Abstract
SugR, RamA, GlxR, GntR1, and a MarR-type transcriptional regulator bind to the promoter region of the gapA gene encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH), essential for glycolysis in Corynebacterium glutamicum. We previously showed that SugR, a transcriptional repressor of phosphotransferase system genes for the sugar transport system, is involved in the downregulation of gapA expression in the absence of sugar. In this study, the role of RamA in the expression of the gapA gene was examined. Comparing the gapA expression and GAPDH activity of a ramA mutant with those of the wild type revealed that RamA is involved in upregulation of gapA expression in glucose-grown cells. DNase I footprint analyses and electrophoretic mobility shift assays revealed that RamA binds with different affinities to three sites in the gapA promoter. lacZ reporter assays with mutated RamA binding sites in the gapA promoter showed that the middle binding site is the most important for RamA to activate gapA expression and that binding of RamA to the gapA promoter activates the gene expression not only in glucose-grown cells but also in acetate-grown cells. Furthermore, RamA also directly activates sugR expression, indicating that two global regulators, RamA and SugR, are coordinately involved in the complex regulation of gapA expression in C. glutamicum.
d-Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), catalyzing the oxidation of d-glyceraldehyde-3-phosphate into 1,3-diphosphoglycerate by using NAD+ as a coenzyme, is a key enzyme of glycolysis and plays a crucial role in catabolic and anabolic carbohydrate metabolism due to the reversibility of its catalysis. Regulation of the GAPDH-encoding genes in bacteria has been studied most in Escherichia coli and Bacillus subtilis. In E. coli, the expression of the GAPDH-encoding gene, gapA, is upregulated in the presence of glucose and this induction depends upon the EIIGlc glucose-permease protein, a component of the phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS) (3). E. coli gapA expression is repressed by catabolite repressor activator (Cra), and its repression is relieved in the presence of sugar metabolite fructose-1-phosphate or fructose-1,6-bisphosphate (36, 39). The B. subtilis GAPDH-encoding gene, gapA, is induced in the presence of sugar (29), and this induction indirectly depends on catabolite control protein A (CcpA), the major transcriptional regulator for catabolite repression (11, 29, 30). In addition, B. subtilis gapA expression is repressed by central glycolytic gene regulator (CggR), and the repression by CggR is relieved by fructose-1,6-bisphosphate (7, 29). Thus, gapA expression in both these organisms is repressed by transcriptional regulators sensing sugar phosphate and upregulated by alleviation of the repression in the presence of sugar.
Corynebacterium glutamicum is a high-GC gram-positive bacterium widely used in the industrial production of amino acids, e.g., l-glutamate and l-lysine, as well as organic acids (18, 22, 25, 27, 42). C. glutamicum additionally produces organic acids and ethanol under oxygen-deprived conditions, under which cells retain the ability to excrete product despite cessation of growth (20, 21, 34). Glycolysis is one of the most important targets to improve the yield of production from sugar. C. glutamicum possesses two GAPDHs, GapA and GapB. GapA is essential for glycolysis, and GapB is dispensable for glycolysis and important for gluconeogenesis (35). The susceptibility of GapA activity to the intracellular NADH/NAD+ ratio (8) suggests that the enzyme may well catalyze the rate-limiting step of glycolysis.
The expression of gapA is controlled at the transcriptional level in C. glutamicum. It is upregulated in glucose-grown cells compared to that in acetate-grown cells (15, 17, 33). The gapA expression level is increased at the onset of the stationary phase during growth on glucose and remains high upon oxygen deprivation (21). SugR, a transcriptional repressor of genes involved in PTS (10, 13, 41), acts as a repressor of gapA expression in the absence of sugar (43). This repression of gapA is relieved by fructose-1-phosphate and fructose-1,6-bisphosphate (43), a pattern similar to the regulation of E. coli gapA expression by Cra and B. subtilis gapA by CggR. However, gapA expression in a sugR deletion mutant is still upregulated in the presence of glucose at the onset of the stationary phase, as it is in the wild-type strain, suggesting the involvement of other transcriptional regulators (43). GlxR, a cyclic AMP receptor protein-type transcriptional regulator involved in many physiological reactions in C. glutamicum (23, 24, 26, 28), binds to the gapA promoter region, although its physiological function remains to be elucidated (15).
