Abstract
Xanthomonas oryzae pv. oryzae, the causal agent of bacterial leaf blight in rice, contains a regulator that is encoded in the genome, designated OryR, which belongs to the N-acyl homoserine lactone (AHL)-dependent quorum-sensing LuxR subfamily of proteins. However, we previously reported that X. oryzae pv. oryzae does not make AHLs and does not possess a LuxI-family AHL synthase and that the OryR protein is solubilized by a compound present in rice. In this study we obtained further evidence that OryR interacts with a rice signal molecule (RSM) and that the OryR concentration increases when rice is infected with X. oryzae pv. oryzae. We also describe three OryR target promoters which are regulated differently: (i) the neighboring proline iminopeptidase (pip) virulence gene, which is positively regulated by OryR in the presence of the RSM; (ii) the oryR promoter, which is negatively autoregulated independent of the RSM; and (iii) the 1,4-β-cellobiosidase cbsA gene, which is positively regulated by OryR independent of the RSM. We also found that the RSM for OryR is small, is not related to AHLs, and is not able to activate the broad-range AHL biosensor Agrobacterium tumefaciens NT1(pZLQR). Furthermore, OryR does not regulate production of the quorum-sensing diffusible signal factor present in the genus Xanthomonas. Therefore, OryR has unique features and is an important regulator involved in interkingdom communication between the host and the pathogen.
The species Xanthomonas oryzae includes two pathovars, X. oryzae pv. oryzae and X. oryzae pv. oryzicola, which are pathogens of rice, are closely related, and were initially considered pathovars of Xanthomonas campestris (28). X. oryzae pv. oryzae is a gram-negative rod-shaped bacterium that causes bacterial leaf blight (BLB), one of the most important diseases of rice. BLB is a vascular disease in which X. oryzae pv. oryzae grows and colonizes the xylem vessels, eventually clogging them; several virulence-associated determinants have been found, including exopolysaccharide production, hypersensitive response, and pathogenicity (hrp) genes (7, 20, 26).
Many gram-negative bacteria possess a form of gene regulation involving cell-cell communication, also known as quorum sensing (QS), that occurs via the production of and response to N-acyl homoserine lactone (AHL) signaling molecules. A typical AHL QS system is most commonly mediated by two proteins belonging to the LuxI and LuxR protein families; LuxI-type proteins are AHL synthases, and LuxR-family proteins are modular sensor-response regulators. In an AHL QS system, AHLs interact directly at a high bacterial cell density (i.e., at a quorum concentration) with the cognate LuxR-type protein, and the protein-AHL complex can then bind at specific gene promoter sequences called lux boxes and affect expression of QS target genes (15). AHL QS has been studied in many bacterial species and has been shown to provide a significant advantage to a community of bacteria by allowing it to adapt to environmental conditions, which enhances its defense against other microorganisms or eukaryotic resistance mechanisms (4, 34).
X. oryzae pv. oryzae does not produce AHL QS signaling molecules; however, we recently reported that it possesses a protein, designated OryR, which is related to the LuxR family of AHL QS regulators (13). In fact, OryR is a modular protein that has an AHL domain and a helix-turn-helix domain, both of which are typical of the LuxR-family subgroup of QS regulators (13, 15). There is not a cognate AHL LuxI-family synthase gene in the genome, and therefore OryR can be considered an unpaired or orphan LuxR-type response regulator (14, 33). OryR has been shown not to bind the most common AHLs; however, it appears to bind a compound present in rice plants. This conclusion was reached following the observation that the OryR protein was not solubilized by many of the structurally different AHLs but OryR solubilization occurred in the presence of rice extract (13). It was also determined that OryR plays a role in X. oryzae pv. oryzae rice virulence since an oryR mutant is less able to cause the BLB symptoms (13). A protein very similar to OryR, designated XccR, has been found in the plant pathogen Xanthomonas campestris pv. campestris, and this protein has been associated with X. campestris pv. campestris pathogenicity and regulates the neighboring proline iminopeptidase (PIP) virulence gene (pip) in planta (35). Studies of the xccR/pip locus revealed that XccR associates with a plant factor and functions as a transcriptional activator that binds to the lux box present in the promoter of the pip gene.
Plants have been reported to produce compounds that are able to act as agonists or antagonists to bacterial AHL QS systems and hence have been called AHL mimics (2). Halogenated furanones from the marine red alga Delisea pulchra, which are structurally similar to the C4-AHL molecule, were able to competitively bind the LuxR homologue proteins having inhibitory functions (16, 21). Additionally, AHL mimics from the unicellular green alga Chlamydomonas reinhardtii and several other plants, including rice, were able to stimulate gene expression via LuxR-family AHL sensors/regulators (9, 29). To date, the structure of these plant compounds is unknown, and the possibility that similar molecules are involved in interkingdom signaling with OryR of X. oryzae pv. oryzae and rice cannot be excluded.
In this study we obtained further evidence of the presence of a molecule in rice which interacts with OryR and showed that the amount of this molecule increases when rice is infected with X. oryzae pv. oryzae. We also obtained evidence that there are three OryR target genes and examined how these genes are regulated in response to the presence of macerated rice. It was also established that the OryR regulatory network does not affect production of diffusible signal factor (DSF), the signal molecule that was found in X. campestris pv. campestris, was characterized, and is present in multiple Xanthomonas species. OryR therefore has unique features and is an important factor in the plant-bacterium interaction through the detection of and response to a small diffusible plant compound.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
X. oryzae pv. oryzae strain XKK.12 was grown at 28°C in PYS liquid medium (13), PS (30) solid medium, and M9 minimal medium (24) supplemented with Casamino Acids. Escherichia coli DH5α (24) was grown at 37°C in Luria-Bertani medium (22), and Agrobacterium tumefaciens NT1(pZLQR) was grown at 28°C in AB minimal medium (5). The following media contained macerated rice. (i) Rice medium was prepared by macerating healthy rice frozen in liquid nitrogen; the powder obtained was added to water, autoclaved to sterilize it, and filtered (Millipore) to remove rice tissue. (ii) Infected-rice medium was prepared by using macerated X. oryzae pv. oryzae-infected rice frozen in liquid nitrogen; the powder obtained was added to water, autoclaved to sterilize it, and filtered (Millipore) to remove rice tissue. (iii) PYS-rice medium was prepared by macerating healthy rice frozen in liquid nitrogen; the powder obtained was added to PYS medium, autoclaved to sterilize it, and filtered (Millipore) to remove rice tissue. When necessary, antibiotics were added at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; and gentamicin, 30 μg/ml. Infected-rice medium was fractionated by ultrafiltration using YM10, YM3, and YM1 membranes (Amicon Inc.). AHLs were acquired from the laboratory of Paul Williams (University of Nottingham, Nottingham, United Kingdom).
