bacilliformis a small, hydrophobic molecule named deformin was identified, which causes deformation and invagination of RBC membranes, leading to the formation of intraerythrocytic vacuoles formed by a process similar to endocytosis (32).
In a study carried out by Murphy and coworkers, the chemical composition of drug-induced endovesicle membranes of erythrocytes was compared with that of the host-derived parasitophorous vacuolar membrane in malaria-infected RBCs (36). The results clearly showed that in pathogen-induced endovacuolation specific raft and cytoskeletal proteins, as well as lipids, are actively excluded from the vacuole, e.g., PIP2, which displays a major phosphoinositide in erythrocyte membranes influencing membrane junctional complex stability. Interestingly, PIP2 is present in the drug-induced endovesicle whereas it cannot be found in the parasitophorous membrane, suggesting an unknown erythrocyte phospholipid remodeling in the malarial vacuole during invasion of P. falciparum. Further studies are necessary to examine whether the M. suis-induced endovacuole has a composition similar to that of the parasitophorous vacuolar membrane in malaria.
Sometimes we found that M. suis cells were located free in the RBC cytoplasm. These bacterial cells might have corresponded to M. suis cells that either used a different mechanism for erythrocyte invasion or escaped from the initial vacuole. Further investigation (e.g., staining of infected erythrocytes with FM4-64 coupled to CLSM) will be done to clearly distinguish M. suis located within RBC-derived vacuoles from free forms located in the RBC cytoplasm. FM4-64 is an amphiphilic plasma membrane stain used to investigate endocytosis and vesicle trafficking in living eukaryotic cells.
The central considerations arising from our observations are unquestionable. Does the intraerythrocytic lifestyle of M. suis provide any benefit to the organism, and are there any hints that it has an impact on the life cycle of this organism?
Obviously, the intracellular localization of M. suis can prevent damage of the bacteria by antibiotics. This conclusion is supported by the fact that it was impossible to cure the high level of bacteremia with tetracycline, the antibiotic which is generally used to treat pigs with acute IAP. Tetracycline is, however, the drug of choice for elimination of obligate intracellular bacteria (22, 29). Thus, it is more probable that M. suis developed tetracycline resistance mechanisms. In a study of M. synoviae, an increase in resistance to enrofloxacin could be demonstrated after several in vivo treatments of experimentally infected hens (27). However, when we used a microarray-based detection assay used to screen for antibiotic resistance genes in gram-positive bacteria (39), we did not find any common tetracycline resistance genes (tetP, tetS, tetT, tetU, tetW, tetZ, tetK, tetL, and tetM) in M. suis. Therefore, M. suis 08/07 might have unknown or uncommon resistance mechanisms.
The presence of M. suis within porcine erythrocytes nonetheless has significant clinical implications. It is possible that the internalized bacteria are able to exploit host cell functions and elude host defense mechanisms, such as complement-mediated lysis and circulating phagocytic cells and antibodies (5, 6, 10, 13). Moreover, an intraerythrocytic localization of M. suis aggravates the natural turnover of infected RBCs in the spleen (30). The RBCs carrying only cytoplasmic M. suis cells do not have modified surfaces and cannot be recognized by spleen macrophages. This conclusion was supported by the findings obtained with experimentally infected pigs that obviously had constant numbers of RBCs that were invaded by M. suis in the peripheral blood and the duration of the severe clinical symptoms. Nevertheless, further verification is needed.
The bacterial invasion of erythrocytes is a special feature that has been described previously only for members of the genera Bartonella (3, 7, 31, 43) and Anaplasma (12) and for M. gallisepticum (47). In contrast to the establishment of a persistent stage of Bartonella, erythrocyte invasion by M. suis 08/07 leads to severe hemolysis and to greater virulence. Furthermore, in contrast to previously described infections with common M. suis isolates, a chronic infection is not established upon infection with M suis 08/07 due to the fatal course of the disease. To further define whether internalization of M. suis occurs in naturally chronically infected pigs and the potential impact of this phenomenon in these animals, TEM studies, as well as CSLM, will have to be performed with samples collected from such pigs. Detection of intracellular M. suis bacteria in asymptomatic persistently infected pigs would be a milestone in research on HM, as it would help explain the long-term survival of the organisms and the associated persistence.