In this study, we show that RamA, which is involved in various physiological reactions, including acetate metabolism (6), synthesis of a cell surface layer protein (16), and the expression of resuscitation-promoting factor (23), binds to three sites in the gapA promoter region with different affinities and that RamA is important for upregulation of gapA expression at the onset of the stationary phase. Finally, we show that RamA directly activates sugR expression. These results indicate a complex regulation of gapA expression by two different types of global regulators in C. glutamicum.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F′[traD36 proAB+lacIqlacZΔM15] | Takara |
| JM110 | dam dcm supE44 hsdR17 tih leu rpsL lacy galK galT ara tonA thr tsx Δ(lac-proAB)/F′[traD36 proAB+ lacIq lacZΔM15] | 37 |
| BL21(DE3) | F−ompT gal dcm lon hsdSB(rB− mB−) λ(DE3) | 40 |
| C. glutamicum | ||
| R | Wild-type strain | 45 |
| KT1 | R with deletion in sugR | 43 |
| KT2 | R with gapA-lacZ | 43 |
| KT3 | KT1 with gapA-lacZ | 43 |
| KT6 | R with deletion in ramA | This study |
| KT7 | R with deletion in glxR | This study |
| KT8 | R with deletion in gntR1 | This study |
| KT9 | R with deletion in marR | This study |
| KT10 | R with deletions in ramA and sugR | This study |
| KT11 | KT6 with gapA-lacZ | This study |
| KT12 | KT10 with gapA-lacZ | This study |
| KT13 derivatives | R with gapA-lacZ with mutations in the RamA binding sites in the gapA promoter | This study |
| Plasmids | ||
| pCold I | Apr; cold-inducible expression vector | Takara |
| pCRC604 | Apr; pColdI with an 846-bp fragment of the ramA gene | This study |
| pCRA725 | Kmr; the suicide vector containing the B. subtilis sacB gene | 19 |
| pCRC605 | Kmr; pCRA725 with 2.3-kb XbaI-SacI PCR fragment containing the ramA gene | This study |
| pCRC606 | Kmr; pCRC604 with a 1.6-kb fragment containing a truncated ramA gene | This study |
| pCRA741 | Kmr; pCRA725 with a 2.0-kb PCR fragment from strain-specific island 7 and a 3.1-kb PCR fragment containing the E. coli lacZ gene | 21 |
| pCRC602 | Kmr; pCRA741 with a 0.6-kb PCR fragment containing the C. glutamicum R gapA promoter region | 43 |
| pCRC607 | Kmr; pCRA741 with a 0.6-kb PCR fragment containing the gapA promoter region with mutations in RamA binding site P | This study |
| pCRC608 | Kmr; pCRA741 with a 0.6-kb PCR fragment containing the gapA promoter region with mutations in RamA binding site M | This study |
| pCRC609 | Kmr; pCRA741 with a 0.6-kb PCR fragment containing the gapA promoter region with mutations in RamA binding site D | This study |
| pCRC610 | Kmr; pCRA741 with a 0.6-kb PCR fragment containing the gapA promoter region with mutations in RamA binding sites P and D | This study |
| pCRC611 | Kmr; pCRA741 with a 0.6-kb PCR fragment containing the gapA promoter region with mutations in RamA binding sites M and D | This study |
| pCRC612 | Kmr; pCRA741 with a 0.6-kb PCR fragment containing the gapA promoter region with mutations in RamA binding sites P and D | This study |
| pCRC613 | Kmr; pCRA741 with a 0.6-kb PCR fragment containing the gapA promoter region with mutations in all RamA binding sites | This study |
Culture conditions.
For genetic manipulation, E. coli strains were grown at 37°C in Luria-Bertani (LB) medium. C. glutamicum strains were grown at 33°C in A medium (21) with 4% glucose. When appropriate, the medium was supplemented with antibiotics. The final antibiotic concentrations for E. coli were 50 μg of ampicillin ml−1 and 50 μg of kanamycin ml−1; for C. glutamicum, kanamycin (50 μg ml−1) was used. Growth experiments with C. glutamicum were performed using A medium containing 1% glucose or acetate as described previously (43).
DNA manipulations.
The oligonucleotides used in this study (see Table S1 in the supplemental material) were obtained from Gene Design (Osaka, Japan). Plasmid DNA was isolated with a QIAprep spin miniprep kit (Qiagen). Chromosomal DNA was isolated from C. glutamicum with Genomic prep (GE Healthcare Bioscience), modified by using 4 mg ml−1 lysozyme at 37°C for 2 h. Restriction endonucleases were purchased from Takara (Osaka, Japan) and used according to the manufacturer's instructions. PCR was performed with a GeneAmp PCR system (Applied Biosystems) using LA Taq polymerase (Takara) or Phusion polymerase (New England Biolabs). The resulting PCR fragments were purified with a QIAquick PCR purification kit (Qiagen). E. coli was transformed by the CaCl2 procedure (37). C. glutamicum was transformed by electroporation as described previously (44). The nucleotide sequence of cloned DNA fragments was determined with a genetic analyzer 3130xl (Applied Biosystems).
DNA affinity purification.