Recombinant DNA techniques.
DNA manipulations, including digestion with restriction enzymes, agarose gel electrophoresis, purification of DNA fragments, ligation with T4 ligase, and transformation of E. coli, were performed as described previously (24). Plasmids were purified using Jet star columns (Genomed GmbH, Löhne, Germany) or by the alkaline lysis method (3). Genomic DNA from X. oryzae pv. oryzae was isolated by Sarkosyl-pronase lysis as previously described (3). X. oryzae pv. oryzae promoters were amplified by PCR and cloned in the pMOSBlue cloning vector (Amersham-Pharmacia). All DNA sequencing was performed by Macrogen (www.macrogen.com). Reporter plasmid pSS122 was transferred to X. oryzae pv. oryzae cells by electroporation as previously described (11).
pSS122 promoter-probe plasmid construction.
Plasmid pSS122 (IncW replicon) was constructed from pUFR047 (8), a stably maintained plasmid that is present at low copy numbers in both E. coli and Xanthomonas. The reporter gene uidA was amplified from E. coli K-12 genomic DNA by PCR using primers UIDAS and UIDAR and cloned with KpnI-EcoRI in pUFR047. The resulting plasmid, which was approximately 10.5 kb long, had unique restriction sites for HindIII, PstI, SalI, SmaI, and KpnI. pSS122 contained the ampicillin and gentamicin resistance genes but did not contain the lacZ+ marker.
β-Glucuronidase assay.
The β-glucuronidase activities of overnight cultures of X. oryzae pv. oryzae with the pSS122 reporter plasmid carrying different promoters were determined. X. oryzae pv. oryzae cells were pelleted and resuspended in 600 μl of GUS buffer (50 mM sodium phosphate [pH 7.0], 1 mM EDTA, 14.3 mM 2-mercaptoethanol). After this 23 μl of 3% Triton X-100 in GUS buffer and 23 μl of 3% sodium lauryl sarcosinate in GUS buffer were added to the samples, the preparations were incubated at 30°C for 10 min, and then 100 μl of 25 mM p-nitrophenyl-β-d-glucuronic acid (PNPG) (Sigma) was added. The reaction was stopped by adding 280 μl of a 1 M Na2CO3 solution after sufficient yellow color had developed. Both the optical densities at 595 nm (OD595) of the X. oryzae pv. oryzae cultures and the OD415 of the yellow color that developed after the β-glucuronidase reaction (OD415 PNPG) of the samples were determined, and 1 Miller unit of β-glucuronidase activity was defined as follows: 1 Miller unit = 1,000 × {[OD415 PNPG − (1.75 × OD595)]/(t × v × OD595)}, where t is the time of the reaction (in minutes), v is the volume of the culture assayed (in milliliters), OD595 is the cell density just before the assay, and 1.75 is the correction factor. All measurements were done in triplicate.
Rice infection and xylem sap collection.
X. oryzae pv. oryzae XKK.12 was grown on PS medium plates (30) at 28°C, and single colonies were transferred to liquid PYS medium (13). A 1-day-old culture whose concentration was adjusted to 109 CFU/ml was used to inoculate 6-week-old rice plants (cultivar IR24) by the clipping method as previously described (13). To collect xylem sap, infected plants were placed in a humid chamber after the dried blighted parts of the infected leaves were removed. Drops of the xylem were collected continously during the subsequent 8 h and placed in sterile tubes.
OryR overexpression and Western blot analysis.
E. coli M15/pQEORYR (13) was grown in 10 ml of Luria-Bertani medium containing 20 μl of xylem sap collected from X. oryzae pv. oryzae-infected rice plants. OryR expression was induced with 1 mM isopropyl-β-d-thiogalactoside at an OD600 of 0.6, and the preparation was incubated for 1 h at 28°C. The culture was rapidly chilled on ice, and soluble His6-OryR was extracted under native conditions according to the supplier's instructions (Qiagen). Proteins were transferred onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore) using a tank system according to the manufacturer's instructions. The membrane was subjected to Western blot analysis using an anti-six-His-tag monoclonal antibody (BD Biosciences, San Jose, CA), and after incubation with a second horseradish peroxidase-labeled antibody, the protein was detected with 3,3′-diaminobenzidine tetrahydrochloride tablets (Sigma, St. Louis, MO).
DSF measurement.
DSF signaling regulates the production of protease and endoglucanase in X. campestris pv. campestris (1). The protease activities of the X. oryzae pv. oryzae XKK.12 parental strain and X. oryzae pv. oryzae XKK.12ORY were assayed on skim milk plates as previously described (1). Endoglucanase activity was visualized on carboxymethyl cellulose agar plates due to the ability of crude DSF extracts from X. oryzae pv. oryzae to restore the DSF production of the X. campestris pv. campestris indicator strain (1). X. oryzae pv. oryzae XKK.12 and XKK.12ORY were grown in PYS rich medium, in rice medium, and in infected-rice medium. DSF was extracted from different culture volumes in order to normalize the number of cells of XKK.12 compared to the number of cells of X. oryzae pv. oryzae XKK.12ORY for each medium.
RESULTS
The presence of xylem sap that was collected from X. oryzae pv. oryzae-infected rice in the growth media increased OryR protein solubility.
The OryR primary structure includes domains typical of QS LuxR-family regulators, including an AHL-binding domain at its N terminus and a helix-turn-helix DNA-binding motif at the C terminus. It was previously shown that when LuxR-family QS proteins are overexpressed, they are highly insoluble, while in the presence of and bound to the cognate AHL molecule, they are soluble (25, 31, 32). OryR, like other LuxR-family regulators, was found to be highly insoluble, but it was soluble when it was expressed in E. coli grown in the presence of macerated rice (13). Therefore, it was postulated that an unknown rice signal molecule (RSM) was present in the rice and was able to interact with OryR, solubilizing the protein. Many structurally different AHLs were unable to solubilize OryR, indicating that OryR likely did not bind AHLs (13).
