Abstract
Some cholate derivatives that are normal components of bile can act with glycine to induce the germination of Clostridium difficile spores, but at least one bile component, chenodeoxycholate, does not induce germination. Here we show that chenodeoxycholate inhibits the germination of C. difficile spores in response to cholate and taurocholate.
The anaerobic human pathogen Clostridium difficile must be in the spore form to survive for extended periods of time outside the colonic environment (6). Spores are also the form of the organism most likely to be ingested by a host. To cause disease, however, C. difficile spores must germinate in the gastrointestinal tract and reach the anaerobic environment of the colon, where they can grow out as vegetative bacteria (2). The vegetative form produces two toxins that damage the colonic epithelium and lead to C. difficile-associated diseases, such as diarrhea, pseudomembranous colitis, and toxic megacolon (4, 15). Extending the work of Wilson and colleagues (17, 18), we have shown that certain bile salts and glycine act as cogerminants for C. difficile spores (13). Primary bile salts produced by the liver are composed mainly of cholate (CA) and chenodeoxycholate (CDCA) derivatives conjugated with either taurine or glycine (11). Since CA derivatives are found in the relatively aerobic proximal ileum (9), we reasoned that C. difficile might benefit if its germination were inhibited until the spores reached the anaerobic environment of the large intestine.
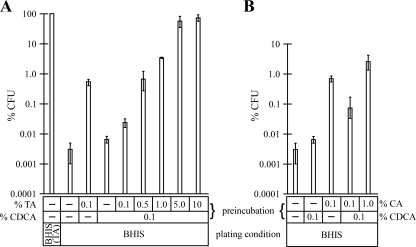
Inhibitors of germination are typically structurally similar to the germinant whose activities they inhibit. For example, l-alanine-mediated germination of Bacillus subtilis spores is inhibited by d-alanine (16) and 6-thioguanosine inhibits inosine-mediated germination in Bacillus anthracis (1, 16). Since CA and CDCA are structurally similar but CA induces the germination of C. difficile spores (13) and CDCA does not, we tested whether CDCA could act as an inhibitor of germination. C. difficile strain CD196 (10) spores were produced and their concentration determined as described previously (13). After the vegetative bacteria were killed by incubation at 60°C for 20 min, spores were incubated in water containing various concentrations and combinations of bile salts for 10 min. Here we took advantage of the finding by Wilson et al. that C. difficile spores germinate very inefficiently on rich medium plates lacking bile salts (18) unless they are preincubated with bile salts (13, 17). After incubation, spores were serially diluted and plated on brain heart infusion agar supplemented with 5 g yeast extract per liter-0.1% l-cysteine (BHIS) (Difco) in the absence of any bile salt (BHIS contains enough glycine to act as a cogerminant). After overnight growth at 37°C, colonies were enumerated. As a positive or negative control, spores were plated on BHIS containing 0.1% taurocholate (TA) [BHIS(TA)] or on BHIS agar alone, respectively. Preincubation of spores with 0.1% TA in water resulted in the recovery of approximately 0.5% of the total number of spores as colonies compared to results for spores plated directly on BHIS(TA). These results are similar to our previous findings that spores germinate and grow out as colonies more efficiently on agar medium containing TA (13). As reported previously, 0.1% CDCA poorly stimulated colony formation by C. difficile spores (13), yielding only 0.006% spore recovery (Fig. 1A). When TA and CDCA were combined, both at 0.1%, colony formation by C. difficile spores was reduced 21-fold to 0.024% compared to the effect of TA alone. This result indicates that CDCA blocks TA-stimulated colony formation and suggests that CDCA may be an inhibitor of C. difficile spore germination. Increasing the ratio of TA to CDCA suppressed the inhibitory effect of CDCA, increasing colony formation by spores (Fig. 1A). Thus, CDCA seems to block colony formation by competing with TA.
FIG. 1.
CDCA inhibits colony formation by C. difficile spores in response to TA and CA. (A) Spores were prepared and preincubated with TA or CDCA or both in water for 10 min before serial dilution and plating on BHIS agar in the absence of TA. Spores plated on BHIS(TA) served as a positive control for 100% colony formation (CFU). Based on comparisons of total spore counts obtained by microscopy and by colony formation on BHIS(TA) plates, the efficiency of colony formation on BHIS(TA) was estimated at 83%. (B) Spores were prepared as described for panel A and exposed to CA or CDCA or both. Values shown are the averages for three independent experiments, and error bars represent one standard deviation from the mean.
