Abstract
SecA is the ATPase that provides energy for translocation of precursor polypeptides through the SecYEG translocon in Escherichia coli during protein export. We showed previously that when SecA receives the precursor from SecB, the ternary complex is fully active only when two protomers of SecA are bound. Here we used variants of SecA and of SecB that populate complexes containing two protomers of SecA to different degrees to examine both the hydrolysis of ATP and the translocation of polypeptides. We conclude that the low activity of the complexes with only one protomer is the result of a low efficiency of coupling between ATP hydrolysis and translocation.
The general secretory or Sec system in Escherichia coli translocates precursors of proteins across the cytoplasmic membrane into the periplasmic space (for a review, see reference 20). Some proteins, such as alkaline phosphatase and the binding proteins for amino acids and sugars, function as soluble proteins in the periplasm; others, such as OmpA, are inserted into the outer membrane. The path across the cytoplasmic membrane barrier is provided by a translocation channel comprising a heterotrimeric complex, SecY, SecE, and SecG (SecYEG). The energy for the movement is supplied by protonmotive force and the hydrolysis of ATP by SecA, which is a peripheral component of the membrane-associated translocon. The Sec system can export polypeptides only if they are devoid of stable tertiary structure. SecB, a small cytosolic chaperone, acts to capture precursors before they acquire stable structure and introduces them into the secretory pathway by delivering them to SecA. SecA can also bind precursors directly, as evidenced by the viability of strains of E. coli that lack SecB. However, efficiency of export is drastically reduced for many proteins (12).
Crystal structures of both SecB and SecA have been solved. SecB is a tetramer (monomer, 17 kDa) organized as a dimer of dimers (6, 33). There are six dimeric forms of SecA (2, 10, 19, 26, 30, 34) that differ greatly in the contacts that stabilize the dimeric interface. However, the structures of the protomers are all closely related and display only two different conformations, an open state (17) and a closed state (10). In free solution, SecA exists in a dynamic equilibrium between monomer and dimer characterized by an equilibrium constant of 0.1 μM to 1 μM, depending on the ionic strength and temperature of the solution (31). When SecA interacts with SecB, two protomers must be bound to SecB for the complex to be active, as assessed in vitro (23).
In this study, we asked why two protomers of SecA are required. We examined the translocation into inverted cytoplasmic membrane vesicles of two natural ligands, one a soluble periplasmic protein, galactose-binding protein, and the other an outer membrane protein, OmpA. We conclude that two protomers of SecA in the complex are required to achieve maximal coupling efficiency between ATP hydrolysis and translocation.
MATERIALS AND METHODS
Materials.
[γ-32P]ATP was purchased from either PerkinElmer (Boston, MA) or GE Healthcare (Pittsburgh, PA), [35S]methionine from PerkinElmer, precoated polyethyleneimine cellulose thin-layer chromatography plates from Merck KGaA (Darmstadt, Germany), trypsin from Millipore Corporation (Freehold, NJ), bovine pancreatic trypsin inhibitor (aprotinin) and DNase I from Sigma-Aldrich (St. Louis, MO), NAP10 and HiTrap Blue HP columns from GE Healthcare Bio-sciences AB (Uppsala, Sweden), N-[(2-pyridyldithio)ethyl]-4-azidosalicylamide (AET) from Toronto Research Chemicals Inc. (Toronto, Canada), Precision Plus protein standards (all blue) from Bio-Rad (Hercules, CA), and Staphylococcus aureus micrococcal nuclease from Worthington (Lakewood, NJ).
Protein purification.
SecA, SecAC4, SecAdN7, SecAdN10, SecB, and SecBL75Q were purified from strains of E. coli harboring plasmids that express the proteins as previously described (23, 24), except that cells expressing wild-type SecA and those expressing SecAdN10 were suspended at 2 g (wet weight) of cell pellet per ml of buffer and disrupted using a French press at 8,000 lb/in2. The P11 column was omitted from the SecAdN10 purification. For cross-linking experiments, SecAC4S350C and SecAC4I641C were purified from strains harboring plasmids derived from plasmid pT7secAC4, which carries a gene for SecA in which the four native cysteine codons are replaced by serine codons (22). For each SecA species, a single cysteine was introduced at the site of interest by site-directed mutagenesis (QuikChange; Stratagene). Proteins were purified as described previously (23) with minor changes. Micrococcal nuclease (314 units/ml [final]) was included along with DNase I (5,000 units/ml [final]) to degrade nucleic acids and decrease the viscosity of the lysate. SecAC4S350C was purified by chromatography on a HiTrap QAE column followed by a HiTrap Blue HP column instead of P11. For purification of SecAC4I641C, only a HiTrap Blue HP column was used. Precursor galactose-binding protein was purified as described previously (28). The precursor of OmpA labeled with [35S]methionine was produced from strains harboring plasmid pET503, which encodes pro-OmpA with the substitution C290S, and was purified as described previously (29). The culture was grown in M9 minimal medium supplemented with glycerol (0.4% wt/vol) as the carbon source, thiamine (4 μg/ml), and ampicillin (0.1 mg/ml). When the culture reached an optical density of 0.6 at 560 nm, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to 0.1 mM to induce pro-OmpA. Eighty minutes after induction, 1 mCi [35S]methionine (90 μl) mixed with 900 μl of 30 μM nonradioactive methionine was added to the culture. For use in the cross-linking experiments, a species of pro-OmpA with both native cysteines changed to serine (C290S and C302S) was constructed by site-directed mutagenesis (QuikChange; Stratagene) and purified as described previously (29). The precursors were stored in buffers containing denaturant: 1 N guanidine hydrochloride (GuHCl), 10 mM HEPES-KOH (pH 7.6), 0.3 M potassium acetate (KOAc), 5 mM Mg(OAc)2, 1 mM EGTA for precursor galactose-binding protein and 4 M urea, 10 mM HEPES-KOH (pH 7.6), 0.1 M KOAc for pro-OmpA. All proteins were stored at −80°C.