To verify the presence of the RSM and possibly to determine its concentration in infected rice, an OryR solubilization assay was performed using rice previously infected with X. oryzae pv. oryzae. As X. oryzae pv. oryzae is a pathogen that colonizes and infects the xylem, it is most likely that the RSM is present in this part of the plant. Rather than using total macerated rice, which was done previously (13), we harvested the xylem sap from X. oryzae pv. oryzae XKK.12-infected rice plants 3, 6, 10, and 14 days after infection and from noninfected rice plants as a control, as described in Materials and Methods. E. coli M15/pQEORYR overexpressing His6-OryR was then grown in the presence of xylem sap isolated at these four times. The presence of a soluble form of OryR was established via Western blot analysis using an anti-His6 antibody. The largest amount of soluble OryR was found when bacteria were grown in the presence of xylem sap collected 10 days after X. oryzae pv. oryzae infection. This result indicated that in the xylem maximum concentrations of RSM and/or maximum OryR levels were reached approximately 10 days after X. oryzae pv. oryzae infection (Fig. 1).
FIG. 1.
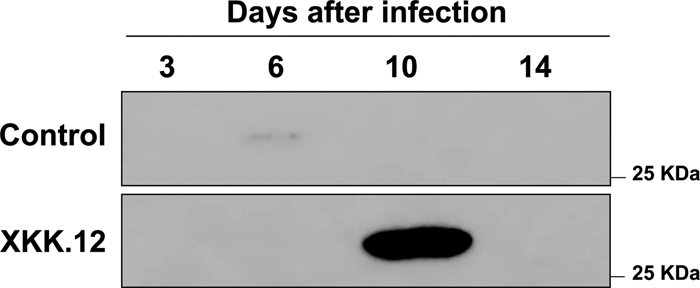
Western blot analysis of soluble His-tagged OryR expressed in E. coli grown in media containing infected xylem sap recovered from rice at various time points (3, 6, 10, or 14 days). A soluble form of OryR was most detectable when it was expressed in E. coli in the presence of xylem sap recovered from rice 10 days after infection (see text for details).
Gene promoter studies of the oryR/pip region: OryR regulates pip in response to an RSM.
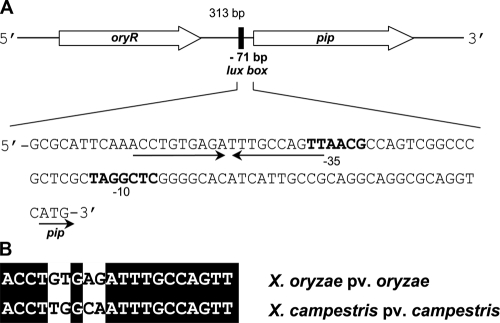
PIP production in X. campestris pv. campestris is regulated by XccR, a homolog of OryR (35). The biological function of PIP is currently unclear; this enzyme can catalyze the removal of the N-terminal proline from small peptides or proteins and is widely distributed in bacteria. PIP in X. campestris pv. campestris has been shown to be a virulence factor as pip mutants were less pathogenic to cabbage because they were less able to spread and grow in the vascular system (35). The pip gene in X. campestris pv. campestris and X. oryzae pv. oryzae is genetically linked to the xccR and oryR genes, respectively (Fig. 2). Interestingly, pip promoters of both X. oryzae pv. oryzae and X. campestris pv. campestris contain well-conserved lux boxes typically found in AHL QS-regulated target genes in gram-negative bacteria. The putative lux box sequence in the X. oryzae pv. oryzae pip promoter is centered at position −71 from the start codon (Fig. 2A) and was found to be highly similar to the experimentally determined lux box in the X. campestris pv. campestris pip promoter (Fig. 2B) (35).
FIG. 2.
(A) Analysis of the pip promoter locus in X. oryzae pv. oryzae. The 313-bp intergenic region upstream from the pip gene contains a putative palindromic lux box sequence centered at position −71 bp from the start codon, as indicated by the arrows. The hypothetical −35 and −10 regions are indicated by bold type. (B) Alignment of the two putative pip lux boxes identified in X. oryzae pv. oryzae and X. campestris pv. campestris.
To verify that OryR is able to regulate the pip promoter in X. oryzae pv. oryzae, an IncW gusA promoter probe plasmid designated pSS122, which was stable in Xanthomonas, was constructed as described in Materials and Methods. The pip promoter was then cloned upstream of the promoterless β-glucuronidase reporter gene in pSS122, generating pPIP122. X. oryzae pv. oryzae strain XKK.12 and the oryR mutant derivative XKK.12ORYR carrying pPIP122 were grown under different conditions in the presence and absence of macerated rice. Our previous studies showed that no OryR protein was detected when X. oryzae pv. oryzae was grown in M9 minimal medium; however, the protein was highly expressed when macerated rice was added to the minimal medium, demonstrating that oryR expression was most likely induced in planta (13). We observed, however, that the OryR protein was present when X. oryzae pv. oryzae was grown in PYS rich medium, indicating that some component(s) in this complex medium probably partially induced oryR expression. We therefore performed pip promoter activity studies using PYS rich medium with and without macerated rice; this ensured that OryR was always present and that the only difference was the presence of macerated rice.
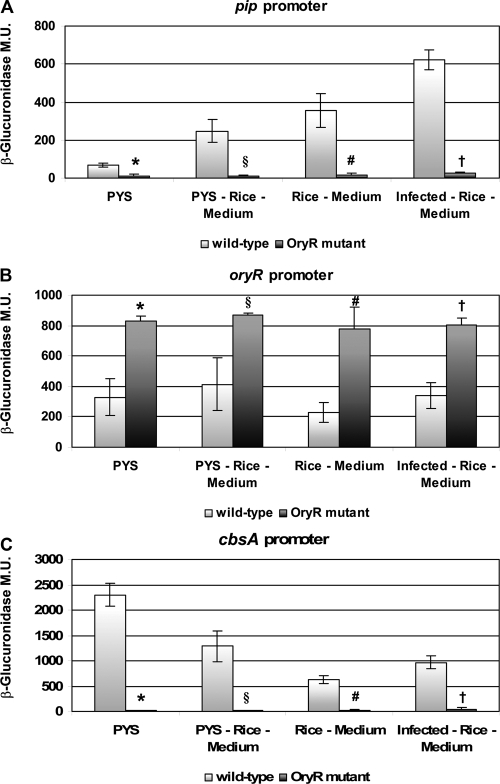
It was determined that pip promoter activity in the wild-type strain was approximately five times higher when macerated rice was present in the medium, indicating that most likely a compound present in rice was pivotal for pip transcription. Significantly, no promoter activity under any of the conditions tested was detected in the X. oryzae pv. oryzae oryR mutant, indicating that the compound (RSM) present in macerated rice was necessary to activate the pip promoter via OryR (Fig. 3A).
FIG. 3.
