Abstract
IcsA is an outer membrane protein in the autotransporter family that is required for Shigella flexneri pathogenesis. Following its secretion through the Sec translocon, IcsA is incorporated into the outer membrane in a process that depends on YaeT, a component of an outer membrane β-barrel insertion machinery. We investigated the role of the periplasmic chaperone Skp in IcsA maturation. Skp is required for the presentation of the mature amino terminus (alpha-domain) of IcsA on the bacterial surface and contributes to cell-to-cell spread of S. flexneri in cell culture. A mutation in skp does not prevent the insertion of the β-barrel into the outer membrane, suggesting that the primary role of Skp is the folding of the IcsA alpha-domain. In addition, the requirement for skp can be partially bypassed by disrupting icsP, an ortholog of Escherichia coli ompT, which encodes the protease that processes IcsA between the mature amino terminus and the β-barrel outer membrane anchor. These findings are consistent with a model in which Skp plays a critical role in the chaperoning of the alpha-domain of IcsA during transit through the periplasm.
Type V secretion apparatuses (also called autotransporters) consist of an extensive class of large, outer membrane proteins of gram-negative bacteria, typically virulence factors, found in all subdivisions of proteobacteria (28). Although originally designated as “autotransporters” because they were thought to mediate their own insertion into and translocation across the outer membrane, more recent evidence suggests that autotransporter secretion and insertion requires the aid of accessory factors (21, 29). Secretion involves the insertion of the carboxy-terminal β-barrel domain into the outer membrane and translocation of the mature passenger (alpha) domain across the outer membrane (Fig. 1). Whether these two events occur sequentially or simultaneously is unclear. Analysis of crystal structures indicates that the carboxy-terminal end of the passenger domain is present within the central pore of the β-barrel (4, 27). Several studies provide evidence that at least some autotransporters are partially folded in the periplasm (7, 20), and one of these studies provides strong evidence that the passenger domain may be partially or fully incorporated into the β-barrel prior to incorporation of the mature protein into the outer membrane (20).
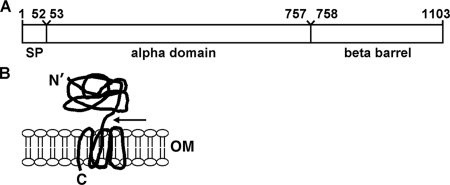
FIG. 1.
Schematic of the autotransporter IcsA. (A) Linear diagram showing the signal peptide (SP), alpha-domain (IcsA53-757), and carboxy-terminal β-barrel domain. (B) IcsA in the outer membrane. The carboxy-terminal β-barrel is inserted into the outer membrane, and the mature amino-terminal alpha-domain is exposed on the bacterial surface. N′, mature amino terminus; C, carboxyl terminus; OM, outer membrane; arrow, proposed site of cleavage between residues 757 and 758 by IcsP.
Shigella flexneri is a gram-negative human pathogen which, upon passage through the lower digestive tract, gains entry into colonic epithelial cells. Once S. flexneri is intracellular, it spreads to adjacent cells by secreting IcsA, a surface-associated autotransporter that is required for the polymerization of host cell actin on the bacterial surface. Actin polymerization occurs at a single pole of the bacterium and is required for infection of adjacent cells and disease pathogenesis (5, 24, 33).
IcsA is encoded on a large virulence plasmid. The full-length protein is approximately 120 kDa and has three assigned functional and structural domains (25): an atypical Sec secretion signal (IcsA1-52), the alpha-domain (IcsA53-757), which is exposed on the bacterial surface and contains sequences that are required for actin polymerization, and the beta-domain (IcsA758-1102), which forms a β-barrel structure in the outer membrane (Fig. 1A) (21, 25). In vivo, a fraction of IcsA molecules are proteolytically processed at the junction between the alpha- and beta-domains by the protease IcsP (SopA), a protein which is also encoded on the virulence plasmid (14, 34). IcsA53-757 is found in the supernatant of liquid cultures, while mature full-length IcsA (IcsA53-1102), IcsA758-1102 (14, 34), and some IcsA53-757 (this work) remain cell associated. IcsA, like other autotransporters, is secreted at the bacterial pole (22), the site at which actin tail assembly occurs. As it is for other β-barrel-containing outer membrane proteins, insertion of IcsA and other autotransporters into the outer membrane requires the outer membrane insertase YaeT (BamA, Omp85) (21).
Skp, DegP, and SurA are periplasmic chaperones that, like YaeT, appear to function in the targeting and/or insertion of outer membrane proteins (35). Evidence based on synthetic phenotypes suggests that during outer membrane protein insertion Skp and DegP act in one pathway and that SurA acts in a distinct but parallel pathway (35).