DNA affinity purification of proteins binding to the gapA promoter region and N-terminal sequencing of the proteins were performed as described previously (43).
Construction of ramA and ramA-sugR mutants.
A DNA fragment containing the ramA gene was amplified by using primer ramAFWXba and primer ramARVSac and genomic DNA from C. glutamicum strain R as template. The PCR product was cloned into pCRA725, a suicide vector for markerless gene disruption (19), yielding pCRC605. An internal 759-bp segment of the ramA gene was removed by inverse PCR using primers ramAinvMunFW and ramAinvMunRV and pCRC605 as template. The PCR product was digested with MunI and self-ligated, yielding pCRC606. Plasmid pCRC606 was isolated as nonmethylated DNA from E. coli JM110 for efficient gene introduction into C. glutamicum R (44). C. glutamicum R and the sugR mutant strain KT1 (43) were transformed by electroporation with pCRC606, and screening for the deletion mutants was performed as described previously (19). Deletion of the ramA gene was checked by PCR using primers ramAFWXba and ramARVSac. The ramA and ramA-sugR mutant strains obtained were designated KT6 and KT10, respectively. Similarly, the gntR1, glxR, and marR deletion mutants were constructed and designated KT7, KT8, and KT9, respectively. The gntR1, glxR, or marR gene was amplified by using primer pair gntRFW-gntRRV, glxRFW-glxRRV, or marRFW-marRRV, respectively. The PCR products were cloned into pCRA725. The cloned gene was truncated by inverse PCR using the following primer pairs: gntRinvFW-gntRinvRV, glxRinvFW-glxRinvRV, and marRinvFW-marRinvRV. The PCR product was digested with appropriate restriction enzymes and self-ligated. C. glutamicum R was transformed by electroporation with the resulting plasmids as described above.
qRT-PCR.
Total RNA was extracted from C. glutamicum cells by using a Qiagen RNeasy mini kit (Qiagen) as described previously (43). Isolated RNA samples were checked for purity by both agarose gel electrophoresis and spectrophotometric analysis and stored at −80°C. Quantitative reverse transcription-PCR (qRT-PCR) was performed using an Applied Biosystems 7500 fast real-time PCR system (Applied Biosystems) and power SYBR green PCR master mix with MuLV reverse transcriptase and RNase inhibitor from a GeneAmp RNA PCR kit (Applied Biosystems) as described previously (43). Specific primers (see Table S1 in the supplemental material) were designed by using Primer Express Software version 3.0 (Applied Biosystems). The specificity of the amplicons was checked by qRT-PCR dissociation curve analysis. The comparative threshold cycle method (7500 Fast System SDS software version 1.4.0.; Applied Biosystems) was used to quantify relative expression.
GAPDH assay.
C. glutamicum cells were harvested by centrifugation, washed with 50 mM Tris-HCl buffer, pH 7.5, and resuspended with buffer containing 50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, and 10% (vol/vol) glycerol. The cells were mechanically disrupted by using a FastPrep FP120 instrument (BIO 101; Thermo Savant) as described previously (43), and the supernatant after centrifugation was used as crude extract for the enzyme assay. The activity of GAPDH was determined as described previously (35).
Overexpression and purification of His-tagged RamA protein.
The ramA gene was amplified from chromosomal DNA of C. glutamicum R by PCR with primers RamAHisFW and RamAHisRV. The PCR product was cloned into the expression vector pColdI (Takara), yielding pCRC604. pCRC604 was used to transform E. coli BL21(DE3), and the resulting strain was grown at 37°C in LB medium to an optical density at 600 nm of 0.5. The culture was incubated for 30 min at 15°C, and then expression of the His-tagged RamA was induced by the addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The culture was shaken overnight at 15°C. The cells were harvested by centrifugation and frozen at −80°C until ready for use. The His-tagged protein was purified by affinity chromatography on Ni-nitrilotriacetic acid agarose (Qiagen) according to the instruction manual. For desalting, the eluted protein was loaded to a PD-10 column (GE Healthcare Bioscience) and eluted with buffer A (50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1 mM EDTA, and 1 mM dithiothreitol). The concentration of the purified protein was determined by a Bio-Rad protein assay (Bio-Rad Laboratories) using bovine serum albumin as a standard.
EMSA.