Gene promoter activity in X. oryzae pv. oryzae XKK.12 harboring the reporter plasmid and grown in different media with and without macerated rice. PYS, rich PYS medium; PYS-Rice-Medium, PYS rich medium with macerated rice; Rice-Medium, macerated rice in distilled sterile water; Infected-Rice-Medium, rice which was infected with X. oryzae pv. oryzae for 10 days prior to maceration (see text for details). (A) pip gene promoter activity in X. oryzae pv. oryzae XKK.12 harboring the reporter plasmid pPIP122. The highest pip promoter activity was observed when X. oryzae pv. oryzae XKK.12(pPIP122) was grown in the presence of infected rice. No promoter activity was detected in the oryR mutant X. oryzae pv. oryzae XKK.12ORY(pPIP122). The results are expressed as means ± standard deviations (n = 3). *, P < 0.002 compared to the parental strain; §, P < 0.003 compared to the parental strain; #, P < 0.003 compared to the parental strain; †, P < 4 × 10−5 compared to the parental strain. (B) oryR promoter activity in X. oryzae pv. oryzae XKK.12(pORY122) and in the oryR mutant X. oryzae pv. oryzae XKK.12ORY(pORY122). The results are expressed as means ± standard deviations (n = 3). *, P < 0.002 compared to the parental strain; §, P < 0.012 compared to the parental strain; #, P < 0.002 compared to the parental strain; †, P < 0.001 compared to the parental strain. (C) cbsA promoter activity in X. oryzae pv. oryzae XKK.12(pCBS122) in different growth media. No promoter activity was detected in the oryR mutant X. oryzae pv. oryzae XKK.12ORY(pCBS122). The results are expressed as means ± standard deviations (n = 3). *, P < 6 × 10−5 compared to the parental strain; §, P < 0.002 compared to the parental strain; #, P < 2 × 10−4 compared to the parental strain; †, P < 3 × 10−4 compared to the parental strain.
Having established that 10-day xylem sap from X. oryzae pv. oryzae-infected rice resulted in the greatest OryR solubilization (see above), we examined whether pip promoter activity increased further in the presence of infected rice in the growth medium. X. oryzae pv. oryzae XKK.12(pPIP122) cells were grown in medium containing macerated 10-day-old X. oryzae pv. oryzae-infected rice, and β-glucuronidase assays were then performed. As shown in Fig. 3A, the activity of the pip promoter in the presence of macerated, infected rice was approximately 10 times higher than the activity of the control, and there was a further twofold increase when uninfected macerated rice was used, confirming that rice infected probably contained larger amounts of RSM for 10 days recognized by OryR. No β-glucuronidase expression was observed in the oryR mutant X. oryzae pv. oryzae XKK.12ORY(pPIP122), further confirming that the pip gene was tightly regulated by OryR (Fig. 3A).
To further verify that the pip promoter was also functional in planta, X. oryzae pv. oryzae XKK.12(pPIP122) was used for rice infection; bacterial cells were then recovered from rice plants 1 week after infection, and β-glucuronidase assays were performed. Although most of the bacterial cells recovered from the infected plants had lost the promoter probe plasmid, significant β-glucuronidase activity was detected, clearly indicating that there was a high level of pip promoter expression in planta (data not shown).
OryR negatively regulates its own expression in a rice-independent manner.
In order to determine whether OryR was able to regulate its own activity, the β-glucuronidase assay was performed with PYS rich medium using X. oryzae pv. oryzae XKK.12 and the oryR mutant derivative XKK.12ORY containing the pORY122 plasmid, which had a transcriptional fusion of the oryR promoter with the promoterless uidA gene encoding β-glucuronidase. The expression from the oryR promoter was twofold higher in the X. oryzae pv. oryzae oryR mutant than in the wild-type X. oryzae pv. oryzae strain, showing that OryR acted as a negative autoregulator. The promoter activity profiles were similar with and without macerated rice in the medium (Fig. 3B), indicating that OryR was able to negatively regulate its own expression and thus act as a transcriptional regulator in the absence of the RSM.
1,4-β-Cellobiosidase expression is OryR dependent.
1,4-β-Cellobiosidase (CbsA) catalyzes the hydrolysis of 1,4-β-d-glucosidic linkages in cellulose, releasing cellobiose from the nonreducing ends of the chains. This hydrolytic enzyme was identified as one of the X. oryzae pv. oryzae secreted proteins involved in virulence, as the ability of X. oryzae pv. oryzae cbsA mutants to cause lesions in rice was reduced (19). Our previous studies showed that the maximal production of secreted CbsA by X. oryzae pv. oryzae wild-type strain KACC10331 occurred when macerated rice was present in the culture medium in the presence of a functional oryR gene (13).
To verify that OryR regulated the expression of cbsA, we performed the β-glucuronidase assays with X. oryzae pv. oryzae XKK.12 and with XKK.12ORY containing the pCBS122 plasmid, in which the cbsA promoter was cloned upstream of the promoterless uidA gene. Interestingly, the cbsA promoter activity in X. oryzae pv. oryzae wild-type strain XKK.12 in rich PYS medium was reduced approximately 50% when macerated rice was added to the medium; however, no β-glucuronidase expression was observed in the oryR mutant X. oryzae pv. oryzae XKK.12ORY(pCBS122) (Fig. 3C). This result indicated that OryR regulated cbsA expression and hence 1,4-beta-cellobiosidase (CbsA) production independent of the RSM. The reduction in cbsA promoter activity in rice medium was surprising since the CbsA protein is most abundant when X. oryzae pv. oryzae is grown in the presence of macerated rice (13). Therefore, the possibility that cbsA/CbsA expression is subject to posttranscriptional regulation cannot be excluded.
OryR does not regulate production of DSF: the QS signal molecule produced by X. oryzae pv. oryzae.
QS has been reported to occur in X. campestris pv. campestris via a signaling molecule designated DSF (1, 27). DSF has been identified as cis-11-methyl-2-dodecenoic acid and is encoded by the rpfF gene; DSF signaling is involved in the regulation of biofilm dispersal and the production of virulence factors (1, 12). A two-component regulatory system designated RpfC/RpfG is involved in perception and transduction of the DSF signal to target genes (17). Since X. oryzae pv. oryzae also contains the rpf cluster in its genome and produces DSF (6), it was of interest to determine whether OryR was involved in and connected with DSF production in X. oryzae pv. oryzae. Using a previously described DSF sensor strain (1), we established that DSF is produced in X. oryzae pv. oryzae XKK.12 and XKK.12ORY as described in Materials and Methods. The DSF levels were comparable in the wild-type and oryR mutant derivative, demonstrating that OryR was not involved in the regulation of QS via DSF production (data not shown). To further confirm that OryR was not involved in DSF production, the rpfF promoter controlling the DSF biosynthesis gene was cloned in pSS122 upstream of the promoterless uidA gene, generating pRPFF122. β-Glucuronidase assays were then performed with XKK.12(pRPFF122) and X. oryzae pv. oryzae XKK.12ORY(pRPFF122) cells. No differences in activity were observed under any of the growth conditions tested, further confirming that OryR does not regulate DSF production (data not shown).