We investigated the role of the periplasmic chaperone Skp in the folding and secretion of IcsA in S. flexneri. We found that in the absence of skp, IcsA is inefficiently presented on the surface of S. flexneri, leading to a cellular spread defect. Surprisingly, the protein was still efficiently cleaved by the outer membrane protease IcsP, as wild-type levels of IcsA53-757 were detected in the culture supernatants. We found that introduction of the icsP mutation into the skp strain background led to an increase in the levels of full-length IcsA presented on the bacterial cell surface of the skp mutant, and we present models that could explain our results.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli and S. flexneri strains were maintained in LB medium at 37°C unless otherwise indicated. Antibiotics, where appropriate, were added to the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; kanamycin, 25 μg/ml. When used, arabinose and glucose were added at a final concentration of 0.2% (wt/vol).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description or construction | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | BRL Life Technologies | |
| BW25113 | skp::kan | |
| S. flexneri strains | ||
| 2457T | Wild type, serotype 2a | 23 |
| MBG283 | 2457T pWR100 icsA::Ω, Spr | 36 |
| MBG341 | 2467T icsP::amp, Ampr | 34 |
| skp mutant | 2457T skp::kan, generated by P1 transduction of skp::kan allele from BW25113 background into 2457T, Kanr | This study |
| skp icsP mutant | 2457T skp::kan icsP::amp, generated by P1 transduction of skp mutant with lysate from MBG341, Kanr Ampr | This study |
| Plasmids | ||
| p-skp | pACYC184 containing P;yaeT-skp (see Materials and Methods), Cmr | This study |
| pACYC184 | Tetr Cmr | 8 |
To generate derivatives of wild-type strain 2457T (23) that contained a mutation in skp or mutations in both skp and icsP, 2457T and 2457T icsP (34) were each transduced with a P1 phage lysate carrying a nonpolar kanamycin insertion in skp (2). To avoid heat stress, which could make the skp strains unstable, cells were transduced at 30°C. Transductants were then patched onto agar containing the dye Congo red and grown at 37°C to confirm the presence of the virulence plasmid. Preliminary analyses examining IcsA levels were carried out with independent transductants.
Since the precise location of the skp promoter has not been mapped, a complementation plasmid (p-skp) was generated that encodes a region upstream of yaeT, the first 36 bp and last 12 bp of yaeT, and the entire putative promoter and coding region of skp. The plasmid was generated by overlap extension PCR (38). The full-length product was generated by using the upstream and downstream products as PCR template. The product was cloned into the HindIII and XbaI sites of pACYC184 (a p15A derivative, medium-copy-number plasmid) and introduced into 2457T skp::kan by electroporation. The sequences of oligonucleotide primers used in this study are available from the authors upon request.
Distribution of IcsA on the bacterial surface and dot blot analysis.
The distribution of IcsA on the bacterial surface was determined by immunofluorescence on intact cells that were fixed in 1.85% (vol/vol) paraformaldehyde (Ted-Pella). All centrifugations were carried out at 4,500 × g for 5 min. All washes were with 1-ml volumes. Briefly, 1.0 ml of fixed cells was centrifuged at 4,500 × g for 5 min in a tabletop centrifuge and washed twice with phosphate-buffered saline (PBS). Cells were blocked for 30 min at 37°C in 100 μl 10% bovine serum albumin (BSA). Polyclonal antibody to IcsA (18) was added to a final dilution of 1:100, and cells were incubated for 1 h. Cells were then washed twice with PBS containing 0.05% Tween 20, resuspended in 100 μl 10% bovine serum albumin containing a 1:200 dilution of Alexa-Fluor 488 (Invitrogen), and incubated for 30 min at 37°C. After incubation, cells were washed three times with PBS containing 0.05% Tween 20. Labeled bacteria were placed onto glass slides, and images were captured as described previously (9).
Immuno-dot blot assays were performed on nitrocellulose membranes. Briefly, cells were grown to exponential phase in LB at 37°C. One ml of cells was centrifuged for 1 min at 15,600 × g in a tabletop microcentrifuge, and the supernatant was discarded. Cells were resuspended to equivalent volumes (normalizing to the optical density at 600 nm [OD600]) in PBS, and 5.0 μl of each sample was spotted onto a nitrocellulose membrane (Whatman) and allowed to air dry. Membranes were rinsed briefly in Western transfer buffer with shaking for 5 min. The membranes were then treated according to standard Western blotting procedures. Densitometry was performed as previously described (19).
Western blot analysis.
Samples were always collected during exponential growth and normalized based on the OD600. Samples for the β-barrel folding assay were treated as described previously (21). All sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels consisted of 4% stacking and 10% separating layers. Western blot analysis was carried out using standard protocols. For primary antibodies, the following dilutions were used: 1:10,000 of rabbit polyclonal antibody to IcsA (18), 1:2,500 of rabbit polyclonal antibody to β-lactamase (ABR; Affinity Bioreagents), 1:5,000 of rabbit polyclonal antibody to DsbA (gift of J. Beckwith), 1:1,000 of rabbit polyclonal antibody to β-galactosidase (Sigma), 1:3,000 of rabbit polyclonal antibody to Skp (gift of T. Silhavy), 1:5,000 of rabbit polyclonal antibody to IcsP (36), 1:1,000 of rabbit polyclonal antibody to OmpA (gift of D. Kahne), and 1:5,000 of rabbit polyclonal to DsbA (gift of J. Beckwith).
Cell fractionation and proteinase K susceptibility analysis.
Periplasmic fractionation was carried out as described previously (1). Supernatant proteins were prepared as follows. One ml of culture supernatant from exponentially growing cells was collected and concentrated by adding 1.0 μl of 10 mg/ml BSA (as a carrier protein) followed by 500 μl of ice-cold 3:2 trichloroacetic acid-acetone. Supernatants were placed on ice for 20 min to overnight and then centrifuged at 15,600 × g at 4°C for 20 min to pellet the precipitate. Pellets were washed twice with 1.0 ml ice-cold 100% acetone, centrifuging 15 min with each wash. Precipitates were resuspended in 20.0 μl of 4× SDS-PAGE buffer and loaded according to normalized OD600 readings. Generally, approximately 5.0 μl of a preparation corresponding to an OD600 of 0.6 gave a good signal. To ensure that the amount loaded was approximately equivalent across all samples, the BSA protein band was visualized on membranes following Western transfer by staining with Ponceau S (0.1% [wt/vol] in 5.0% acetic acid) and a brief destaining with distilled deionized H2O. The preparation of proteins for the analysis of heat-modifiable mobility of IcsA was performed as described previously (21).