Cytoplasmic membrane vesicles.
Inverted cytoplasmic membrane vesicles were prepared as described previously (32) from E. coli strain HB3616, harboring plasmids that express SecE and SecG (pMAN809 with a tac-secG insertion [15]) and SecY (pMAN510 [15]) under the control of the tac promoter, except that to induce the proteins, IPTG was added to 1 mM. In order to remove endogenous SecA, the isolated vesicles were exposed to 5 M urea in 50 mM Tris-Cl (pH 8), 2 mM dithiothreitol (DTT) on ice for 30 min, centrifuged (65,000 rpm, 30 min, 4°C, type 65 rotor; Beckman Coulter, Fullerton, CA), and suspended in 10 mM HEPES-KOH (pH 7.6), 0.3 M KOAc, 5 mM Mg(OAc)2, 2 mM DTT. The vesicles were tested for translocation of precursors and for ATPase activity in the absence of added SecA. There was no protection of either precursor ligand and no detectable hydrolysis of ATP within the first 2 min. All calculations of efficiency of coupling were done within this time frame.
Translocation and ATPase assays.
Both assays were done in the same reaction mixture, which was made up in glass tubes (12 by 75 mm) so that temperature equilibration would occur rapidly. All mixtures contained 13 mM HEPES-KOH (pH 7.6), 250 mM KOAc, 5 mM Mg(OAc)2, 2 mM DTT, 3.3 mM [γ-32P]ATP (specific activity, 1.8 Ci/mol), and 2 μM SecA dimer. When specified, SecB was added to 2 μM tetramer and urea-treated inverted membrane vesicles to a final concentration of 0.6 mM lipid. The reactions were initiated by dilution of precursor (precursor galactose-binding protein or [35S]methionine pro-OmpA) to 2 μM from denaturant, and the glass tubes were immediately transferred to a water bath at 30°C. For reaction mixtures containing precursor galactose-binding protein, the final concentration of GuHCl was 12 mM, and for those containing pro-OmpA, the final urea concentration was 36 mM. For the experiments in which we determined the ratio of ATP hydrolysis to translocation, we did not provide NADH or an ATP-regenerating system because we were measuring hydrolysis of ATP using [γ-32P]ATP.
For assessment of both translocation and ATPase activity from the same reaction mixture, samples of 10 μl (from a total mixture of 100 μl) were taken into tubes held on ice at various times, from which samples of 2 μl were immediately removed to tubes with 2 μl 0.1 M EDTA on ice for the ATPase assay. To assess translocation, trypsin (5 mg/ml in 1 mM HCl) was added to a final concentration of 0.5 mg/ml to the tubes containing 8 μl reaction mixture, and the samples were incubated on ice for 15 min, at which time proteolysis was stopped by addition of bovine pancreatic trypsin inhibitor (44 mg/ml in H2O) to a final concentration of 5 mg/ml. For determination of the total amount of precursor added to the assay, 10-μl samples were taken at 1 min and 6 min and no trypsin was added. Nonreducing sodium dodecyl sulfate (SDS) gel sample buffer containing N-ethylmaleimide (8 mM) was added, and the samples were boiled immediately for 3 min and analyzed by SDS-polyacrylamide gel electrophoresis on the same day to avoid sample degradation.
Analyses by SDS-gel electrophoresis, immunoblotting, and thin-layer chromatography.
Polyacrylamide (10%, wt/wt) gels for experiments with [35S]methionine-labeled pro-OmpA were dried and exposed to an imaging plate (Fuji Film, Stamford, CT) overnight, scanned using a PhosphorImager (Fuji Film, Stamford, CT), and analyzed with ImageGauge 4.0 (Fuji Film, Stamford, CT). Immunoblots of 10% (wt/wt) gels were used to analyze the results of all precursor galactose-binding protein experiments and the results presented in Fig. 3. Blots were processed by incubation with a rabbit antiserum raised to the appropriate purified protein and then with goat antibodies raised to rabbit immunoglobulin Gs and conjugated with horseradish peroxidase (Bio-Rad), followed by staining with a 4-chloro-1-naphthol-hydrogen peroxide solution. All samples for precursor galactose-binding protein were electrophoresed on the same gel as the purified precursor, which was applied in quantities of 5 ng, 10 ng, and 20 ng to generate a standard curve. The amount of protein at each time point was determined using only those intensities that were within the linear range of the standard. A Kodak EDAS 290 digital camera was used to capture images of the immunoblots, and TotalLab software (version 2.01; Nonlinear Dynamics Ltd.) was used to quantify the band intensities. The concentration of the precursor added to the assay in combination with the percentage of protein protected from trypsin digestion was used to determine the concentration of precursor protected for both ligands.