DNA fragments of various lengths covering the gapA promoter region (P1 to P6) were obtained by amplifying regions of the gapA promoter from chromosomal DNA using the following primer pairs: pgapAFW2-pgapARV3Cy3, pgapAFW3-pgapARV3Cy3, pgapAFW5-pgapARV3Cy3, pgpaAFW5Cy3-pgapARV4, pgapAFW5Cy3-pgapARV5, and pgapAFW5Cy3-pgapARV6. DNA fragments P1, P3, and P4 containing mutations in the RamA binding sites were amplified by PCR using the following primer pairs and plasmids as template: pgapAFW5-pgapARV3Cy3, pgapAFW3-pgapARV3Cy3, and pgapAFW5Cy3-pgapARV4 and pCRC607, pCRC608, pCRC609, and pCRC610 containing the gapA promoter region with mutations in the RamA binding sites as described for construction of the lacZ fusion. The DNA fragments were purified with a QIAquick column (Qiagen). Electrophoretic mobility shift assay (EMSA) was performed using the purified His-tagged RamA as described previously (43). The resulting DNA-protein complex was loaded onto a 5% polyacrylamide gel in 0.5× Tris-borate-EDTA and separated at 125 V for 40 min. The gel was stained with ethidium bromide and visualized with a UV transilluminator. When Cy3-labeled probes were used, DNA and DNA-protein complexes were visualized by using a Typhoon TRIO variable mode imager (GE Healthcare Bioscience).
DNase I footprinting.
Labeled DNA fragments were obtained by amplification with 5′-IRD700-labeled oligonucleotides. The gapA promoter region was amplified by using the following primer pairs: gapAIRD FW and pgapA RV2 (coding strand), and gapAIRD RV and pgapA RV (noncoding strand). An IRD700-labeled DNA fragment (4 nM) was mixed with purified RamA in a total volume of 50 μl of binding buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, 3 mM MgCl2, 5 mM CaCl2, 0.1 mM EDTA, 0.5 mM dithiothreitol, 10% [vol/vol] glycerol, and 1.5 μg/μl of bovine serum albumin). The mixture was incubated for 25 min at room temperature. The reaction mixture volume was increased to 100 μl with 1× binding buffer, and then DNase I (Takara) was added at 35 mU, and incubation was continued for 1 min at 25°C. The mixture was treated with phenol, precipitated with ethanol, and resuspended with IR2 stop solution (Li-Cor, Lincoln, NE). The dissolved sample was separated on a 5.5% KB plus gel matrix (Li-Cor) using a Li-Cor 4300 DNA analyzer. The DNA-sequencing reaction mixtures were set up using the same IRD-700-labeled primers and a DYEnamic direct cycle sequencing kit with 7-deaza-dGTP (GE Healthcare/Bioscience).
Construction of the gapA promoter-lacZ fusions.
The DNA fragment containing the gapA promoter region was generated by PCR using primers pgapAFW5 and pgapARV and cloned upstream of the lacZ gene in pCRA741 (21), yielding pCRC602. The direction and sequence of the inserted fragment were confirmed by DNA sequencing. Plasmid pCRC602 was isolated as nonmethylated DNA from E. coli JM110 for efficient gene introduction into C. glutamicum R (44) and was subsequently integrated into the chromosome of C. glutamicum R, the ramA mutant strain KT6, and the ramA-sugR mutant strain KT10 by markerless gene insertion methods, as described previously (19), yielding the resulting strains KT2 (43), KT11, and KT12, respectively. Mutations in RamA binding sites were constructed by overlapping PCR using overlapping primer pairs ramAmutPFW-ramAmutPRV, ramAmutMFW-ramAmutMRV, and ramAmutDFW-ramAmutDRV for the proximal, middle, and distal sites, respectively, together with primers pgapAFW5 and pgapARV and genomic DNA from strain R as a template. The overlapping PCR products were purified and used as template for PCR using primer pgapAFW5 and primer pgapARV. The resulting fragments were cloned into pCRA741, generating pCRC607, pCRC608, and pCRC609 containing mutations in the proximal, middle, and distal sites in the gapA promoter, respectively, hereafter referred to as sites P, M, and D. Double and triple mutations in the RamA binding sites were introduced by overlapping PCR using the primer pairs described above and plasmids pCRC607 to pCRC609 instead of genomic DNA as template, and the resulting fragments were cloned into pCRA741, generating pCRC610, pCRC611, pCRC612, and pCRC613. Plasmids from pCRC607 to pCRC613 were individually integrated into the chromosome of C. glutamicum R. The resulting strains were designated KT13P, KT13M, KT13D, KT13PM, KT13MD, KT13PD, and KT13PMD, where the last letter(s) indicates the RamA binding site mutated. The mutations introduced in the gapA promoter-lacZ fusion on the chromosome were confirmed by direct sequencing of a PCR fragment which was amplified using a primer specific for lacZ and one specific for the gapA promoter (pgapAFW5), with genomic DNA extracted from the mutants obtained as a template.
β-Galactosidase assay.