CA and other cholate derivatives (e.g., TA, glycocholate, and deoxycholate [DCA]) are also germinants for C. difficile spores (13, 17). To test if CDCA prevents colony formation induced by CA, spores were preexposed to 0.1% CA with and without CDCA. Exposure to CA alone resulted in approximately 1% spore recovery, whereas exposure to 0.1% CA and 0.1% CDCA together led to a decrease in colony formation to 0.075% (Fig. 1B). The effect of CDCA on CA-mediated colony formation was relieved by increasing the concentration of CA to 1.0%, raising colony formation to 2.6% (Fig. 1B). These results indicate that CDCA blocks colony formation induced by CA, as well as that induced by TA, and may be an inhibitor of germination by C. difficile spores that acts competitively in both cases.
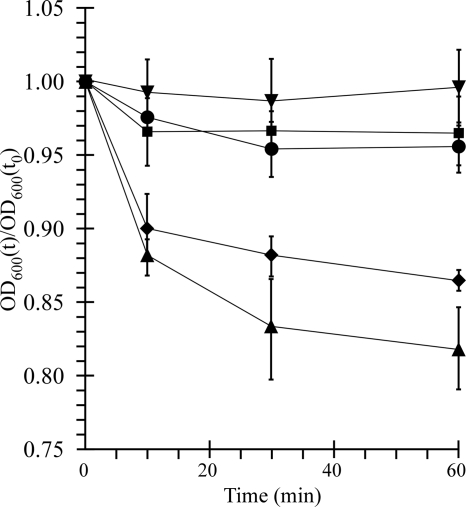
Spore germination per se is classically measured as a decrease in the optical density of a spore suspension occurring concomitantly with a release of Ca2+-dipicolinate from the spore core, rehydration of the core, and degradation of the cortex (8, 12). As determined by this assay, TA is the most effective bile salt for inducing rapid germination (13). To test if CDCA is an inhibitor of germination as opposed to an inhibitor of some other step between germination and colony formation, spores were purified as described previously (13). Spores did not germinate in BHIS medium alone or when this medium was supplemented with 0.1% CDCA (Fig. 2). When C. difficile spores were suspended in BHIS containing 0.1% TA, the optical density of the suspension rapidly decreased, indicating that the spores were germinating. However, the optical density of the spores suspended in BHIS with 0.1% TA plus 0.1% CDCA did not decrease over time, indicating that CDCA inhibited TA-mediated germination (Fig. 2). When the concentration of TA was increased from 0.1% to 1.0% in the presence of 0.1% CDCA, spores were able to germinate (Fig. 2). After overnight incubation in BHIS with 0.1% TA plus 0.1% CDCA, 84% of the spores remained phase bright, while only 11% of spores remained phase bright in BHIS with 1.0% TA plus 0.1% CDCA, indicating that CDCA blocks germination at a very early step. Thus, CDCA is an inhibitor of germination by C. difficile spores that functions by competing with TA and possibly with CA.
FIG. 2.
CDCA inhibits germination of Clostridium difficile spores. Spores were prepared as described previously (13). C. difficile spores were suspended in BHIS alone (•), BHIS plus 0.1% CDCA (▾), BHIS plus 0.1% TA (⧫), BHIS plus 0.1% TA-0.1% CDCA (▪), or BHIS plus 1.0% TA-0.1% CDCA (▴). The ratio of the OD600 at the various time points to the OD600 at time zero is plotted versus time. Data points are the averages of three independent experiments, and error bars represent one standard deviation from the mean.
We previously suggested a role for bile salts in determining the ability of C. difficile to colonize and cause disease (13). In this model, germination of C. difficile spores depends on interaction with glycine and certain bile salts. We show here that the primary bile salt CDCA inhibits germination of C. difficile spores. As mentioned above, germination inhibitors are commonly structurally related to the germinant they inhibit. The structures of CA derivatives and CDCA derivatives are very similar; they differ only insofar as CDCA lacks the 12α hydroxyl group (11).
CDCA and CA derivatives are present in approximately equal concentrations in the cecum (5). Under such conditions, CDCA would compete with CA derivatives for binding to putative germinant receptors on C. difficile spores. Mekhjian and colleagues measured the colonic absorption rates of CDCA, CA, and DCA that were introduced into the cecum and collected at the distal colon (7). They found that CDCA was absorbed by the colon at 10 times the rate for CA (7). Thus, when spores reach the distal large intestine, they encounter a decreased ratio of CDCA to CA. Such a change in ratio might allow CA derivatives to act as effective germinants. Thus, C. difficile spores would not be expected to germinate until they reach the colon, which also provides the anaerobic environment required for C. difficile growth.