FIG. 3.

Translocation of pro-OmpA in the presence and absence of SecB. In vitro translocation of 2 μM pro-OmpA by wild-type SecA (circles) or SecAdN7 (triangles) at 2 μM SecA expressed as a dimer was carried out in the presence (filled symbols) or absence (open symbols) of 2 μM SecB expressed as a tetramer. The efficiency of translocation without SecB is extremely low; therefore, to maximize activity for these experiments, 1.7 mM NADH, 7.5 mM phosphocreatine, and 37 mg/ml creatine phosphokinase were included in the in vitro system to regenerate ATP. The samples were analyzed by immunoblotting.
Thin-layer chromatography was used to analyze the hydrolysis of ATP. One microliter of the samples taken into EDTA for the ATPase assay as described above was applied to a precoated thin-layer chromatography plate and dried. After application of all samples, the plate was developed in 125 mM KH2PO4 (13). After drying, the plates were exposed and scanned using the PhosphorImager. The ATPase activity was estimated from the proportion of total radioactivity that migrated as inorganic phosphate (Rf ∼ 0.59).
Calculation of the efficiency of coupling ATP hydrolysis to translocation.
The efficiency of the coupling of the hydrolysis of ATP to the translocation of precursor polypeptides was calculated using time points taken within the first 2 min. Early time points were used for two reasons. Firstly, ADP has higher affinity for SecA than does ATP (8). Therefore, the accumulation of ADP at later times would be expected to suppress activity. We did not include an ATP-regenerating system, since we use the appearance of [32P]phosphate as the assay for hydrolysis. Secondly, as the process of translocation approaches a plateau, the translocation ATPase activity is replaced by membrane ATPase activity, since SecA without precursor bound still binds SecYEG. This would result in false values for the coupling.
Cross-linking with a photoactivatable reagent.
Each of the SecA variants, SecAC4S350C and SecAC4I641C, has a single cysteine as specified. Each variant was labeled with the sulfhydryl-specific photoactivatable reagent AET (1). The protein to be labeled was exchanged into 100 mM Na2B4O7, 100 mM KOAc (pH 8.3) using a NAP10 column. All subsequent steps were done in the dark. AET (stored at 45 mM in dimethyl sulfoxide at −80°C) was added at a 10-fold molar excess over cysteine in the SecA, and the mixture was incubated for 2 h at room temperature followed by 1 h on ice. Free AET was removed by exchange of the protein into 10 mM HEPES-HOAc (pH 6.7), 300 mM KOAc, 5 mM Mg(OAc)2 using a NAP10 column. The AET modification was confirmed by MALDI (matrix-assisted laser desorption ionization) mass spectrometry. Mixtures (∼30 μl) of the AET-labeled SecA (12 μM dimer) and precursor (either precursor galactose-binding protein or pro-OmpA at 12 μM) in 10 mM HEPES-HOAc (pH 6.7), 300 mM KOAc, 5 mM Mg(OAc)2 were prepared, placed in the shallow spots of a porcelain spot plate held on ice, and irradiated with a mercury lamp for 1 min. Samples were analyzed by SDS-polyacrylamide (10%, wt/wt) gel electrophoresis using both reducing and nonreducing sample buffers. Gels (both reduced and nonreduced samples) were run in duplicate and subjected to immunoblotting using antisera to purified SecA as well as to the relevant precursor.
Characterization of protein preparations and determination of lipid concentration.
Protein concentrations were determined spectrophotometrically at 280 nm using extinction coefficients of 47,600 M−1 cm−1 for SecB tetramer, 157,800 M−1 cm−1 for SecA dimer, 52,955 M−1 cm−1 for pro-OmpA, and 37,410 M−1 cm−1 for precursor galactose-binding protein. Since modification by AET changes the absorbance of the SecA variants, those concentrations were determined by electrophoresis of at least three samples of the protein in increasing quantities on the same SDS-polyacrylamide (14%, wt/wt) gel as three quantities of pure SecA of known concentration to generate a standard curve. The amounts of protein in the standards and the variants were determined as described for quantification of protein on immunoblots. Concentration of lipid in the vesicle preparation was determined as described on the Avanti Polar Lipids website using an average molar mass for E. coli lipids of 741 Da.
All protein preparations used in this study were subjected to rigorous characterization. They were analyzed by mass spectrometry. The only degradation detected was in SecAdN10, where approximately 10% of the protein was cleaved to generate two fragments of 67,000 Da and 33,000 Da. The ratio of absorbance at 280 nm to 260 nm for all preparations was 2.0 except that for SecAC4, which was 1.8; thus, there was no significant contamination by nucleic acid or nucleotides. Column chromatography carried out with a static light scatter detector and analytical centrifugation showed that the protein preparations contained no aggregation and formed the expected complexes with stoichiometry of either the A1:B4 or A2:B4 complex, consistent with our previously published results (23). The purity of all preparations was between 80% and 90%.