We found many erythrocytic precursor cells in the circulating blood using TEM. It is well known from former studies that RBC precursors can be found in the circulation of patients with chronic and acute anemia (48) and in animals infected with HM (11). However, the presence of M. suis-infected reticulocytes and normoblasts in the bloodstream is a novel finding for the pathogenesis of HM (data not shown). Further investigations of the bone marrow of infected pigs should reveal whether M. suis 08/07 can propagate within the blood-building tissue.
In conclusion, this is the first report to show that a member of the HM group is able to invade the erythrocytes of its host. Our studies clearly showed that the intracellular life cycle of M. suis dramatically increased the virulence of the organism. Further studies are required to understand the cellular interactions and factors that contribute to host cell invasion by M. suis 08/07 and to investigate the ability of common M. suis isolates to invade erythrocytes.
Acknowledgments
We thank Gery Barmettler for assistance with electron microscopic preparation of our blood samples.
This work was supported in part by the Forschungskredit of the University of Zurich.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Archer, G. L., P. H. Coleman, R. M. Cole, R. J. Duma, and C. L. Johnston, Jr. 1980. Hemotropic bacteria. N. Engl. J. Med. 3021151-1152. [DOI] [PubMed] [Google Scholar]
- 2.Baseman, J. B., M. Lange, N. L. Criscimagna, J. A. Giron, and C. A. Thomas. 1995. Interplay between mycoplasmas and host target cells. Microb. Pathog. 19105-116. [DOI] [PubMed] [Google Scholar]
- 3.Benson, L. A., S. Kar, G. McLaughlin, and G. M. Ihler. 1986. Entry of Bartonella bacilliformis into erythrocytes. Infect. Immun. 54347-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohlander, S. K., R. Espinosa III, M. M. Le Beau, J. D. Rowley, and M. O. Diaz. 1992. A method for the rapid sequence-independent amplification of microdissected chromosomal material. Genomics 131322-1324. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, N. R. 1988. Complement and infectious agents. Rev. Infect. Dis. 10(Suppl. 2)S447-S449. [DOI] [PubMed] [Google Scholar]
- 6.Dallo, S. F., and J. B. Baseman. 2000. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb. Pathog. 29301-309. [DOI] [PubMed] [Google Scholar]
- 7.Dehio, C. 2004. Molecular and cellular basis of bartonella pathogenesis. Annu. Rev. Microbiol. 58365-390. [DOI] [PubMed] [Google Scholar]
- 8.Duarte, M. I., M. S. Oliveira, M. A. Shikanai-Yasuda, O. N. Mariano, C. F. Takakura, C. Pagliari, and C. E. Corbett. 1992. Haemobartonella-like microorganism infection in AIDS patients: ultrastructural pathology. J. Infect. Dis. 165976-977. [PubMed] [Google Scholar]
- 9.Elsinghorst, E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236405-420. [DOI] [PubMed] [Google Scholar]
- 10.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276718-725. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick, J. L., R. C. J. Barron, L. Andrew, and H. Thompson. 1998. Eperythrozoon ovis infection of sheep. Comp. Haematol Int. 8230-234. [Google Scholar]
- 12.Foote, L. E., J. C. Geer, and Y. E. Stich. 1958. Electron microscopy of the Anaplasma body: ultra-thin sections of bovine erythrocytes. Science 128147-148. [DOI] [PubMed] [Google Scholar]
- 13.Gotschlich, E. C. 1983. Thoughts on the evolution of strategies used by bacteria for evasion of host defenses. Rev. Infect. Dis. 5(Suppl. 4)S778-S783. [DOI] [PubMed] [Google Scholar]
- 14.Haldar, K., and N. Mohandas. 2007. Erythrocyte remodeling by malaria parasites. Curr. Opin. Hematol. 14203-209. [DOI] [PubMed] [Google Scholar]
- 15.Haldar, K., B. U. Samuel, N. Mohandas, T. Harrison, and N. L. Hiller. 2001. Transport mechanisms in Plasmodium-infected erythrocytes: lipid rafts and a tubovesicular network. Int. J. Parasitol. 311393-1401. [DOI] [PubMed] [Google Scholar]
- 16.Heinritzi, K. 1984. A contribution on splenectomy in swine. Tierarztl. Prax. 12451-454. [PubMed] [Google Scholar]
- 17.Heinritzi, K. H. 1990. Untersuchungen zur Pathogenese und Diagnostik der Infektion mit Eperythrozoon suis. Professorial dissertation. Ludwig Maximilians University of Munich, Munich, Germany.