For proteinase K susceptibility analysis, exponential-phase cells were harvested by centrifugation for 3 min at 6,000 × g. Cell pellets were resuspended at a normalized equivalent OD600 of 6.25 in 10 mM Tris, pH 8.0; periplasmic fractions were extracted with chloroform as described previously (1) and recovered in equivalent volumes of 10 mM Tris, pH 8.0. Proteinase K was added to each sample at a final concentration of 10 μg/ml, and samples were incubated at 55°C for 15 min, at which time phenylmethylsulfonyl fluoride was added to a final concentration of 5 mM and samples were incubated for 15 min at room temperature to inhibit further proteolysis. After addition of 5× SDS-PAGE buffer, samples were immediately boiled for 10 min and then were analyzed by SDS-PAGE (10% separating layer) and Western blotting.
Bacterial invasion and intercellular spread.
Analysis of intercellular spread of wild-type and mutant S. flexneri strains was performed essentially as described previously (26). Confluent monolayers of HeLa cells grown in 60-mm dishes were infected at a multiplicity of infection of 0.001 at 37°C. Following an initial invasion period of 1.5 h, monolayers were washed with fresh minimal essential medium and overlaid with 0.5% agarose in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, nonessential amino acids, and 25 μg/ml gentamicin. Forty-eight h later, monolayers were stained with neutral red, and images of the infected monolayers were acquired using an Epson Perfection 4990 photo desktop scanner and Adobe Photoshop Elements 2.0 software. The area of individual bacterial plaques within the monolayers was measured using IPLab software (Scanalytics). To verify that the skp mutant was invasive, HeLa cells seeded on acetone-washed glass coverslips were infected with wild-type S. flexneri or the skp mutant at a multiplicity of infection of 10. Bacteria were allowed to invade for 1.5 h, after which cells were washed and overlaid with fresh minimal essential medium supplemented with 10% fetal bovine serum, nonessential amino acids, and 25 μg/ml gentamicin. At 1.5 h later, cells were washed and fixed with 3.7% p-formaldehyde. Light microscopy was performed using a Nikon Eclipse TE300 microscope equipped with Chroma Technology filters and a Photometrics CoolSNAP HQ charge-coupled-device camera (Roper Scientific), and images were acquired using IPLab software.
RESULTS
Skp mutants display less IcsA on the cell surface.
Although IcsA is designated an autotransporter protein, previous work from our laboratory indicated that at least one other protein, YaeT, is required for the incorporation of IcsA into the outer membrane (21). IcsA is translocated across the inner membrane via the Sec secretion apparatus (6). In transit from the Sec apparatus to the outer membrane, IcsA must pass through the periplasmic space, and the periplasmic chaperone DegP has been shown to be required for the proper presentation of IcsA on the bacterial surface (29, 30). Evidence suggests that Skp, a second periplasmic chaperone that is encoded immediately downstream of yaeT, acts in the same pathway as DegP (31). We hypothesized that Skp would also be required for presentation of IcsA on the bacterial surface.
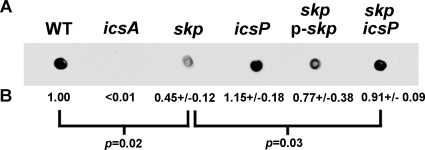
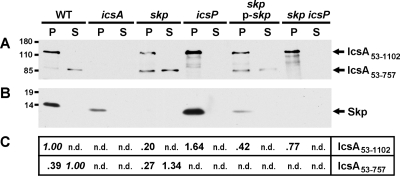
We tested whether Skp contributed to the presentation of IcsA on the bacterial surface by determining the amount of IcsA on the surface of intact bacteria. First, we compared the amount of IcsA on the surface of wild-type S. flexneri strain 2457T to that on the surface of a skp mutant of 2457T by whole-cell, quantitative dot blot analysis (Fig. 2), using polyclonal antibodies that recognize epitopes present in IcsA53-757, which is displayed on the bacterial surface, but that do not recognize the outer membrane β-barrel component (18, 36). The levels of IcsA on the surface of the skp mutant were reduced to 45 ± 0.12% (mean ± standard deviation) of the wild-type control (Fig. 2) (P = 0.02). Introduction of skp on a plasmid (p-skp) increased these levels to 77% of wild type (Fig. 2), indicating that the reduction observed in the skp mutant was due to the mutation in skp.
FIG. 2.
Detectable IcsA on the surface of the skp mutant compared to other S. flexneri strains. (A) Dot blot of pellet of intact bacteria, probed with antiserum to IcsA. Blot is representative. (B) Mean, standard deviations, and P values for the means of two replicates in each of two independent experiments.
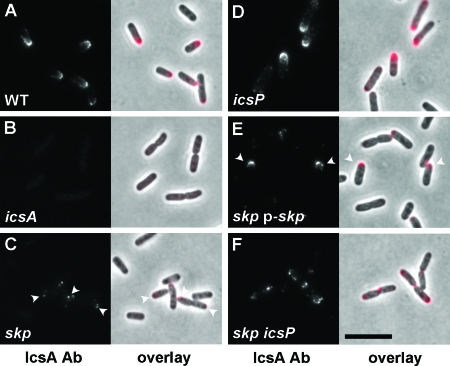
A limitation of the whole-cell dot blot analysis is that even though the bacterial cells are intact at the time they are applied to the membrane, there is a possibility of some cell lysis occurring during the procedure. Consequently, a portion of the signal could be from protein that is intracellular and not normally exposed on the cell surface. Therefore, to more directly examine IcsA localized to the bacterial surface, we performed immunolocalization on intact bacteria, using polyclonal antibodies that specifically recognize epitopes present in IcsA53-757 (Fig. 3). In addition, this allowed us to determine whether there was an alteration in the distribution of IcsA on the bacterial surface. In wild-type cells, IcsA is distributed as a cap over the bacterial pole (18). Relative to the wild-type control, only a small amount of IcsA was detectable on the surface of the skp mutant (Fig. 3A and C), consistent with the decrease in IcsA levels observed by dot blot analysis (Fig. 2). The distribution of IcsA on the surface of the skp mutant was distinctly polar (Fig. 3A and C). In a mutant icsA background, there was no signal (Fig. 3B), indicating that the antibodies specifically recognize the IcsA protein. A skp mutant harboring a plasmid-borne copy of skp, p-skp, showed a recovery of IcsA surface presentation in a subpopulation of bacteria within the population (Fig. 3E). Taken together, we conclude that skp mutants have reduced levels of IcsA on the bacterial surface.