RESULTS
Translocation of precursors and ATP hydrolysis mediated by SecA variants with different dimer equilibria.
Complexes between wild-type SecA and SecB as well as between variants were assayed for both translocation of precursors and the associated translocation ATPase activity to determine the effect of varying the number of protomers of SecA in the complex. The species of SecA studied were wild-type SecA and three variants of SecA that were characterized previously (5, 23): SecAdN10 (amino acids 2 through 11 deleted), SecAdN7 (amino acids 2 through 8 deleted), and SecAC4, which lacks zinc. In addition to the complex between wild-type SecA and SecB, we studied a complex containing a SecB variant, SecBL75Q, which carries a substitution that interferes with binding the zinc site on SecA. In earlier work (23), we used size exclusion chromatography coupled with static light scatter to demonstrate that the complex between SecB and SecA has a mass of 272 kDa, indicating that the stoichiometry of the wild-type complex is two protomers of SecA (mass, 204 kDa for a dimer) bound to a tetramer of SecB (mass, 69 kDa), referred to herein as A2:B4. It is possible to populate complexes containing only one protomer of SecA because although SecA and SecB both display twofold symmetry, the A2:B4 complex is stabilized by contacts that are distributed asymmetrically. Only one protomer of SecA can bind if the area of contact that is between residues in the β-sheets that form the flat sides of SecB and the C-terminal zinc domain of SecA is eliminated. Here we examined complexes that have that contact disrupted in two ways: wild-type SecA bound to a mutant of SecB (SecBL75Q) that has a residue located on the flat β-sheet changed and wild-type SecB bound to a SecA variant (SecAC4) that lacks zinc because the cysteines that coordinate the zinc are replaced by serines. Both complexes were shown to have a stoichiometry of A1:B4 (23), i.e., a molar mass of 171 kDa.
Complexes with a stoichiometry of A1:B4 can also be populated by using species of SecA that have the monomer-dimer equilibrium altered by truncation at the N terminus. Deletion of amino acids 2 through 8 (SecAdN7) shifts the equilibrium toward monomer (equilibrium dissociation constant [KD] ∼ 24 μM) (5), whereas deletion of amino acids 2 through 11 results in a species, SecAdN10, that exists only as a monomer (KD > 230 μM) (5). SecAdN10 was shown to form a complex with SecB with a mass of 169 kDa, corresponding to a stoichiometry of A1:B4 (23), whereas the mass observed for a mixture of SecAdN7 and SecB was 235 kDa, indicating an equilibrating population that contains both A1:B4 and A2:B4. It is not possible to estimate the amount of any one species in such a mixture because it would contain not only complexes with stoichiometry A1:B4 and A2:B4 but also free SecA, which would itself be in equilibrium between monomer and dimer.
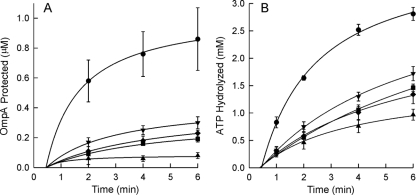
The various combinations of SecA and SecB displaying different equilibrating mixtures of complexes with A1:B4 and A2:B4 stoichiometries were tested for the translocation of precursor into inverted membrane vesicles and for the coupled hydrolysis of ATP. Translocation of the precursor of the outer membrane protein OmpA, pro-OmpA, mediated by SecAdN10-SecB complexes (A1:B4) is 22% of that mediated by complexes containing wild-type SecA (A2:B4), 0.19 μM versus 0.86 μM, respectively, at the 6-min time point; whereas ATP hydrolysis is 52% of the activity of the wild-type complex (Fig. 1A and 1B). Similar results were seen for translocation of precursor galactose-binding protein (Fig. 2A and 2B); translocation of precursor by the SecAdN10-SecB complex is approximately 24% of that mediated by a wild-type complex, whereas ATP hydrolysis is 37% of wild-type activity. These data indicate that the efficiency of coupling of hydrolysis of ATP to movement of the precursor through the translocon is much poorer for the SecA-SecB complex, which has a stoichiometry of A1:B4, than it is for complexes having two protomers bound to SecB as in the wild-type complexes. The translocation activity of complexes containing SecB and SecAdN7 (Fig. 1A and 2A) lies between the activity of wild-type complexes and that of complexes containing SecAdN10. As described above, the equilibrating population of complexes between SecAdN7 and SecB contains both A1:B4 and A2:B4; therefore, we conclude that shifting the equilibrium to complexes containing two protomers of SecA results in more robust translocation. In addition the efficiency of coupling of ATP hydrolysis to translocation is improved. The data in Fig. 1 and 2 were used to calculate the efficiency of coupling of hydrolysis of ATP to translocation of a precursor polypeptide (Table 1; see Materials and Methods for calculations). The efficiency of coupling for pro-OmpA was the highest for the A2:B4 wild-type complex, which hydrolyzed approximately 2,500 mol of ATP per mol of precursor protected. Complexes with SecAdN7, which contain a mixture of A1:B4 and A2:B4, displayed a coupling of 3,600 mol ATP hydrolyzed per mol precursor protected, and the lowest efficiency was observed with A1:B4 complexes: SecAdN10 plus SecB, zincless SecAC4 plus SecB, and SecA plus SecBL75Q. The coupling efficiency for precursor galactose-binding protein showed the same trend: highest for wild-type complexes, intermediate for complexes formed with SecAdN7, and lowest for those complexes which populate only A1:B4 complexes (Table 1).