C. glutamicum cells carrying the gapA promoter-lacZ fusion were harvested, washed once with Z buffer (32), resuspended with the same buffer, and treated with toluene. The permeabilized cells were then incubated with o-nitrophenyl-β-galactoside, and activity was measured in Miller units as previously described (32).
RESULTS
Transcriptional regulatory proteins binding to the gapA promoter region of C. glutamicum.
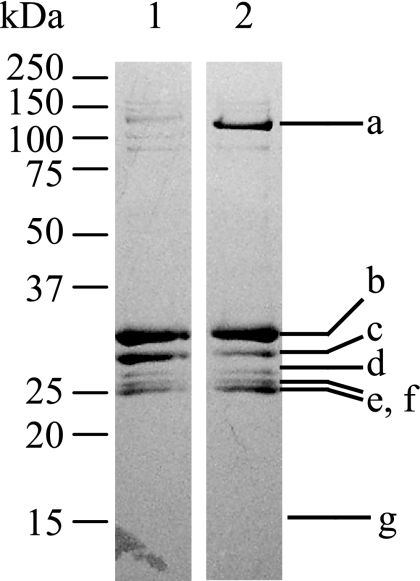
To identify proteins binding to the gapA promoter region, we previously performed DNA affinity purification with magnetic streptavidin beads (43). A 334-bp biotinylated promoter region (positions between −304 and +30 relative to the transcriptional start point [TSP] of gapA) was linked to streptavidin-coated magnetic beads and incubated with cell extracts from C. glutamicum grown on minimal medium containing glucose or acetate as the sole carbon source. Figure 1 shows the proteins eluted from the beads. We identified the proteins by comparing the N-terminal amino acid sequence of the proteins (Fig. 1, a to g) with the entire genome sequence of C. glutamicum R (45). In addition to SugR, which was previously identified and shown to act as a repressor of gapA expression (43), four other transcriptional regulatory proteins were identified in this study (Fig. 1). Three of them, RamA (CgR2464), GntR1 (CgR2434), and GlxR (CgR0377), were previously identified as regulators of genes involved in various physiological reactions other than glycolysis (6, 10, 12, 13, 16, 23, 24, 28, 41). The last one, a MarR-type transcriptional regulator (CgR2877), has not been fully characterized to date.
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of proteins binding to the gapA promoter region. Cell extracts of C. glutamicum R grown in minimal medium containing 1% acetate (lane 1) or glucose (lane 2) were incubated with magnetic beads linked to biotin-labeled gapA promoter DNA fragment, and bound proteins were eluted with 1 M NaCl. The N-terminal amino acid sequence of the proteins in bands a to g was analyzed: band a, termination factor Rho (CgR1278); band b, RamA (CgR2464); band c, SugR (CgR1761); band d, GntR1 (CgR2434); bands e and f, GlxR; band g, MarR-type transcriptional regulator (CgR2877). A molecular mass standard is shown to the left.
RamA is involved in upregulation of gapA expression in the presence of glucose.
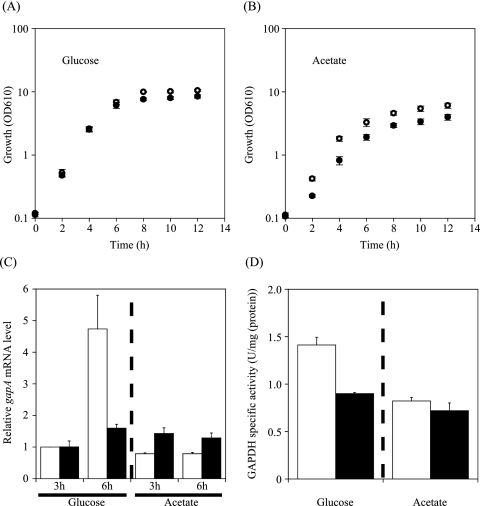
To clarify in vivo roles of the transcriptional regulators identified, the corresponding genes were inactivated by in-frame deletion. Deletion mutants of each of the ramA, glxR, gntR1, and cgR2877 genes were grown on nutrient-rich A medium containing glucose or acetate. We consequently compared the expression level of gapA of each of the mutant strains with that of the wild-type strain by qRT-PCR. Total RNA was extracted from the cells grown on glucose or acetate at the mid-exponential phase (3 h) and the onset of the stationary phase (6 h) (43). Deletion of the gntR1 or cgR2877 gene had no effect on growth and gapA expression under the conditions tested (data not shown). In contrast, the glxR mutant grew too slowly to analyze. The ramA mutant strain grew at a rate similar to that of the wild type in the presence of glucose, although the final cell density of the mutant culture was slightly lower than that of the wild type (Fig. 2A), which is consistent with the results of a previous study (6). As the ramA mutant strain is unable to grow on acetate as the sole carbon source (6), it grew more slowly than the wild type in A medium with acetate (Fig. 2B).