- 18.Hoelzle, L. E. 2008. Haemotrophic mycoplasmas: recent advances in Mycoplasma suis. Vet. Microbiol. 130215-226. [DOI] [PubMed] [Google Scholar]
- 19.Hoelzle, L. E. 2007. Significance of haemotrophic mycoplasmas in veterinary medicine with particular regard to the Mycoplasma suis infection in swine. Berl. Muench. Tieraerztl. Wochenschr. 12034-41. [PubMed] [Google Scholar]
- 20.Hoelzle, L. E., M. Helbling, K. Hoelzle, M. Ritzmann, K. Heinritzi, and M. M. Wittenbrink. 2007. First LightCycler real-time PCR assay for the quantitative detection of Mycoplasma suis in clinical samples. J. Microbiol. Methods 70346-354. [DOI] [PubMed] [Google Scholar]
- 21.Hoelzle, L. E., K. Hoelzle, A. Harder, M. Ritzmann, H. Aupperle, H. A. Schoon, K. Heinritzi, and M. M. Wittenbrink. 2007. First identification and functional characterization of an immunogenic protein in unculturable haemotrophic mycoplasmas (Mycoplasma suis HspA1). FEMS Immunol. Med. Microbiol. 49215-223. [DOI] [PubMed] [Google Scholar]
- 22.Hoerauf, A., K. Nissen-Pahle, C. Schmetz, K. Henkle-Duhrsen, M. L. Blaxter, D. W. Buttner, M. Y. Gallin, K. M. Al-Qaoud, R. Lucius, and B. Fleischer. 1999. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Investig. 10311-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kihlstrom, E. 1977. Infection of HeLa cells with Salmonella typhimurium 395 MS and MR10 bacteria. Infect. Immun. 17290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kixmoeller, M., M. Ritzmann, and K. H. Heinritzi. 2006. Reference ranges for laboratory parameters in pigs of different breeds. Prakt. Tierärztl. 87204-213. [Google Scholar]
- 25.Kraft, W., U. M. Dürr, M. Fürll, H. Bostedt, and K. Heinritzi. 2005. Hämatologie, vol. 6. Schattauer-Verlag, Stuttgart, Germany.
- 26.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-147. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, United Kingdom.
- 27.Le Carrou, J., A. K. Reinhardt, I. Kempf, and A. V. Gautier-Bouchardon. 2006. Persistence of Mycoplasma synoviae in hens after two enrofloxacin treatments and detection of mutations in the parC gene. Vet. Res. 37145-154. [DOI] [PubMed] [Google Scholar]
- 28.Lo, S. C., M. M. Hayes, H. Kotani, P. F. Pierce, D. J. Wear, P. B. Newton III, J. G. Tully, and J. W. Shih. 1993. Adhesion onto and invasion into mammalian cells by Mycoplasma penetrans: a newly isolated mycoplasma from patients with AIDS. Mod. Pathol. 6276-280. [PubMed] [Google Scholar]
- 29.McOrist, S. 2000. Obligate intracellular bacteria and antibiotic resistance. Trends Microbiol. 8483-486. [DOI] [PubMed] [Google Scholar]
- 30.Mebius, R. E., and G. Kraal. 2005. Structure and function of the spleen. Nat. Rev. 5606-616. [DOI] [PubMed] [Google Scholar]
- 31.Mehock, J. R., C. E. Greene, F. C. Gherardini, T. W. Hahn, and D. C. Krause. 1998. Bartonella henselae invasion of feline erythrocytes in vitro. Infect. Immun. 663462-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mernaugh, G., and G. M. Ihler. 1992. Deformation factor: an extracellular protein synthesized by Bartonella bacilliformis that deforms erythrocyte membranes. Infect. Immun. 60937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mernaugh, G. R., S. F. Dallo, S. C. Holt, and J. B. Baseman. 1993. Properties of adhering and nonadhering populations of Mycoplasma genitalium. Clin. Infect. Dis. 17(Suppl. 1)S69-S78. [DOI] [PubMed] [Google Scholar]
- 34.Messick, J. B. 2004. Hemotrophic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential. Vet. Clin. Pathol. 332-13. [DOI] [PubMed] [Google Scholar]