We were also interested in determining whether DSF production in X. oryzae pv. oryzae was influenced by the presence of macerated rice in the growth medium. The results of extraction of DSF from X. oryzae pv. oryzae XKK.12 and XKK.12ORY grown in PYS rich medium, in rice medium, and in infected-rice medium established that there were no differences in DSF production between X. oryzae pv. oryzae XKK.12 and XKK.12ORY under any of the growth conditions tested (data not shown), meaning that DSF production most likely does not change in planta.
RSM is a small molecule that is probably not related to AHLs.
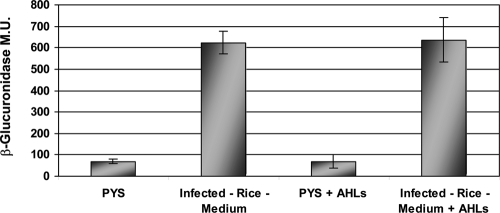
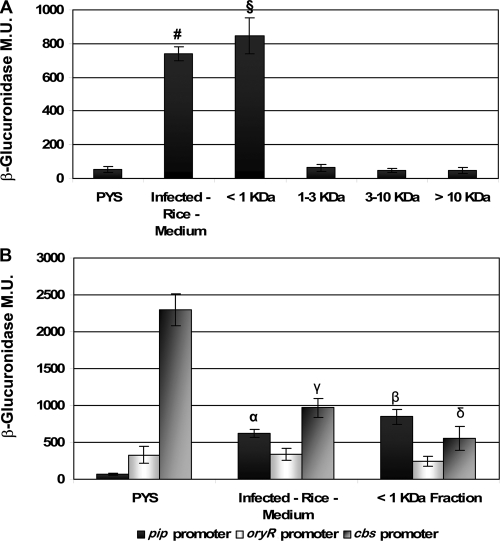
Previous studies of OryR solubility showed that the RSM which was able to bind OryR was probably not an AHL-type molecule (13). This conclusion was based on the fact that structurally different AHLs could not solubilize OryR. To further confirm these data, we analyzed the OryR target pip promoter activity when structurally different AHLs (C4-, C6-, C8-, C10-, C12-, C6-3-oxo-, C8-3-oxo-, C10-3-oxo-, C12-3-oxo-, C6-3-OH-, C8-3-OH-, C10-3-OH-, and C12-3-OH-AHLs) were added to the culture medium at a final concentration of 2 μM in independent experiments. No pip gene promoter induction was observed, and there was no competition for the OryR binding site, since the β-glucuronidase production by X. oryzae pv. oryzae XKK.12(pPIP122) cells was not reduced in the presence of both the RSM and any of the structurally different AHLs (Fig. 4). To verify that the RSM was a small molecule, media containing the RSM were fractionated by molecular size using progressive filtration (see Materials and Methods). A β-glucuronidase assay with X. oryzae pv. oryzae XKK.12(pPIP122) was then performed using the four fractions obtained, and the results clearly showed that strong pip promoter activation occurred only with the <1-kDa fraction, indicating the RSM was a small molecule (Fig. 5A). The <1-kDa fraction was also tested using the oryR and cbsA promoters with X. oryzae pv. oryzae XKK.12(pORY122) and XKK.12(pCBS122). It was determined that the two gene promoter activities measured using the β-glucuronidase reporter gene when X. oryzae pv. oryzae was grown in the presence of the <1-kDa fraction were statistically similar to the activity when X. oryzae pv. oryzae was grown in infected-rice medium (Fig. 5B).
FIG. 4.
β-Glucuronidase pip promoter activity in X. oryzae pv. oryzae XKK.12(pPIP122) in the presence of a mixture of structurally different AHLs. PYS, rich PYS medium; Infected-Rice-Medium, rice which was infected with X. oryzae pv. oryzae for 10 days prior to maceration; PYS+AHLs, rich PYS medium containing 2 μM each of the 15 most structurally common AHLs; Infected Rice Medium+AHLs, rice which was infected with X. oryzae pv. oryzae for 10 days prior to maceration and contained 2 μM each of the 15 most structurally common AHLs (see the text for details). No pip promoter activation and no binding competition were observed in the presence of AHLs. The results are expressed as means ± standard deviations (n = 3). Statistical analysis revealed no statistically significant differences (P ≥ 0.05) between PYS and PYS+AHLs values or between Infected-Rice-Medium and Infected-Rice-Medium+AHLs values.
FIG. 5.
(A) pip promoter activity in X. oryzae pv. oryzae XKK.12(pPIP122) in rich medium in the presence of macerated rice or in different fractions of filtered macerated infected-rice medium. PYS, X. oryzae pv. oryzae with plasmid pPIP122 grown in rich PYS medium; Infected-Rice-Medium, X. oryzae pv. oryzae with plasmid pPIP122 grown in rice which was infected with X. oryzae pv. oryzae for 10 days prior to maceration; <1 KDa, X. oryzae pv. oryzae with plasmid pPIP122 grown in the presence of a filtrate from macerated rice that lacked all molecules larger than 1 kDa; 1-3 KDa, X. oryzae pv. oryzae with plasmid pPIP122 grown in the presence of a filtrate from macerated rice that included molecules with molecular masses ranging from 1 to 3 kDa; 3-10 KDa, X. oryzae pv. oryzae with plasmid pPIP122 grown in the presence of a filtrate from macerated rice that included molecules with molecular masses ranging from 3 to 10 kDa; >10 KDa, X. oryzae pv. oryzae with plasmid pPIP122 grown in the presence of a filtrate from macerated rice that included molecules with molecular masses larger than 10 kDa (see text for details). Promoter activity as determined by β-glucuronidase activity was detected only in the medium containing the <1-kDa fraction, indicating that the molecular mass of the RSM is less than 1 kDa. The results are expressed as means ± standard deviations (n = 3). §, P < 1.5 × 10−5 compared to the PYS value; #, P < 2 × 10−4 compared to the PYS value. Analyses in which the 1-3 KDa, 3-10 KDa, and >10 KDa values were compared to the PYS values revealed no statistically significant differences (P ≥ 0.05). (B) The pip promoter, oryR promoter, and cbsA promoter activities in the <1-kDa fraction were assayed and compared to the β-glucuronidase activities obtained for X. oryzae pv. oryzae with plasmid pPIP122 grown in rich PYS medium and for X. oryzae pv. oryzae with plasmid pPIP122 grown in rice which was infected with X. oryzae pv. oryzae for 10 days prior to maceration (see above). The results are expressed as means ± standard deviations (n = 3). α, P < 5.7 × 10−5 compared to the PYS value; β, P < 2.1 × 10−5 compared to the PYS value; γ, P < 8.3 × 10−5 compared to the PYS value; δ, P < 4 × 10−5 compared to the PYS value.