FIG. 2.
Effect of deletion of the ramA gene on growth (A, B), gapA expression (C), and GAPDH activity (D). (A, B) Growth of the wild-type R (white circles) and the ramA mutant KT6 (black circles) in nutrient-rich A medium containing 1% glucose (A) or acetate (B). Mean values from at least four independent cultures are shown, with standard deviations. OD610, optical density at 610 nm. (C) The level of gapA mRNA in the wild-type R (white) and ramA mutant KT6 (black) during growth in A medium with 1% glucose or acetate at the exponential (3 h) and the stationary (6 h) phase was analyzed by qRT-PCR, and the levels relative to that in the wild type grown in the presence of glucose at 3 h were determined. Mean values from three independent cultures are shown, with standard deviations. (D) Activities of GAPDH in the wild-type R (white) and the ramA mutant KT6 (black) grown in A medium with 1% glucose or acetate. The activity was determined at the stationary (6 h) phase. Mean values from at least three independent cultures are shown, with standard deviations.
The level of gapA mRNA of the wild-type strain grown on glucose was comparable to that of cells grown on acetate at the exponential phase (Fig. 2C). In the presence of glucose, the level of gapA mRNA in the wild type was increased nearly fivefold at the onset of the stationary phase (21), while the level was constant throughout growth in the presence of acetate (Fig. 2C). In the presence of glucose, the level of gapA mRNA in the ramA mutant was almost the same as that in the wild-type strain at the exponential phase, while gapA expression was downregulated in the ramA mutant by 60% compared to that in the wild type strain at the onset of the stationary phase (Fig. 2C). In the presence of acetate, the level of gapA mRNA in the ramA mutant was relatively low compared with that in the presence of glucose throughout growth (Fig. 2C). As observed with the mRNA level, the corresponding activity of GAPDH encoded by the gapA gene in the ramA mutant was lower than that of the wild type in the glucose-grown cells at the onset of the stationary phase (Fig. 2D). In the presence of acetate, the GAPDH activity of the ramA mutant was comparable to that of the wild type. These results suggested that, in the presence of glucose, RamA is involved in upregulation of gapA expression at the onset of the stationary phase.
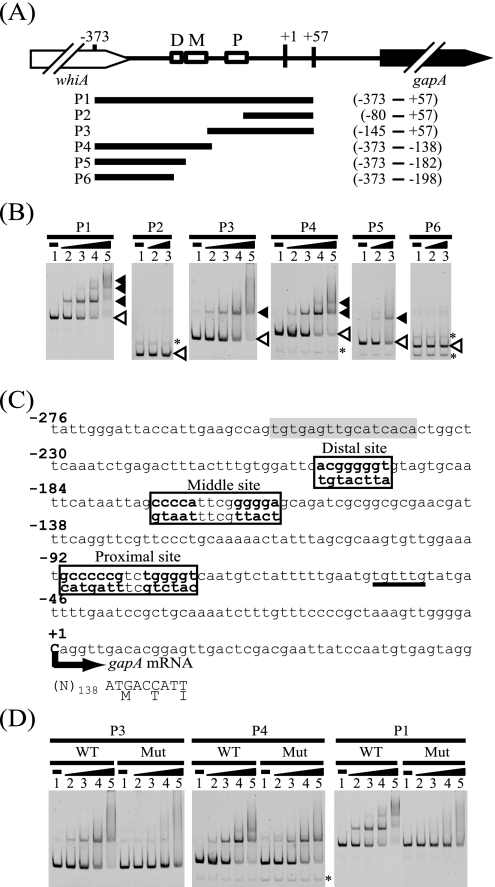
RamA binds to three distinct sites within the gapA promoter region.