- 35.Much, P., F. Winner, L. Stipkovits, R. Rosengarten, and C. Citti. 2002. Mycoplasma gallisepticum: influence of cell invasiveness on the outcome of experimental infection in chickens. FEMS Immunol. Med. Microbiol. 34181-186. [DOI] [PubMed] [Google Scholar]
- 36.Murphy, S. C., S. Fernandez-Pol, P. H. Chung, S. N. Prasanna Murthy, S. B. Milne, M. Salomao, H. A. Brown, J. W. Lomasney, N. Mohandas, and K. Haldar. 2007. Cytoplasmic remodeling of erythrocyte raft lipids during infection by the human malaria parasite Plasmodium falciparum. Blood 1102132-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neimark, H., K. E. Johansson, Y. Rikihisa, and J. G. Tully. 2001. Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of ‘Candidatus Mycoplasma haemofelis,’ ‘Candidatus Mycoplasma haemomuris,’ ‘Candidatus Mycoplasma haemosuis’ and ‘Candidatus Mycoplasma wenyonii.’ Int. J. Syst. Evol. Microbiol. 51891-899. [DOI] [PubMed] [Google Scholar]
- 38.Neimark, H., K. E. Johansson, Y. Rikihisa, and J. G. Tully. 2002. Revision of haemotrophic Mycoplasma species names. Int. J. Syst. Evol. Microbiol. 52683. [DOI] [PubMed] [Google Scholar]
- 39.Perreten, V., L. Vorlet-Fawer, P. Slickers, R. Ehricht, P. Kuhnert, and J. Frey. 2005. Microarray-based detection of 90 antibiotic resistance genes of gram-positive bacteria. J. Clin. Microbiol. 432291-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pospischil, A., and R. Hoffmann. 1982. Eperythrozoon suis in naturally infected pigs: a light and electron microscopic study. Vet. Pathol. 19651-657. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds, E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ristic, M., and J. P. Kreier. 1979. Hemotropic bacteria. N. Engl. J. Med. 301937-939. [DOI] [PubMed] [Google Scholar]
- 43.Seubert, A., R. Schulein, and C. Dehio. 2002. Bacterial persistence within erythrocytes: a unique pathogenic strategy of Bartonella spp. Int. J. Med. Microbiol. 291555-560. [DOI] [PubMed] [Google Scholar]
- 44.Stipkovits, L., and I. Kempf. 1996. Mycoplasmoses in poultry. Rev. Sci. Tech. Off. Int. Epizoot. 151495-1525. [DOI] [PubMed] [Google Scholar]
- 45.Tasker, S., C. R. Helps, M. J. Day, D. A. Harbour, S. E. Shaw, S. Harrus, G. Baneth, R. G. Lobetti, R. Malik, J. P. Beaufils, C. R. Belford, and T. J. Gruffydd-Jones. 2003. Phylogenetic analysis of hemoplasma species: an international study. J. Clin. Microbiol. 413877-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor-Robinson, D., H. A. Davies, P. Sarathchandra, and P. M. Furr. 1991. Intracellular location of mycoplasmas in cultured cells demonstrated by immunocytochemistry and electron microscopy. Int. J. Exp. Pathol. 72705-714. [PMC free article] [PubMed] [Google Scholar]
- 47.Vogl, G., A. Plaickner, S. Szathmary, L. Stipkovits, R. Rosengarten, and M. P. Szostak. 2008. Mycoplasma gallisepticum invades chicken erythrocytes during infection. Infect. Immun. 7671-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weick, J. K., A. B. Hagedorn, and J. W. Linman. 1974. Leukoerythroblastosis. Diagnostic and prognostic significance. Mayo Clin. Proc. 49110-113. [PubMed] [Google Scholar]
- 49.Winner, F., R. Rosengarten, and C. Citti. 2000. In vitro cell invasion of Mycoplasma gallisepticum. Infect. Immun. 684238-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, D., X. Tai, Y. Qiu, and S. Yun. 2000. Prevalence of Eperythrozoon spp. infection and congenital eperythrozoonosis in humans in Inner Mongolia, China. Epidemiol. Infect. 125421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]