FIG. 3.
Localization of IcsA on the surface of the skp mutant compared to other S. flexneri strains. Images are from immunofluorescence labeling of IcsA on the surface of intact bacteria. (A) Wild type; (B) icsA mutant; (C) skp mutant. Arrowheads, foci of IcsA at the cell poles. (D) icsP mutant. (E) skp mutant complemented with a plasmid carrying skp. Arrowheads, subpopulations of cells that display rescue of IcsA presentation on the bacterial surface. (F) skp icsP mutant. Antibody labeling was performed after fixation of the cells. Left panels, fluorescent signal; right panels, overlay of fluorescence (pseudocolored red) and phase microscopy. Increased contrast was uniformly applied to every panel of the fluorescence overlay so as to permit the visualization of the fluorescent signal on top of the phase image. Bar (in panel F), 10 μm.
The decrease in IcsA on the bacterial surface is associated with a decrease in cell-to-cell spread.
During cellular infection by Shigella, IcsA is required for the assembly of actin tails and the movement of the bacteria to the cell periphery and into adjacent cells (5, 24). Efficient actin-based motility depends on sequences within the surface-exposed portion of IcsA (15). Since we found that in the absence of skp the levels of IcsA on the surface of S. flexneri were reduced, we hypothesized that S. flexneri lacking skp would be defective in cell-to-cell spread.
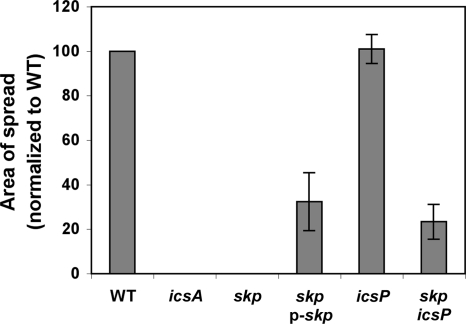
To determine whether a mutation in skp led to an effect on S. flexneri cell-to-cell spread, the extent of spread of the skp mutant through a tissue culture monolayer was compared to that of the wild-type strain using a plaque assay. Although the skp mutant was able to invade cells in a monolayer, as found by microscopic analysis (data not shown), it did not form plaques in the monolayer (Fig. 4). The reduction in cell-to-cell spread could be due to the lower levels of surface-presented IcsA (Fig. 2 and 3) or to a less direct effect of the skp mutation. Complementation of the skp mutant strain with the plasmid encoding skp restored plaque formation. The plaques formed by the complemented strain were only 32 ± 13% of the area of those formed by the wild-type strain (Fig. 4); the lack of full complementation is likely due to overall reduced levels of IcsA on the bacterial surface in this background compared to the wild-type strain (Fig. 2). These data indicate that Skp plays an important role in S. flexneri cell-to-cell spread, at least partially through a reduction in surface presentation of IcsA. We do not exclude the possibility that Skp may also play an important role in the folding or secretion of other factors important for cell-to-cell spread.
FIG. 4.
Area of spread of the skp mutant and other strains. The histogram shows the area of spread of various strains used in this study in a plaque assay on monolayers of HeLa cells. Error bars represent the standard deviations of the mean areas of spread normalized to the wild-type strain for three independent experiments.
Skp mutants have reduced levels of IcsA53-1102 but wild-type levels of secreted IcsA53-757.
The reduction in surface presentation of IcsA could correspond to overall changes in IcsA protein levels in the cell. Alternatively, the overall levels of IcsA may remain constant, but the amount detectable by antibody on the bacterial surface could be reduced. To investigate these different possibilities, we examined the levels of total IcsA protein by Western blot analysis of protein preparations from bacterial cell pellets and culture supernatants.
In the absence of skp, levels of cell-associated IcsA53-1102 were reduced to 20 ± 11% of the wild-type control (P = 0.00009) (Fig. 5A and C). A portion of secreted IcsA (IcsA53-757) is released into culture supernatants following cleavage by the outer membrane protease IcsP (14, 34). Surprisingly, the levels of IcsA53-757 present in culture supernatants of the skp mutant were similar to those present in the wild-type control (Fig. 5). As expected, neither IcsA53-757 nor IcsA53-1102 was detectable in the icsA mutant (Fig. 5A and 6A, below). The levels of IcsA53-1102 were significantly restored to 42 ± 3% of wild-type levels by introducing into the skp mutant the complementation plasmid p-skp (P = 0.009 for the comparison of IcsA53-1102 in the skp mutant versus IcsA53-1102 in the complemented strain) (Fig. 5A and 5C), indicating that the reduced levels of IcsA in the skp mutant can be attributed to the mutation in skp. Introduction of p-skp restored Skp levels only partially toward wild-type levels, as judged by Western blot analysis (Fig. 5B); thus, the absence of complete rescue of IcsA levels in this strain is likely due to the relatively reduced levels of Skp.
FIG. 5.