FIG. 1.
Translocation of pro-OmpA mediated by A1:B4 and A2:B4 complexes. (A) Protection of pro-OmpA; (B) associated ATPase activity of the translocation. In vitro assay mixtures contained pro-OmpA, SecA species, and SecB species at 2 μM each, expressed as dimeric SecA and tetrameric SecB, as follows: wild-type SecA (circles), SecAdN7 (down triangles), and SecAdN10 (squares) with wild-type SecB; SecAC4 with wild-type SecB (up triangles); and wild-type SecA with SecBL75Q (diamonds). The data here and in Fig. 2 and 3 were fitted to a hyperbola using SigmaPlot 2001 software. The error bars show standard deviations. All assays were done at least three times. Where error bars are not obvious, they fall within the symbols. For all fits, 0.45 min was used as the intercept on the x axis.
FIG. 2.
Translocation of precursor galactose-binding protein mediated by A1:B4 and A2:B4 complexes. (A) Protection of precursor galactose-binding protein (pGBP); (B) associated ATPase activity of the translocation. Concentrations of proteins and symbols are the same as in Fig. 1. The samples were analyzed by immunoblotting.
TABLE 1.
Coupling of ATP hydrolysis to translocation
| Precursor | Hydrolysisa with complex
|
||||
|---|---|---|---|---|---|
| SecA-SecB | SecAdN7-SecB | SecAdN10-SecB | SecAC4-SecB | SecA-SecBL75Q | |
| Pro-OmpA | 2,500 | 3,600 | 5,800 | 5,800 | 5,600 |
| pGBPb | 4,200 | 6,800 | 11,500 | 11,500 | ND |
Moles of ATP hydrolyzed per mole of precursor protected. SecA and SecB are the wild-type species. ND, not determined (reliable results could not be generated because of the low level of precursor protected).
Precursor galactose-binding protein.
Since the stoichiometry between SecA and SecB was varied both by using full-length SecA as well as a truncated species and by using wild-type SecB as well as a variant of SecB, it is safe to conclude that the observed decrease in efficiency of the coupling of ATP hydrolysis to translocation results from a decrease in occupancy of complexes having a stoichiometry of A2:B4 and not from either the truncations of SecA or the mutation of SecB.
Stoichiometry of complexes between SecA and precursors.
Although SecA mediates a higher level of translocation when it receives precursors via a SecB-precursor complex as opposed to binding precursor directly, it does function in vitro in the absence of SecB (Fig. 3). SecAdN10, which is monomeric, shows no detectable processing in the absence of SecB, whereas SecAdN7, which does populate dimers but to a lesser extent than does the wild type (23), shows a low level of translocation which is greatly enhanced by addition of SecB. SecA can mediate translocation in the absence of SecB in vivo, as evidenced by the viability of SecB-null strains (12). Therefore, it is of interest to determine the stoichiometry of a complex between SecA and precursors in the absence of SecB. We were not able to demonstrate a complex between SecA and precursors using size exclusion chromatography; therefore, we could not use the approaches we have applied previously to reveal stoichiometry by determination of molar mass using static light scatter (23). As an alternative, we used cross-linking. We chose a photoactivatable cross-linking reagent, AET, to determine whether one protomer of SecA or two interact with one precursor polypeptide. The reagent was attached via a disulfide exchange reaction to two SecA variants, each having a single cysteine, one at position 350 and the other at position 641. These sites were identified as contact sites between SecA and its precursor ligands by site-directed spin labeling and electron paramagnetic resonance spectroscopy (3). Irradiation of solutions that contained complexes between the derivatized SecA variants and either pro-OmpA or precursor galactose-binding protein generated covalent linkages between the proteins. Analyses of the irradiated samples by SDS-polyacrylamide gel electrophoresis followed by immunoblotting using antisera to SecA and to each of the precursors revealed that the complex formed between SecA and either pro-OmpA or precursor galactose-binding protein had a molar mass of approximately 250 kDa (Fig. 4). This was true whether the reagent was linked to SecA at amino acid position 350 or at position 641. Addition of reducing agent to the SDS-gel sample buffer caused disappearance of the high-molecular-weight cross-linked species (data not shown), which was expected since the AET moiety is linked to SecA via a disulfide bond. The ability of the AET-labeled SecAC4I641 to cross-link to SecB was tested as a control for specificity. Electron paramagnetic resonance studies (3) have shown that SecAC4I641 is not a site of contact within the SecA-SecB complex. As expected, no cross-linked species was observed (data not shown). Bands migrating with an apparent mass of approximately 150 kDa that appeared upon irradiation even in the absence of a precursor ligand are likely to be cross-linked dimers of the two SecA species, SecAC4S350AET and SecAC4I641AET. The aberrant migration of the dimers can be explained because the pairs of SecA polypeptides are tethered at two internal positions, thereby preventing the chain from becoming fully extended in SDS.
FIG. 4.