To examine the binding of RamA to the gapA promoter region, we performed EMSAs with a DNA fragment containing the gapA promoter region, which encompasses positions +57 to −373 relative to the TSP of gapA, and the recombinant RamA protein (Fig. 3A). The results of the EMSAs showed that RamA specifically bound to the gapA promoter (Fig. 3B, P1 fragment). Three shifted bands were observed when 800 nM of RamA was added to the reaction mixture, suggesting that there are three RamA binding sites in the gapA promoter. To identify the RamA binding sites in the gapA promoter region, we performed EMSAs with a series of the gapA promoter fragments from P2 to P6 (Fig. 3B). The results showed that RamA bound to P3 and P4 but not to P2 or P6, indicating that RamA specifically binds to the two regions between positions −86 and −148 and positions −152 and −197 relative to the TSP of the gapA gene. We found a putative RamA consensus binding site consisting of tandem A/C/TG4-6T/C or AC4-5A/G/T in each region (Fig. 3C) (6). The two sites are located between positions −77 and −91 and positions −160 and −174 relative to the TSP. The significance of these sites for RamA binding was tested by mutational analysis, in which G/C stretches were changed (Fig. 3C). RamA failed to bind to the mutated P3 fragment (Fig. 3D), whereas RamA was still able to bind to the mutated P4 fragment but only one shifted band was observed, unlike in the case of the wild-type fragment (Fig. 3D, P4 fragments). RamA binds to not only tandem but also single GC-rich stretches (5). We found a single GC-rich stretch, ACGGGGGT, similar to the RamA binding site, at position −198 relative to the TSP (Fig. 3C), and showed that this sequence was sufficient for RamA binding (Fig. 3B, P5 fragment). Simultaneous mutation in each of these three sites diminished the binding of RamA to the gapA promoter (Fig. 3D, P1 fragments). These results demonstrated that RamA binds to the three sites centered at positions −84, −168, and −198 with respect to the TSP (sites P, M, and D, respectively) in the gapA promoter.
FIG. 3.
Binding of RamA to the gapA promoter region. (A) DNA fragments (P1 to P6) used to determine the location of the RamA binding site in the gapA promoter are shown. The numbers above the diagram indicate the position relative to the TSP (+1) of the gapA gene. The regions included in the DNA fragments are indicated, with positions with respect to the TSP at the right side. The gapA gene is indicated with a black arrow. The whiA gene, which encodes a WhiA-like protein, is located upstream of gapA with the same direction and is indicated with a white arrow. Three RamA binding sites (P, M, and D sites) are indicated with white boxes. (B) Results of EMSAs using the gapA promoter region and His-tagged RamA. The DNA fragments (P1 to P6) used are indicated at the top of the gels. Each well contained 10 nM of DNA fragment. Lanes 1 to 5 show EMSA results using 0, 200, 400, 800, and 1,600 nM of RamA protein, respectively. Probe DNA and DNA-RamA complexes are indicated with white and black arrowheads, respectively. Nonspecific bands are indicated with asterisks. (C) DNA sequence of the gapA promoter showing the putative RamA binding sites. The TSP (+1) is indicated with a bold letter and an arrow. The numbers indicate the position relative to the TSP (+1). The coding region of gapA is indicated with capital letters and the amino acid sequence. Three RamA binding sites (P, M, and D sites) and the mutated sequences shown under the sites are boxed. The mutated sequences are indicated with bold letters. The binding sites of SugR and GlxR are underlined and shaded in gray, respectively. (D) The results of EMSAs using DNA fragments without (WT) or with (Mut) the mutations in the RamA binding site are shown. The DNA fragments (P1, P3, and P4) used are indicated at the top of the gels. Each well contained 10 nM of DNA fragment. Lanes 1 to 5 show results of EMSAs using 0, 200, 400, 800, and 1,600 nM of RamA protein, respectively. The mutated P3 fragment contains the mutated P binding site. The mutated P4 fragment contains the mutated middle and native distal binding sites. The mutated P1 fragment contains mutations in all the binding sites. A nonspecific band is indicated with an asterisk.
The three RamA binding sites have different affinities for RamA.
To confirm the RamA binding site in the gapA promoter, DNase I footprinting assays were performed. A DNA fragment encompassing the gapA promoter (from position +56 to −316 with respect to the TSP) was labeled on one strand or the other, incubated with an increasing quantity of RamA, and then hydrolyzed by using DNase I. RamA protected three regions on either strand (Fig. 4). The regions that were protected were positions −74 to −93, −155 to −176, and −187 to −203 on the coding strand, and −71 to −97, −156 to −181, and −186 to −205 on the noncoding strand. These regions included the three RamA binding sites described above (Fig. 3C). As indicated by the appearance of DNase I-hypersensitive sites in the P and M sites and between the two sites, conformational changes might occur in the DNA upon binding of RamA (Fig. 4). At low concentrations, RamA preferentially bound to the region containing the M site, while simultaneous protection of the other binding sites was observed at increasing concentrations (Fig. 4). The results suggested that RamA has the highest affinity for the M site.
FIG. 4.
DNase I footprint analysis of the interaction between RamA and the gapA promoter regions examined on the coding and the noncoding strands. A DNA fragment (4 nM) was incubated with different concentrations of RamA: lanes 1 and 7, no protein; lane 2, 200 nM; lane 3, 400 nM; lane 4, 800 nM; lane 5, 1,600 nM; lane 6, 3,200 nM. The three RamA binding sites are indicated with brackets on the left side of each panel. Protected regions are indicated by bars, and hypersensitive sites are indicated by arrows. The DNA sequencing reactions were set up using the same labeled primer and plasmid as for generating labeled footprinting probes.