RSM does not act as an AHL QS mimic.
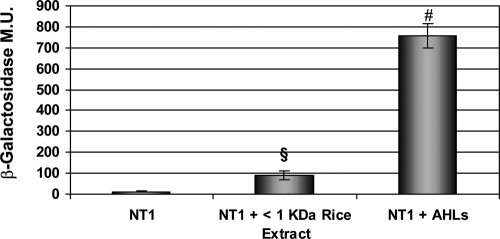
Several studies have reported that plants contain molecules that are able to activate bacterial AHL QS systems; however, the structure of these molecules is currently unknown (2, 17). To examine whether RSM could act as an AHL mimic, activating a QS LuxR-family protein, A. tumefaciens NT1(pZLQR) was used as an AHL biosensor strain because of its ability to recognize a broad range of different AHL molecules (5). In the presence of an active AHL molecule, TraR activates transcription of the β-galactosidase reporter gene present in the pZLQR plasmid. A. tumefaciens NT1(pZLQR) was grown in the presence of the <1-kDa fraction containing the active RSM as described above. As a positive control, a mixture of different AHL molecules was added to the culture medium, whereas medium alone was used as the negative control. As expected, β-galactosidase activity was detected in the presence of AHLs, while no background activity was found in the medium alone. A statistically significant increase in β-galactosidase activity was observed in the presence of RSM (Fig. 6). This increase in activity did not appear to be significant. However, we cannot exclude the possibility that this fraction from the rice plant contained molecules able to weakly activate AHL QS systems.
FIG. 6.
β-Galactosidase activity in the A. tumefaciens NT1(pZLQR) AHL biosensor grown in the presence of the <1-kDa fraction obtained from macerated infected rice. Growth media with and without AHLs were used as positive and negative controls, respectively. The results are expressed as means ± standard deviations (n = 3). §, P < 0.004 compared to the NT1 value; #, P < 2.5 × 10−5 compared to the NT1 value.
DISCUSSION
In this study we demonstrated that the LuxR-family OryR regulatory protein present in X. oryzae pv. oryzae responds to a small RSM. OryR has the typical modular structure of QS LuxR-family response regulator proteins; at the N terminus it has an AHL-binding domain, and at the C terminus it has a helix-turn-helix DNA-binding domain. The primary structure of OryR, however, just like the primary structure of XccR of X. campestris pv. campestris, does not have sequence similarity in the AHL-binding domain at two highly conserved amino acids (Trp57 and Tyr61), which have been shown by structural analysis to be involved in AHL binding in TraR of A. tumefaciens (36). Trp57 forms a hydrogen bond with the keto group of AHL, whereas Tyr61 is part of the β-sheet surface (the AHL-binding domain consists of an α/β/α “sandwich”) important for interactions with the fatty acyl chain of the AHL. The lack of conservation in these two important amino acids might have evolved to allow OryR to bind to a structurally different molecule present in the rice plant and to allow it to be involved in interkingdom signaling. Very recently, Zhang et al. (35) carefully investigated the presence of X. oryzae pv. oryzae OryR-like and X. campestris pv. campestris XccR-like proteins in other bacterial species and determined that related proteins form a distinct group that includes proteins from, for example, Pseudomonas syringae, Pseudomonas fluorescens, and Rhizobium leguminosarum. All the bacterial species possessing an OryR-related protein live in close association with plants; thus, it is reasonable to postulate that they might interact with similar plant-derived signal molecules.
This work showed that the RSM was present in the xylem sap that was collected; experiments showed that the highest levels of OryR solubility were obtained by adding to the growth media xylem sap from 10-day-old X. oryzae pv. oryzae-infected plants. In addition, the OryR promoter activation of the pip target gene was greatest when macerated X. oryzae pv. oryzae-infected rice was added to the growth media, which resulted in 10-fold activation, compared to the 5-fold activation observed when uninfected macerated rice was added. These results indicate that probably the RSM is present in rice at higher concentrations when the rice is infected by X. oryzae pv. oryzae, possibly due to a defense response to the infection. Plants are known to synthesize an extremely large set of low-molecular-weight secondary metabolites in response to pathogen attack (10), and it is therefore likely that the RSM interacting with OryR is one of these molecules. Since salicylic acid is known to be an important signaling molecule involved in microbial defense, we tested whether it could induce the OryR activity of the pip gene promoter. No induction was observed (data not shown); hence, we concluded that this molecule does not bind OryR. Very recently, it was reported that the algal compound riboflavin and its derivative lumichrome activate the QS LasR protein of P. aeruginosa (23). As these compounds are also secreted by plant cells, we tested whether they could activate OryR by measuring pip promoter activity; we established that addition of neither of these compounds results in OryR activation (data not shown). Due to the very numerous low-molecular-weight secondary metabolites produced by plants, many of which are present at very low concentrations, identifying the molecule(s) that interacts with the OryR subfamily of LuxR-family regulators will be a major challenge. Most likely, the RSM is not related to AHLs since competition experiments with AHLs and RSM did not alter the ability of OryR to activate the pip promoter; in addition, it was previously determined that no AHL was able to solubilize OryR (13).