Cross-linking of AET-SecA to precursors. (A) Cross-linking to pro-OmpA; (B) cross-linking to precursor galactose-binding protein (pGBP). Immunoblots using antiserum to SecA are shown in the upper panels, and those using antisera to the precursors are in the lower panels. Cross-linked complexes are indicated by dots and irradiation by hν. Molecular mass markers (MW) are shown on the right.
We conclude that each precursor species binds two protomers of SecA to give the observed mass, approximately 250 kDa (204 kDa for SecA dimer and 37 kDa for pro-OmpA or 36 kDa for precursor galactose-binding protein). Since chemical cross-linking results in an irreversible reaction, we have no information relating to the affinity of SecA for the precursors or whether the protomers of SecA bind to the precursor sequentially or as a dimer.
DISCUSSION
We previously showed that formation of a complex between SecA and SecB that is capable of mediating a high level of translocation of precursors requires two protomers of SecA bound to a tetramer of SecB. Within the complex there are three areas of contact. One site involves the C-terminal zinc-containing region of SecA, which interacts with the negatively charged region on the flat β-sheets that form the sides of the SecB dimer of dimers (4). A second interaction is between the extreme C-terminal flexible tail of SecB and the amino-terminal 11 amino acids of SecA. A third area of contact that provides energy of stabilization is less defined but involves residues lying on the β-sheets of SecB as well as along the interface of the dimer of dimers (21). Even though each of the binding partners displays twofold symmetry, the contacts between them are distributed asymmetrically. When the contact between the zinc domain on SecA and the side of SecB is disrupted or when 10 amino acyl residues are deleted from the amino terminus of SecA, only one protomer of SecA is bound, yielding a complex with a stoichiometry of A1:B4 that displays a very low activity both in vivo (11) and in vitro (9, 23, 24, 32).
Here we asked why two protomers of SecA must be present for full activity in the reaction cycle of SecA and SecB during translocation. We made use of variants of both SecA and SecB and the complexes they form, all of which are well characterized. Assays of the extent of translocation of precursors and the associated translocation ATPase activity allowed us to establish a correlation between an increase in the efficiency of coupling of ATP hydrolysis to translocation of polypeptides and an increase in the population of complexes containing two protomers of SecA bound to SecB. We established this correlation by varying the population of complexes that have stoichiometries of A1:B4 and A2:B4 by using species of SecA truncated at the extreme amino terminus with wild-type and a truncated SecB, wild-type SecA with a mutant of SecB, and wild-type SecB with full-length SecA containing no zinc. In every case, the higher efficiency of coupling occurs when the A2:B4 complex is more populated. The construct with amino acids 2 through 8 deleted (SecAdN7) was previously studied by Mori et al. (16) under the name of SecA N-8. The two preparations differ in that our purified protein retains the N-terminal methionine, whereas theirs does not. It was shown that SecA N-8 was defective in protein translocation, the translocation ATPase activity, and the topological inversion of SecG. The investigators concluded that the amino-terminal region of SecA is involved in functional interaction with SecG. In our work described here, we eliminated the possibility that the effects we observed are specific for deletions at the amino terminus. It seems possible that the effects observed by Mori et al. (16) also result from the oligomeric state of SecA and not directly from the lack of seven amino acyl residues. Perhaps the inversion of SecG involves two protomers of SecA.
By what mechanism might two protomers of SecA increase the coupling of ATP hydrolysis to movement? Lill et al. (14) showed that in a system using inverted membrane vesicles, such as the one we used here, SecA demonstrates a high rate of nonproductive hydrolysis, hydrolyzing more than 1,000 mol of ATP per mol of precursor translocated, a level similar to that observed here for wild-type SecA (Table 1) as well as that observed by others (27). It was subsequently shown by Schiebel et al. (25) that the poor coupling is the result of a backward slippage of the precursor undergoing translocation. It might be that the two protomers of SecA are required to work together to prevent the backward movement. One protomer might insert a segment of the bound precursor polypeptide into the translocon channel and release it upon hydrolysis of ATP; the second protomer would remain bound to the next more distal segment of the precursor polypeptide and, if also bound to SecYEG, would prevent backward movement of the polypeptide chain. This idea is consistent with observations by others (7, 18). Osborne and Rapoport (18) proposed that, based on cross-linking of SecA and pro-OmpA to a covalent dimeric form of SecYEG, one copy of SecYEG serves as the translocation channel and the other provides a static binding site for SecA. These authors proposed that only a single protomer of SecA is involved, but a slight modification adapts this model to the idea we have put forth here: two protomers of SecA act together to translocate precursors, one staying bound while the other releases the polypeptide into the channel and dissociates from SecYEG. Duong (7) observed association of both monomeric and dimeric SecA to the translocon using the same covalent dimeric SecYEG, but upon addition of ATP, only SecA monomers remained associated.
SecA can mediate translocation of precursors through the Sec secretory pathway in the absence of SecB both in vivo (12) and in vitro. It is currently debated whether when acting in the absence of SecB, SecA functions as a monomer or dimer. Although the in vitro translocation activity is very low without SecB, the trend is the same as that seen with SecB complexes: SecAdN7 shows very low but detectable activity, whereas SecAdN10 shows none. This correlation suggests that although monomers do function, the dimers are more efficient. We show here that even when SecA interacts directly with precursors, two protomers can associate with each precursor polypeptide. Since it is unlikely that SecA has a different mechanism of coupling hydrolysis to movement of the polypeptide through the translocon in the presence and absence of SecB, the two protomers might act together as proposed here to prevent backward slippage.