IcsA expression and secretion into the culture supernatant in the skp mutant and other strains. Western blot analysis of whole-cell proteins in pellets (P) and of proteins in culture supernatants (S) of strains used in this study. (A) Representative blot, probed with antibody to IcsA, showing full-length mature IcsA (IcsA53-1102) and the IcsP-cleaved fragment of IcsA (IcsA53-757). (B) Blot probed with antibody to Skp. (C) Densitometry of bands corresponding to IcsA53-1102 or IcsA53-757; the number reported is the mean value for densitometry of bands on multiple blots, with values normalized to the wild-type strain. Since protein loading for the pellets was not directly proportional to that for the supernatants, values were normalized separately for pellets and supernatants (values of 1.00 for the wild type are shown in italics). n.d., not done. Molecular size markers are indicated in kilodaltons to the left of the blots.
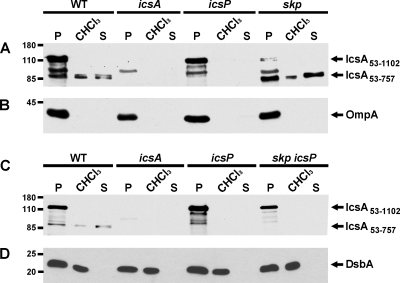
FIG. 6.
IcsP-cleaved alpha-domain of IcsA is present in the chloroform-released protein fraction. Western blot analysis results are for whole-cell proteins in pellets (P), of protein fraction released by chloroform treatment (CHCl3), and of proteins in culture supernatants (S) of strains used in this study. (A and C) Blots probed with antibody to IcsA, showing full-length mature IcsA (IcsA53-1102) and the IcsP-cleaved fragment of IcsA (IcsA53-757). (B) Blot probed with antibody to OmpA. (D) Blot probed with antibody to DsbA. Molecular size markers are indicated in kilodaltons to the left of the blots.
Cell-associated IcsA53-757 is extracted by chloroform.
In addition to the reduction in overall IcsA53-1102 levels in the skp mutant, there was a relative enrichment in the amount of IcsA53-757 relative to the amount of IcsA53-1102 in the cell pellets of the skp mutant compared to the wild-type control (Fig. 5A and C). The cell-associated IcsA53-757 is produced in an IcsP-dependent manner (Fig. 6A and data not shown), indicating that it corresponds to the same cleavage product that is secreted into the supernatants of S. flexneri cultures.
To examine the subcellular distribution of this fragment, we treated cells with chloroform, which permeabilizes the outer membrane and releases periplasmic contents into the soluble fraction (1). This method does not disrupt the cytoplasmic membrane or lead to release of cytoplasmic, inner membrane, or outer membrane β-barrel proteins (1). IcsA53-757 was detected in the soluble fraction following chloroform treatment in both the skp mutant and the wild-type strain (Fig. 6A). We also found that IcsA53-1102, IcsP, OmpA, and β-galactosidase did not fractionate with the chloroform-released fraction (Fig. 6B and data not shown). The presence of IcsA53-757 in the chloroform-released fraction was dependent on IcsP-mediated cleavage, since introduction of a mutation in icsP led to disappearance of this band in both the wild-type and the skp strain background (Fig. 6A and C). As a positive control, the membranes were also probed with an antibody to the soluble, periplasmic protein DsbA (3). DsbA was detected in the whole-cell fractions and the chloroform-released fractions, but not in the supernatants (Fig. 6D). Skp was not released by the chloroform treatment (data not shown), consistent with previous observations that suggest that Skp stably associates with membrane-associated components in the periplasmic compartment (12). We conclude that following IcsP-mediated cleavage in both the wild type and the skp mutant, a portion of IcsA53-757 is either released into the periplasm or remains associated with the outer membrane in a manner that is abrogated by chloroform treatment.
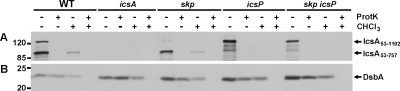
To distinguish between these two possibilities, we tested whether the IcsA53-757 that is present in the cell pellets was susceptible to degradation by proteinase K. We reasoned that if IcsA53-757 is present on the cell surface, then it should be degraded by exogenously added proteinase K. Alternatively, if IcsA53-757 is present in the periplasm, then it should be protected from the exogenously added protease. We tested intact cells of the wild type, the skp mutant, the icsP mutant, and the skp icsP double mutant for susceptibility to proteinase K. In the wild type and the skp mutant, both IcsA53-757 and full-length IcsA were degraded by proteinase K treatment (Fig. 7A). In the icsP derivatives, full-length IcsA was degraded (Fig. 7A). As a control to ensure that periplasmic proteins were protected from the protease under our experimental conditions, we probed our samples for the soluble periplasmic protein DsbA and found that it remained intact (Fig. 7B). In contrast, pretreatment with chloroform led to degradation of DsbA, confirming that when the outer membrane was disrupted, DsbA became susceptible to protease. These results indicate that IcsA53-757 that is present in the cell pellets is localized to the bacterial surface.
FIG. 7.
Proteinase K susceptibility of IcsA on intact and chloroform-treated cells. Western blots are for cell pellets treated with proteinase K (ProtK), either with or without prior disruption the outer membrane with chloroform (CHCl3). (A) Blot probed with antiserum to IcsA. (B) Blot probed with antiserum to DsbA. IcsA53-1102, full-length mature IcsA; IcsA53-757, proteolytically processed mature amino terminus of IcsA. Molecular size markers are indicated in kilodaltons to the left of the blot.
The decrease in IcsA53-1102 protein levels and surface presentation in the skp mutant is partially rescued by introduction of a mutation in icsP.