In this study we found three target promoters of OryR. First, the pip gene target is adjacent to the oryR gene, and the pip promoter contains a very well conserved lux box. The oryR-pip locus with a lux box is very well conserved among plant-associated bacteria which possess an oryR-like gene (35). The lux box in the pip promoter of X. campestris pv. campestris has been shown to be functional and regulated by XccR in planta (35). The pip promoter in X. oryzae pv. oryzae is tightly positively regulated by OryR in response to the RSM, and due to the very high conservation with the xccR-pip locus of X. campestris pv. campestris it is very likely that the lux box is functional and that once OryR is bound to the RSM, it also binds to the lux box and directly activates transcription of the pip gene. The PIP enzyme was shown to be a virulence factor in X. campestris pv. campestris. We did not determine if it is also a virulence factor in X. oryzae pv. oryzae. However, due to the high level of identity of the two loci and because both X. oryzae pv. oryzae and X. campestris pv. campestris are vascular pathogens, it is very probable that in X. oryzae pv. oryzae PIP is also associated with virulence. Second, OryR negatively regulated its own transcription since there was a twofold increase in the oryR promoter activity in the oryR mutant; importantly, this increase was independent of the presence of rice extract, indicating that OryR can probably also influence transcription in the absence of the RSM. It is not known whether this OryR autoregulation is direct or indirect. We could not detect a clear lux box in the oryR promoter. However, this does not exclude the possibility that there is OryR direct regulation since lux boxes can have several sequence variations. Third, OryR regulated the expression of the 1,4-β-cellobiosidase cbsA gene, which encodes a secreted hydrolytic enzyme involved in X. oryzae pv. oryzae virulence (19). Our previous studies showed that in X. oryzae pv. oryzae oryR mutants there was significantly less CbsA in the extracellular medium (13). The cbsA promoter displayed strong promoter activity in rich medium which was dependent on OryR since in the X. oryzae pv. oryzae oryR mutant the promoter activity was very significantly decreased; the reason for this is currently unknown. The cbsA promoter activity decreased by approximately 50% in the wild-type strain when macerated rice was added to the medium. The cbsA promoter was therefore positively regulated by OryR but, unlike the pip promoter, in a rice-independent way; again, we cannot exclude the possibility that OryR regulates the cbsA promoter indirectly as we could not detect a clear lux box in its promoter region. At present, we cannot explain the mechanisms of cbsA/CbsA regulation by OryR, and further studies are needed to determine whether OryR regulates a posttranscriptional mechanism in response to RSM which results in higher CbsA protein levels in the presence of rice extract. In summary, the three OryR promoter targets that we describe here are regulated differently, indicating that OryR can probably function with and without the RSM and act as a positive transcriptional regulator, as well as a negative transcriptional regulator.
QS in Xanthomonas has been associated with the DSF signaling molecule (18); thus, we were interested in determining whether OryR and DSF signaling are interconnected. We established that OryR was not involved in DSF production since the DSF synthesis and rpfF promoter activity of the X. oryzae pv. oryzae oryR mutant were not altered. Furthermore, we determined that the quantity of DSF did not change in the presence of macerated rice. We cannot exclude the possibility that DSF can regulate oryR/OryR levels. However, a recent genome-scale analysis of X. campestris pv. campestris revealed that DSF QS is not involved in the regulation of xccR (17). It is therefore reasonable to assume that it is very likely that in X. oryzae pv. oryzae DSF signaling does not regulate oryR. DSF cell-cell communication and OryR-RSM regulation therefore act independently and are not interconnected; however, the possibility that the two systems might have overlapping regulons cannot be excluded. Experiments described here also showed that the RSM is very small and does not interfere with and/or act as an agonist in AHL QS systems. This suggests that this member of the LuxR family, regardless of the conservation with AHL QS members, does not bind AHLs but binds an unknown RSM and is involved in interkingdom signaling.
TABLE 1.
X. oryzae pv. oryzae strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Characteristics or sequence | Reference or source |
|---|---|---|
| Strains | ||
| X. oryzae pv. oryzae XKK.12 | Wild-type strain | 13 |
| X. oryzae pv. oryzae XKK.12ORYR | Strain XKK.12 OryR mutant | 13 |
| A. tumefaciens NTL4(pZLQR) | Indicator strain for AHL detection | 5 |
| Plasmids | ||
| pMOSBlue | Cloning vector, Ampr | Amersham-Pharmacia |
| pSS122 | Promoter probe vector, IncW, Apr Gmr | This study |
| pORY122 | oryR promoter cloned with HindIII-SmaI in pSS122 | This study |
| pPIP122 | pip promoter cloned with HindIII-PstI in pSS122 | This study |
| pCBS122 | cbsA promoter cloned with HindIII-SalI in pSS122 | This study |
| pRPFF122 | rpfF promoter cloned with PstI-SalI in pSS122 | This study |
| Oligonucleotides | ||
| UIDAS | 5′-CCGGTACCTTGACCAGTATTAT-3′ | This study |
| UIDAR | 5′-CAGAATTCTCATTGTTTGCCTC-3′ | This study |
| ORYPRS | 5′-ATAAGCTTAGACGCCGCCGAAG-3′ | This study |
| ORYPRR | 5′-ATCCCGGGTAGACCAACGACTG-3′ | This study |
| PIPPRS | 5′-TTAAGCTTCGCGTGATGCGCTTG-3′ | This study |
| PIPPRR | 5′-TTCTGCAGTGGCCGCCAGATCCT-3′ | This study |
| CBSPRS | 5′-TTAAGCTTGCGTGTGGGCGTCAG-3′ | This study |
| CBSPRR | 5′-TTGTCGACCGCGCCTGTCAGCAA-3′ | This study |
| RPFFPRS | 5′-AACTGCAGATCGCCACCATGC-3′ | This study |
| RPFFPRR | 5′-CAGTCGACCGTCGAATTCTAT-3′ | This study |
Acknowledgments
S.F. was supported by an ICGEB fellowship. The research activities at the Biosafety Outstation were supported by the Fondazione Cassamarca, Treviso, Italy.
We thank M. Dow for providing the DSF biosensor strain and Sujatha Subramoni for reading the manuscript and for fruitful discussions.
Footnotes
Published ahead of print on 21 November 2008.