We conclude that maximal efficiency of translocation is achieved when two protomers of SecA in complex with SecB act together. A single protomer of SecA can perform the necessary conversion of chemical energy (hydrolysis of ATP) to mechanical work (movement of the precursor through the translocon), but tight coupling of this conversion requires two protomers. An exciting direction for future research is elucidation of the movement within SecA at the resolution of amino acyl side chains that transduces the chemical energy of hydrolysis of ATP to insertion of precursors into and through the translocon.
Acknowledgments
We thank Donald Oliver (Wesleyan University) for supplying the plasmid pT7secAC4, which carries the secA gene with the four native cysteine codons changed to serine codons, Angela A. Lilly for construction of the SecA variants, Beverly DaGue (The Proteomics Center, University of Missouri) for MALDI mass spectrometry, Hilary Roth for assistance with the in vitro activity assays, and Jennine M. Crane for critically reading the manuscript.
This work was supported by NIH research grant GM29798 to L.L.R.
Footnotes
Published ahead of print on 31 October 2008.
REFERENCES
- 1.Cai, K., Y. Itoh, and H. G. Khorana. 2001. Mapping of contact sites in complex formation between transducin and light-activated rhodopsin by covalent crosslinking: use of a photoactivatable reagent. Proc. Natl. Acad. Sci. USA 984877-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, Y., X. Pan, Y. Tang, S. Quan, P. C. Tai, and S.-F. Sui. 2008. Full-length Escherichia coli SecA dimerizes in a closed conformation in solution as determined by cryo-electron microscopy. J. Biol. Chem. 28328783-28787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper, D. B., V. F. Smith, J. M. Crane, H. C. Roth, A. A. Lilly, and L. L. Randall. 2008. SecA, the motor of the secretion machine, binds diverse partners on one interactive surface. J. Mol. Biol. 38274-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crane, J. M., C. Mao, A. A. Lilly, V. F. Smith, Y. Suo, W. L. Hubbell, and L. L. Randall. 2005. Mapping of the docking of SecA onto the chaperone SecB by site-directed spin labeling: insight into the mechanism of ligand transfer during protein export. J. Mol. Biol. 353295-307. [DOI] [PubMed] [Google Scholar]
- 5.Das, S., E. Stivison, E. Folta-Stogniew, and D. Oliver. 2008. Re-examination of the role of the amino terminus of SecA in promoting its dimerization and functional state. J. Bacteriol. 1907302-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dekker, C., B. de Kruijff, and P. Gros. 2003. Crystal structure of SecB from Escherichia coli. J. Struct. Biol. 144313-319. [DOI] [PubMed] [Google Scholar]
- 7.Duong, F. 2003. Binding, activation and dissociation of the dimeric SecA ATPase at the dimeric SecYEG translocase. EMBO J. 224375-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fak, J. J., A. Itkin, D. D. Ciobanu, E. C. Lin, X. J. Song, Y. T. Chou, L. M. Gierasch, and J. F. Hunt. 2004. Nucleotide exchange from the high-affinity ATP-binding site in SecA is the rate-limiting step in the ATPase cycle of the soluble enzyme and occurs through a specialized conformational state. Biochemistry 437307-7327. [DOI] [PubMed] [Google Scholar]
- 9.Fekkes, P., J. G. de Wit, A. Boorsma, R. H. Friesen, and A. J. Driessen. 1999. Zinc stabilizes the SecB binding site of SecA. Biochemistry 385111-5116. [DOI] [PubMed] [Google Scholar]
- 10.Hunt, J. F., S. Weinkauf, L. Henry, J. J. Fak, P. McNicholas, D. B. Oliver, and J. Deisenhofer. 2002. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science 2972018-2026. [DOI] [PubMed] [Google Scholar]
- 11.Kimsey, H. H., M. D. Dagarag, and C. A. Kumamoto. 1995. Diverse effects of mutation on the activity of the Escherichia coli export chaperone SecB. J. Biol. Chem. 27022831-22835. [DOI] [PubMed] [Google Scholar]
- 12.Kumamoto, C. A., and J. Beckwith. 1985. Evidence for specificity at an early step in protein export in Escherichia coli. J. Bacteriol. 163267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levit, M. N., T. W. Grebe, and J. B. Stock. 2002. Organization of the receptor-kinase signaling array that regulates Escherichia coli chemotaxis. J. Biol. Chem. 27736748-36754. [DOI] [PubMed] [Google Scholar]
- 14.Lill, R., K. Cunningham, L. A. Brundage, K. Ito, D. Oliver, and W. Wickner. 1989. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 8961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuyama, S., J. Akimaru, and S. Mizushima. 1990. SecE-dependent overproduction of SecY in Escherichia coli. Evidence for interaction between two components of the secretory machinery. FEBS Lett. 26996-100. [DOI] [PubMed] [Google Scholar]