As described above, to determine whether the IcsA53-757 seen in the supernatant and chloroform-solubilized fractions of the skp mutant was dependent on the IcsP protease, we fractionated proteins of a skp icsP double mutant. As expected, the secreted product was dependent on IcsP. The mutation in icsP also reproducibly increased levels of IcsA in whole-cell protein preparations to 77 ± 6% of those observed in the wild type (P = 0.00008 for the comparison of IcsA53-1102 in the skp mutant versus IcsA53-1102 in the skp icsP double mutant) (Fig. 5). In addition, the introduction of the icsP mutation partially restored the defect of the skp mutant in presentation of IcsA on the bacterial surface, as judged by both dot blot analysis (Fig. 2) and surface immunolocalization (Fig. 3F). Finally, the defect in cell-to-cell spread seen in the skp mutant was partially restored in the skp icsP double mutant (to 23 ± 8% of WT) (Fig. 4). Since a mutation in icsP restores the level of IcsA53-1102 associated with the cell and can partially restore presentation of IcsA on the bacterial surface and cell-to-cell spread to the skp mutant, we conclude that Skp is not absolutely required for translocation of functional IcsA to the bacterial surface.
The β-barrel component of IcsA is properly folded in the skp mutant.
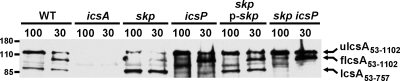
As has been demonstrated for other β-barrel proteins, the mobility of IcsA on a denaturing SDS-PAGE gel is modifiable by heat and is dependent on the proper folding and assembly of the protein in the outer membrane (21). Thus, when cell pellets are heated to only 30°C, IcsA migrates faster than when cell pellets are heated to 100°C. Based on analogy to detailed analyses of the insertion of the LamB β-barrel into the outer membrane and the resultant change in heat-modifiable mobility of LamB on SDS-PAGE (37), the faster-migrating band in the 30°C sample likely represents protein that is folded and inserted into the outer membrane, whereas the slower-migrating band in the 100°C sample likely represents unfolded denatured protein. To determine whether Skp was required for folding of the IcsA β-barrel and its insertion into the outer membrane, we analyzed the heat-modifiable mobility of IcsA in whole-cell protein preparations of the wild type and the skp mutant. Skp was not required for folding of the IcsA β-barrel and its insertion into the outer membrane, since the heat-modifiable migration of IcsA bands for the skp mutant was identical to that of the wild type (Fig. 8), whereas YaeT-depleted cells had altered migration of IcsA in the 30°C sample (21). These results indicate that in the absence of Skp, the β-barrel portion of IcsA is properly folded and properly assembled in the outer membrane. Thus, although the beta-domain is folded in the periplasm either before or in conjunction with its insertion into the outer membrane, this process is independent of Skp.
FIG. 8.
Heat modifiability of the IcsA β-barrel in the skp mutant and other strains. Western blot results are for IcsA in whole-cell protein preparations run on an SDS-PAGE gel after heating to 100°C or 30°C, as indicated above the blot. The blot was probed with antiserum to IcsA. uIcsA53-1102, unfolded full-length mature IcsA; fIcsA53-1102, folded full-length mature IcsA; IcsA53-757, proteolytically processed mature amino terminus of IcsA. Molecular size markers are indicated in kilodaltons to the left of the blot.
DISCUSSION
Our results indicate that Skp is not directly required for IcsA surface presentation. In the absence of Skp, IcsA53-757 is processed by IcsP and released into the supernatant at levels comparable to wild type (Fig. 5). We were unable to determine the mechanism leading to reduced levels of cell-associated IcsA53-1102, although we hypothesize that in the absence of Skp, a portion of the protein is improperly folded and probably degraded in the periplasm. Strikingly, the IcsA53-1102 that is detectable is folded in a conformation consistent with proper β-barrel folding. Moreover, the preservation of IcsA processing by IcsP suggests that the IcsP recognition site is properly folded. Thus, in the absence of Skp, the translocation of IcsA to the bacterial surface is less efficient, but folding of the β-barrel domain and of the IcsP recognition site at the junction of the alpha- and beta-domains is preserved. Therefore, we hypothesize that if misfolding occurs in the absence of the Skp chaperone, then it is occurring in the alpha-domain portion of the protein.
We found that by introducing a mutation in icsP, sufficient IcsA is successfully presented on the cell surface in an active conformation to rescue intercellular spread, at least partially. We envision three models that could account for this result. In the first model, in the absence of skp, a substantial portion of IcsA is misfolded, thus making the protein subject to degradation by periplasmic proteases. A mutation in icsP could allow more IcsA to accumulate on the cell surface by increasing the overall levels of IcsA53-1102. Assuming that at least a portion of this additional uncleaved IcsA achieves a functional conformation, the icsP mutation would then complement the skp phenotypes. In an alternative model, the icsP mutation would lead to a cell envelope stress response, and a commensurate increase in proteins, such as periplasmic chaperones, which could then lead to improved stability and folding of outer membrane proteins such as IcsA. Consistent with this hypothesis, we observed an increase in Skp levels in the icsP mutant (Fig. 5).
In the final model, the absence of skp could lead to slower maturation of IcsA in the outer membrane. As a result of slowing of this process, enhanced IcsP-mediated cleavage would occur. Since the skp mutant did not have significantly increased levels of IcsA53-757 in the cell supernatants, this model predicts that IcsA53-757 would remain associated with the cell. Consistent with this model, we detected relatively enriched amounts of IcsA53-757 associated with the surface of the bacterium. The cell-associated IcsA53-757 is not sufficient to mediate actin-based motility, but in the absence of IcsP cleavage, enough IcsA53-1102 accumulates on the surface to partially rescue the skp mutant.