REFERENCES
- 1.Barber, C. E., J. L. Tang, J. X. Feng, M. Q. Pan, T. J. Wilson, H. Slater, J. M. Dow, P. Williams, and M. J. Daniels. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24555-566. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, W. D., and U. Mathesius. 2004. Plant responses to bacterial quorum sensing signals. Curr. Opin. Plant Biol. 7429-433. [DOI] [PubMed] [Google Scholar]
- 3.Birnboim, H. C. 1983. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 100243-255. [DOI] [PubMed] [Google Scholar]
- 4.Camara, M., P. Williams, and A. Hardman. 2002. Controlling infection by tuning in and turning down the volume of bacterial small-talk. Lancet Infect. Dis. 2667-676. [DOI] [PubMed] [Google Scholar]
- 5.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 111119-1129. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee, S., and R. V. Sonti. 2002. rpfF mutants of Xanthomonas oryzae pv. oryzae are deficient for virulence and growth under low iron conditions. Mol. Plant-Microbe Interact. 15463-471. [DOI] [PubMed] [Google Scholar]
- 7.Cho, H. J., Y. J. Park, T. H. Noh, Y. T. Kim, J. G. Kim, E. S. Song, D. H. Lee, and B. M. Lee. 2008. Molecular analysis of the hrp gene cluster in Xanthomonas oryzae pathovar oryzae KACC10859. Microb. Pathog. 44473-483. [DOI] [PubMed] [Google Scholar]
- 8.De Feyter, R., Y. Yang, and D. W. Gabriel. 1993. Gene-for-genes interactions between cotton R genes and Xanthomonas campestris pv. malvacearum avr genes. Mol. Plant-Microbe Interact. 6225-237. [DOI] [PubMed] [Google Scholar]
- 9.Degrassi, G., G. Devescovi, R. Solis, L. Steindler, and V. Venturi. 2007. Oryza sativa rice plants contain molecules that activate different quorum-sensing N-acyl homoserine lactone biosensors and are sensitive to the specific AiiA lactonase. FEMS Microbiol. Lett. 269213-220. [DOI] [PubMed] [Google Scholar]
- 10.Dixon, R. A. 2001. Natural products and plant disease resistance. Nature 411843-847. [DOI] [PubMed] [Google Scholar]
- 11.do Amaral, A. M., C. P. Toledo, J. C. Baptista, and M. A. Machado. 2005. Transformation of Xanthomonas axonopodis pv. citri by electroporation. Fitopatol. Bras. 30292-294. [Google Scholar]
- 12.Dow, J. M., L. Crossman, K. Findlay, Y. Q. He, J. X. Feng, and J. L. Tang. 2003. Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA 10010995-11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferluga, S., J. Bigirimana, M. Höfte, and V. Venturi. 2007. A LuxR homologue of Xanthomonas oryzae pv. oryzae is required for optimal rice virulence. Mol. lant Pathol. 8529-538. [DOI] [PubMed] [Google Scholar]
- 14.Fuqua, C. 2006. The QscR quorum-sensing regulon of Pseudomonas aeruginosa: an orphan claims its identity. J. Bacteriol. 1883169-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35439-468. [DOI] [PubMed] [Google Scholar]
- 16.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1786618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, Y. W., C. Wang, L. Zhou, H. Song, J. M. Dow, and L. H. Zhang. 2006. Dual signaling functions of the hybrid sensor kinase RpfC of Xanthomonas campestris involve either phosphorelay or receiver domain-protein interaction. J. Biol. Chem. 28133414-33421. [DOI] [PubMed] [Google Scholar]
- 18.He, Y. W., and L. H. Zhang. 2008. Quorum sensing and virulence regulation in Xanthomonas campestris. FEMS Microbiol. Rev. 32842-857. [DOI] [PubMed] [Google Scholar]
- 19.Jha, G., R. Rajeshwari, and R. V. Sonti. 2007. Functional interplay between two Xanthomonas oryzae pv. oryzae secretion systems in modulating virulence on rice. Mol. Plant-Microbe Interact. 2031-40. [DOI] [PubMed] [Google Scholar]
- 20.Lee, S. W., K. S. Jeong, S. W. Han, S. E. Lee, B. K. Phee, T. R. Hahn, and P. Ronald. 2008. The Xanthomonas oryzae pv. oryzae PhoPQ two-component system is required for AvrXA21 activity, hrpG expression, and virulence. J. Bacteriol. 1902183-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manefield, M., R. de Nys, N. Kumar, R. Read, M. Givskov, P. Steinberg, and S. Kjelleberg. 1999. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 145283-291. [DOI] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory,Cold Spring Harbor, NY.
- 23.Rajamani, S., W. D. Bauer, J. B. Robinson, J. M. Farrow III, E. C. Pesci, M. Teplitski, M. Gao, R. T. Sayre, and D. A. Phillips. 2008. The vitamin riboflavin and its derivative lumichrome activate the LasR bacterial quorum-sensing receptor. Mol. Plant-Microbe Interact. 211184-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 25.Schuster, M., M. L. Urbanowski, and E. P. Greenberg. 2004. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc. Natl. Acad. Sci. USA 10115833-15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen, Y., and P. Ronald. 2002. Molecular determinants of disease and resistance in interactions of Xanthomonas oryzae pv. oryzae and rice. Microbes Infect. 41361-1367. [DOI] [PubMed] [Google Scholar]
- 27.Slater, H., A. Alvarez-Morales, C. E. Barber, M. J. Daniels, and J. M. Dow. 2000. A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol. Microbiol. 38986-1003. [DOI] [PubMed] [Google Scholar]
- 28.Swings, J., M. Van den Mooter, L. Vauterin, B. Hoste, M. Gillis, T. W. Mew, and K. Kersters. 1990. Reclassification of the causal agents of bacterial blight (Xanthomonas campestris pv. oryzae) and bacterial leaf streak (Xanthomonas campestris pv. oryzicola) of rice as pathovars of Xanthomonas oryzae (ex Ishiyama 1922) sp. nov., nom. rev. Int. J. Syst. Bacteriol. 40309-311. [Google Scholar]
- 29.Teplitski, M., H. Chen, S. Rajamani, M. Gao, M. Merighi, R. T. Sayre, J. B. Robinson, B. G. Rolfe, and W. D. Bauer. 2004. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 134137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuchiya, K., T. W. Mew, and S. Wakimoto. 1982. Bacteriological and pathological characteristics of wild-types and induced mutants of Xanthomonas campestris pv. oryzae. Phytopathology 7243-46. [Google Scholar]
- 31.Urbanowski, M. L., C. P. Lostroh, and E. P. Greenberg. 2004. Reversible acyl-homoserine lactone binding to purified Vibrio fischeri LuxR protein. J. Bacteriol. 186631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vannini, A., C. Volpari, C. Gargioli, E. Muraglia, R. Cortese, R. De Francesco, P. Neddermann, and S. D. Marco. 2002. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 214393-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walters, M., and V. Sperandio. 2006. Quorum sensing in Escherichia coli and Salmonella. Int. J. Med. Microbiol. 296125-131. [DOI] [PubMed] [Google Scholar]
- 34.Waters, C. M., and B. L. Bassler. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21319-346. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, L., Y. Jia, L. Wang, and R. Fang. 2007. A proline iminopeptidase gene upregulated in planta by a LuxR homologue is essential for pathogenicity of Xanthomonas campestris pv. campestris. Mol. Microbiol. 65121-136. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, R. G., T. Pappas, J. L. Brace, P. C. Miller, T. Oulmassov, J. M. Molyneaux, J. C. Anderson, J. K. Bashkin, S. C. Winans, and A. Joachimiak. 2002. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature 417971-974. [DOI] [PubMed] [Google Scholar]