- 16.Mori, H., H. Sugiyama, M. Yamanaka, K. Sato, M. Tagaya, and S. Mizushima. 1998. Amino-terminal region of SecA is involved in the function of SecG for protein translocation into Escherichia coli membrane vesicles. J. Biochem. 124122-129. [DOI] [PubMed] [Google Scholar]
- 17.Osborne, A. R., W. M. Clemons, Jr., and T. A. Rapoport. 2004. A large conformational change of the translocation ATPase SecA. Proc. Natl. Acad. Sci. USA 10110937-10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osborne, A. R., and T. A. Rapoport. 2007. Protein translocation is mediated by oligomers of the SecY complex with one SecY copy forming the channel. Cell 12997-110. [DOI] [PubMed] [Google Scholar]
- 19.Papanikolau, Y., M. Papadovasilaki, R. B. G. Ravelli, A. A. McCarthy, S. Cusack, A. Economou, and K. Petratos. 2007. Structure of dimeric SecA, the Escherichia coli preprotein translocase motor. J. Mol. Biol. 3661545-1557. [DOI] [PubMed] [Google Scholar]
- 20.Papanikou, E., S. Karamanou, and A. Economou. 2007. Bacterial protein secretion through the translocase nanomachine. Nat. Rev. Microbiol. 5839-851. [DOI] [PubMed] [Google Scholar]
- 21.Patel, C. N., V. F. Smith, and L. L. Randall. 2006. Characterization of three areas of interactions stabilizing complexes between SecA and SecB, two proteins involved in protein export. Protein Sci. 151379-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramamurthy, V., and D. Oliver. 1997. Topology of the integral membrane form of Escherichia coli SecA protein reveals multiple periplasmically exposed regions and modulation by ATP binding. J. Biol. Chem. 27223239-23246. [DOI] [PubMed] [Google Scholar]
- 23.Randall, L. L., J. M. Crane, A. A. Lilly, G. Liu, C. Mao, C. N. Patel, and S. J. S. Hardy. 2005. Asymmetric binding between SecA and SecB two symmetric proteins: implications for function in export. J. Mol. Biol. 348479-489. [DOI] [PubMed] [Google Scholar]
- 24.Randall, L. L., J. M. Crane, G. Liu, and S. J. S. Hardy. 2004. Sites of interaction between SecA and the chaperone SecB, two proteins involved in export. Protein Sci. 131124-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
25.Schiebel, E., A. J. Driessen, F. U. Hartl, and W. Wickner. 1991.
 and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell 64927-939. [DOI] [PubMed] [Google Scholar]
and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell 64927-939. [DOI] [PubMed] [Google Scholar] - 26.Sharma, V., A. Arockiasamy, D. R. Ronning, C. G. Savva, A. Holzenburg, M. Braunstein, W. R. Jacobs, Jr., and J. C. Sacchettini. 2003. Crystal structure of Mycobacterium tuberculosis SecA, a preprotein translocating ATPase. Proc. Natl. Acad. Sci. USA 1002243-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomkiewicz, D., N. Nouwen, R. van Leeuwen, S. Tans, and A. J. M. Driessen. 2006. SecA supports a constant rate of preprotein translocation. J. Biol. Chem. 28115709-15713. [DOI] [PubMed] [Google Scholar]
- 28.Topping, T. B., and L. L. Randall. 1997. Chaperone SecB from Escherichia coli mediates kinetic partitioning via a dynamic equilibrium with its ligands. J. Biol. Chem. 27219314-19318. [DOI] [PubMed] [Google Scholar]
- 29.van der Does, C., J. de Keyzer, M. van der Laan, and A. J. M. Driessen. 2003. Reconstitution of purified bacterial preprotein translocase in liposomes. Methods Enzymol. 37286-98. [DOI] [PubMed] [Google Scholar]
- 30.Vassylyev, D. G., H. Mori, M. N. Vassylyeva, T. Tsukazaki, Y. Kimura, T. H. Tahirov, and K. Ito. 2006. Crystal structure of the translocation ATPase SecA from Thermus thermophilus reveals a parallel, head-to-head dimer. J. Mol. Biol. 364248-258. [DOI] [PubMed] [Google Scholar]
- 31.Woodbury, R. L., S. J. S. Hardy, and L. L. Randall. 2002. Complex behavior in solution of homodimeric SecA. Protein Sci. 11875-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodbury, R. L., T. B. Topping, D. L. Diamond, D. Suciu, C. A. Kumamoto, S. J. S. Hardy, and L. L. Randall. 2000. Complexes between protein export chaperone SecB and SecA. Evidence for separate sites on SecA providing binding energy and regulatory interactions. J. Biol. Chem. 27524191-24198. [DOI] [PubMed] [Google Scholar]
- 33.Xu, Z., J. D. Knafels, and K. Yoshino. 2000. Crystal structure of the bacterial protein export chaperone SecB. Nat. Struct. Biol. 71172-1177. [DOI] [PubMed] [Google Scholar]
- 34.Zimmer, J., W. Li, and T. A. Rapoport. 2006. A novel dimer interface and conformational changes revealed by an X-ray structure of B. subtilis SecA. J. Mol. Biol. 364259-265. [DOI] [PubMed] [Google Scholar]