The mechanism by which the alpha-domain of autotransporters is translocated across the outer membrane and how translocation is coordinated with insertion of the β-barrel into the outer membrane are incompletely understood. Several models for these events have been described (10, 11, 39). At present, two models seem most consistent with published data. In the first of these two models, the β-barrel is inserted into the outer membrane with the alpha-domain on the periplasmic side of the membrane, and then the alpha-domain is threaded through the β-barrel unfolded in a hairpin fashion. Once the alpha-domain is extracellular, it folds into a mature confirmation. In the second model, the alpha-domain is translocated across the outer membrane simultaneously with insertion of the β-barrel into the outer membrane, in a process that requires the outer membrane insertase YaeT (BamA, Omp85). Recent data suggest that partial folding of the β-barrel and insertion of the alpha-domain into the β-barrel may occur in the periplasm prior to outer membrane insertion (20). IcsA forms a disulfide bond in the periplasm, can be detected in an at least partially folded state in the periplasm (7), and requires YaeT for outer membrane insertion (21). These findings are more simply accommodated by the second model, but it is possible that IcsA is unfolded prior to insertion and translocation, and thus we do not rule out the first model. Our findings indicate that Skp is not required for the insertion of the IcsA β-barrel into the outer membrane but that in the absence of Skp, the IcsA alpha-domain is less efficiently translocated and is highly processed by the IcsP protease. We believe that our data suggest that Skp assists in the folding of the alpha-domain in the periplasm in a manner that is important to its proper translocation across the outer membrane. It seems plausible that Skp could hold IcsA in a partially unfolded state prior to translocation, but it seems equally plausible that Skp promotes periplasmic folding; therefore, our data could be consistent with either model for translocation.
IcsP is the functional homolog of E. coli OmpT. What is the function of IcsP in the context of infection? Following cleavage of IcsA, the actin-polymerizing domain (IcsA53-757) is released into the host cell; however, in at least one strain background, an IcsP null mutant is hypermotile and fully supports actin-based motility (34). Furthermore, noncleavable IcsA mutants are still capable of cell-to-cell spread (16), although intracellular actin-based motility of this mutant has been reported as aberrant relative to wild type (13), possibly due to the altered distribution of IcsA on the surface of cells on which IcsP-mediated cleavage is prevented (13, 32). Although we do not exclude that the cleavage of IcsA serves some function during infection, the cleavage of IcsA is apparently not critical for cell-to-cell spread. OmpT, the E. coli ortholog of IcsP, is induced by heat stress (17) and may be involved in processing of misfolded proteins in the envelope. Perhaps one function of IcsP is to process molecules that are inefficiently incorporated into the outer membrane.
Acknowledgments
We thank T. Silhavy for providing Skp antibody, D. Kahne for providing OmpA antibody, J. Beckwith for providing DsbA antibody, and the National BioResource Project (Japan): E. coli for providing the E. coli skp mutant. We also thank members of the Goldberg laboratory for critical reading of the manuscript.
This work was supported by NIH grants AI035817 (to M.B.G.) and AI061073 (to M.B.G.), NIH postdoctoral fellowship T32 AI07061 (to J.K.W.), a predoctoral Harvey Fellowship from the Mustard Seed Foundation (to J.E.H.), and AHA award 0325770T (to S.J.).
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Ames, G. F., C. Prody, and S. Kustu. 1984. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J. Bacteriol. 1601181-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 22006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardwell, J. C., K. McGovern, and J. Beckwith. 1991. Identification of a protein required for disulfide bond formation in vivo. Cell 67581-589. [DOI] [PubMed] [Google Scholar]
- 4.Barnard, T. J., N. Dautin, P. Lukacik, H. D. Bernstein, and S. K. Buchanan. 2007. Autotransporter structure reveals intra-barrel cleavage followed by conformational changes. Nat. Struct. Mol. Biol. 141214-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardini, M. L., J. Mounier, H. d'Hauteville, M. Coquis-Rondon, and P. J. Sansonetti. 1989. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. USA 863867-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandon, L. D., N. Goehring, A. Janakiraman, A. W. Yan, T. Wu, J. Beckwith, and M. B. Goldberg. 2003. IcsA, a polarly localized autotransporter with an atypical signal peptide, uses the Sec apparatus for secretion, although the Sec apparatus is circumferentially distributed. Mol. Microbiol. 5045-60. [DOI] [PubMed] [Google Scholar]
- 7.Brandon, L. D., and M. B. Goldberg. 2001. Periplasmic transit and disulfide bond formation of the autotransported Shigella protein IcsA. J. Bacteriol. 183951-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 1341141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charles, M., M. Perez, J. H. Kobil, and M. B. Goldberg. 2001. Polar targeting of Shigella virulence factor IcsA in Enterobacteriacae and Vibrio. Proc. Natl. Acad. Sci. USA 989871-9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dautin, N., T. J. Barnard, D. E. Anderson, and H. D. Bernstein. 2007. Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J. 261942-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dautin, N., and H. D. Bernstein. 2007. Protein secretion in gram-negative bacteria via the autotransporter pathway. Annu. Rev. Microbiol. 6189-112. [DOI] [PubMed] [Google Scholar]
- 12.De Cock, H., U. Schafer, M. Potgeter, R. Demel, M. Muller, and J. Tommassen. 1999. Affinity of the periplasmic chaperone Skp of Escherichia coli for phospholipids, lipopolysaccharides and non-native outer membrane proteins. Role of Skp in the biogenesis of outer membrane protein. Eur. J. Biochem. 25996-103. [DOI] [PubMed] [Google Scholar]