The colonic microflora, which is known to protect the host against C. difficile infection, plays a significant role in the metabolism of bile salts (3, 11). Many different species express on their cell surfaces bile salt hydrolases that serve to remove the conjugated tauryl or glycyl groups from primary bile salts (11). After deconjugation, CA and CDCA are further metabolized by a small percentage of the bacterial species in the cecum to the secondary bile salts deoxycholate and lithocholate, respectively (11, 14). Deoxycholate is an inhibitor of C. difficile growth (13, 17). CDCA inhibits both germination and growth (13). The use of CDCA either as prophylaxis or as a therapy for C. difficile-associated disease might be helpful for patients who are undergoing antibiotic regimens or who are colonized by this bacterium. For example, when an antibiotic that is known to be associated with an increased risk of inciting C. difficile-associated disease is administered, the coadministration of CDCA might protect that individual from colonization by C. difficile through inhibiting spore germination. Alternatively, administering CDCA to individuals who are already being given vancomycin or metronidazole for C. difficile-associated disease may have the benefit of preventing spore germination and further vegetative growth (13) after antibiotic therapy is stopped. This strategy may reduce the already significant risk of a relapse.
Acknowledgments
We thank B. Belitsky, L. Bouillaut, L. Handke, and S. McBride for helpful discussions and comments on the manuscript.
This project was supported in part by funding from the National Institutes of Health under contract no. N01 AI30050 (S. Tzipori, principal investigator). J.A.S. acknowledges support through NIH Federal Training in Education and Critical Research Skills (TEACRS) fellowship K12 GM074869-02.
Footnotes
Published ahead of print on 5 December 2008.
REFERENCES
- 1.Akoachere, M., R. C. Squires, A. M. Nour, L. Angelov, J. Brojatsch, and E. Abel-Santos. 2007. Identification of an in vivo inhibitor of Bacillus anthracis spore germination. J. Biol. Chem. 28212112-12118. [DOI] [PubMed] [Google Scholar]
- 2.Dawson, A. M., D. Trenchard, and A. Guz. 1965. Small bowel tonometry: assessment of small gut mucosal oxygen tension in dog and man. Nature 206943-944. [DOI] [PubMed] [Google Scholar]
- 3.Freeman, J., and M. H. Wilcox. 1999. Antibiotics and Clostridium difficile. Microbes Infect. 1377-384. [DOI] [PubMed] [Google Scholar]
- 4.Gerding, D. N., C. A. Muto, and J. R. C. Owens. 2008. Treatment of Clostridium difficile infection. Clin. Infect. Dis. 46S32-S42. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton, J. P., G. Xie, J.-P. Raufman, S. Hogan, T. L. Griffin, C. A. Packard, D. A. Chatfield, L. R. Hagey, J. H. Steinbach, and A. F. Hofmann. 2007. Human cecal bile acids: concentration and spectrum. Am. J. Physiol. Gastrointest. Liver Physiol. 293G256-G263. [DOI] [PubMed] [Google Scholar]
- 6.Jump, R. L. P., M. J. Pultz, and C. J. Donskey. 2007. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob. Agents Chemother. 512883-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mekhjian, H. S., S. F. Phillips, and A. F. Hofmann. 1979. Colonic absorption of unconjugated bile acids: perfusion studies in man. Dig. Dis. Sci. 24545-550. [DOI] [PubMed] [Google Scholar]
- 8.Moir, A., and D. A. Smith. 1990. The genetics of bacterial spore germination. Annu. Rev. Microbiol. 44531-553. [DOI] [PubMed] [Google Scholar]
- 9.Northfield, T., and I. McColl. 1973. Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. Gut 14513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popoff, M. R., E. J. Rubin, D. M. Gill, and P. Boquet. 1988. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 562299-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridlon, J. M., D. Kang, and P. B. Hylemon. 2006. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47241-259. [DOI] [PubMed] [Google Scholar]
- 12.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6550-556. [DOI] [PubMed] [Google Scholar]
- 13.Sorg, J. A., and A. L. Sonenshein. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 1902505-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas, L. A., M. J. Veysey, G. French, P. B. Hylemon, G. M. Murphy, and R. H. Dowling. 2001. Bile acid metabolism by fresh human colonic contents: a comparison of caecal versus faecal samples. Gut 49835-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voth, D. E., and J. D. Ballard. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18247-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wax, R., E. Freese, and M. Cashel. 1967. Separation of two functional roles of l-alanine in the initiation of Bacillus subtilis spore germination. J. Bacteriol. 94522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson, K. H. 1983. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J. Clin. Microbiol. 181017-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson, K. H., M. J. Kennedy, and F. R. Fekety. 1982. Use of sodium TA to enhance spore recovery on a medium selective for Clostridium difficile. J. Clin. Microbiol. 15443-446. [DOI] [PMC free article] [PubMed] [Google Scholar]