- 13.d'Hauteville, H., R. D. Lagelouse, F. Nato, and P. J. Sansonetti. 1996. Lack of cleavage of IcsA in Shigella flexneri causes aberrant movement and allows demonstration of a cross-reactive eukaryotic protein. Infect. Immun. 64511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egile, C., H. d'Hauteville, C. Parsot, and P. J. Sansonetti. 1997. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol. Microbiol. 231063-1073. [DOI] [PubMed] [Google Scholar]
- 15.Egile, C., T. P. Loisel, V. Laurent, R. Li, D. Pantaloni, P. J. Sansonetti, and M.-F. Carlier. 1999. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 1461319-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda, I., T. Suzuki, H. Munakata, N. Hayashi, E. Katayama, M. Yoshizawa, and C. Sasakawa. 1995. Cleavage of Shigella surface protein VirG occurs at a specific site, but the secretion is not essential for intracellular spreading. J. Bacteriol. 1771719-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill, R. T., M. P. DeLisa, M. Shiloach, T. R. Holoman, and W. E. Bentley. 2000. OmpT expression and activity increase in response to recombinant chloramphenicol acetyltransferase overexpression and heat shock in E. coli. J. Mol. Microbiol. Biotechnol. 2283-289. [PubMed] [Google Scholar]
- 18.Goldberg, M. B., O. Barzu, C. Parsot, and P. J. Sansonetti. 1993. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J. Bacteriol. 1752189-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman, S. R., Y. Tu, and M. B. Goldberg. 2008. Differential regulation by magnesium of the two MsbB paralogs of Shigella flexneri. J. Bacteriol. 1903526-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ieva, R., K. M. Skillman, and H. D. Bernstein. 2008. Incorporation of a polypeptide segment into the beta-domain pore during the assembly of a bacterial autotransporter. Mol. Microbiol. 67188-201. [DOI] [PubMed] [Google Scholar]
- 21.Jain, S., and M. B. Goldberg. 2007. Requirement for YaeT in the outer membrane assembly of autotransporter proteins. J. Bacteriol. 1895393-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain, S., P. van Ulsen, I. Benz, M. A. Schmidt, R. Fernandez, J. Tommassen, and M. B. Goldberg. 2006. Polar localization of the autotransporter family of large bacterial virulence proteins. J. Bacteriol. 1884841-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaBrec, E. H., H. Schneider, T. J. Magnani, and S. B. Formal. 1964. Epithelial cell penetration as an essential step in the pathogenesis of bacillary dysentery. J. Bacteriol. 881503-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lett, M.-C., C. Sasakawa, N. Okada, T. Sakai, S. Makino, M. Yamada, K. Komatsu, and M. Yoshikawa. 1989. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J. Bacteriol. 171353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loveless, B. J., and M. H. Saier, Jr. 1997. A novel family of channel-forming, autotransporting, bacterial virulence factors. Mol. Membr. Biol. 14113-123. [DOI] [PubMed] [Google Scholar]
- 26.Oaks, E. V., M. E. Wingfield, and S. B. Formal. 1985. Plaque formation by virulent Shigella flexneri. Infect. Immun. 48124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oomen, C. J., P. van Ulsen, P. van Gelder, M. Feijen, J. Tommassen, and P. Gros. 2004. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 231257-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pallen, M. J., R. R. Chaudhuri, and I. R. Henderson. 2003. Genomic analysis of secretion systems. Curr. Opin. Microbiol. 6519-527. [DOI] [PubMed] [Google Scholar]
- 29.Purdy, G. E., C. R. Fisher, and S. M. Payne. 2007. IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp, and SurA. J. Bacteriol. 1895566-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purdy, G. E., M. Hong, and S. M. Payne. 2002. Shigella flexneri DegP facilitates IcsA surface expression and is required for efficient intercellular spread. Infect. Immun. 706355-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizzitello, A. E., J. R. Harper, and T. J. Silhavy. 2001. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J. Bacteriol. 1836794-6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins, J. R., D. Monack, S. J. McCallum, A. Vegas, E. Pham, M. B. Goldberg, and J. A. Theriot. 2001. The making of a gradient: IcsA (VirG) polarity in Shigella flexneri. Mol. Microbiol. 41861-872. [DOI] [PubMed] [Google Scholar]
- 33.Sansonetti, P. J., J. Arondel, A. Fontaine, H. d'Hauteville, and M. L. Bernardini. 1991. OmpB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine 9416-422. [DOI] [PubMed] [Google Scholar]
- 34.Shere, K. D., S. Sallustio, A. Manessis, T. G. D'Aversa, and M. B. Goldberg. 1997. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol. Microbiol. 25451-462. [DOI] [PubMed] [Google Scholar]
- 35.Sklar, J. G., T. Wu, D. Kahne, and T. J. Silhavy. 2007. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev. 212473-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinhauer, J., R. Agha, T. Pham, A. W. Varga, and M. B. Goldberg. 1999. The unipolar Shigella surface protein IcsA is targeted directly to the bacterial old pole: IcsP cleavage of IcsA occurs over the entire bacterial surface. Mol. Microbiol. 32367-377. [DOI] [PubMed] [Google Scholar]
- 37.Ureta, A. R., R. G. Endres, N. S. Wingreen, and T. J. Silhavy. 2007. Kinetic analysis of the assembly of the outer membrane protein LamB in Escherichia coli mutants each lacking a secretion or targeting factor in a different cellular compartment. J. Bacteriol. 189446-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallejo, A. N., R. J. Pogulis, and L. R. Pease. 1994. In vitro synthesis of novel genes: mutagenesis and recombination by PCR. PCR Methods Appl. 4S123-S130. [DOI] [PubMed] [Google Scholar]
- 39.van Ulsen, P., and J. Tommassen. 2006. Protein secretion and secreted proteins in pathogenic Neisseriaceae. FEMS Microbiol. Rev. 30292-319. [DOI] [PubMed] [Google Scholar]