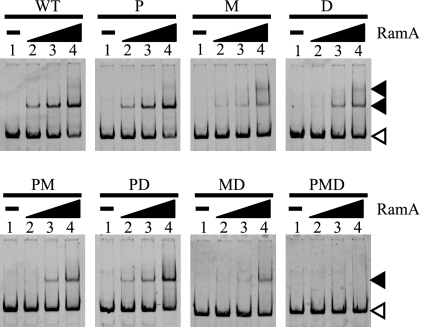
To investigate the affinity of RamA for the binding sites in detail, the effects of mutations in the binding sites on the binding of RamA to the gapA promoter fragment were compared by EMSAs (Fig. 5). With the wild-type promoter (WT) fragment, a shifted band was observed at 100 nM of RamA and a lower-mobility complex was observed at 400 nM of RamA. RamA bound to a fragment containing mutations in the P site with the same affinity as it did to the WT fragment (Fig. 5, WT and P). Mutations in the M site, however, drastically reduced the ability of RamA to bind to the gapA promoter, but the lower-mobility complex was more clearly observed compared with that observed in the WT fragment at 400 nM of RamA (Fig. 5, WT and M). Mutations in the D site also reduced the ability of RamA to bind to the gapA promoter fragment, but the inhibitory effect was smaller than that observed for mutations in the M site (Fig. 5, WT and D). These results supported the results of the footprint analysis, in which the region containing the M site was protected from DNase I at lower concentration of RamA than the other sites. When fragments containing two mutated binding sites were used, the lower-mobility complex was not observed. The effects of the mutations in each binding site on the binding of RamA seemed to be additive, i.e., the affinity of RamA for the fragment with mutations in both the P and D sites (PD) was lower than that for a fragment with mutations in only the P site, and the affinity of RamA for the fragment with mutations in both the M and D sites was lower than that for a fragment with mutations in only the M site (Fig. 5; compare MD and M).
FIG. 5.
RamA has different affinities for the three binding sites in the gapA promoter region. The gapA promoter fragment without (WT) or with mutated RamA binding sites was incubated with different concentrations of RamA: lane 1, no protein; lane 2, 100 nM; lane 3, 200 nM; lane 4, 400 nM. The binding sites mutated are indicated at the top of the gels. Probe DNA and DNA-RamA complexes are indicated with white and black arrowheads, respectively.
Effects of mutations of the RamA binding sites on gapA promoter activity.
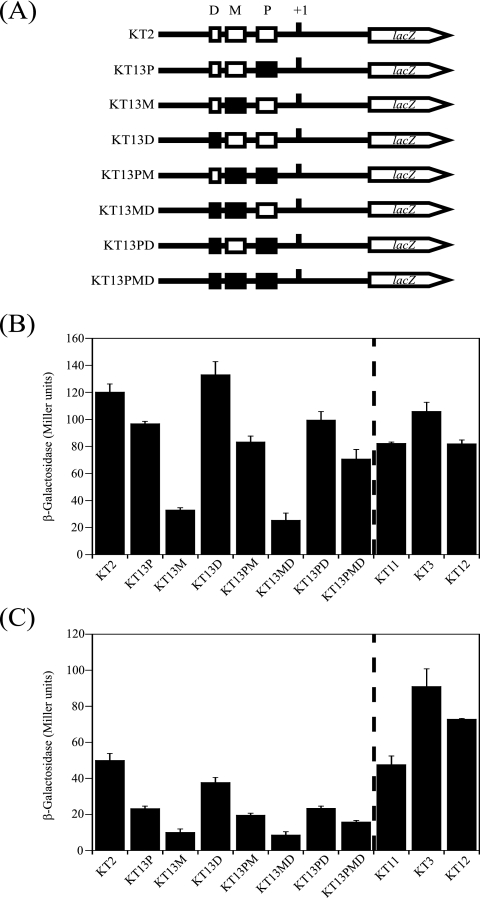
To investigate roles of the three RamA binding sites in the regulation of gapA expression in vivo, the effects of mutations in the RamA binding sites (Fig. 3C) on the gapA promoter activity were examined by using the lacZ reporter assay system (Fig. 6). We integrated the gapA promoter-lacZ translational fusion (PgapA-lacZ), containing mutations in each site; in both the P and M, P and D, and M and D sites; or in all sites, into the chromosome of C. glutamicum R and designated the resulting strains KT13P, KT13M, KT13D, KT13PM, KT13PD, KT13MD, and KT13PMD, respectively (Fig. 6A). In the presence of glucose (Fig. 6B), mutations in the M site decreased the β-galactosidase activity to 30% of that of the wild type. Mutations in the P site also elicited a negative effect on the β-galactosidase activity, but the effect was much smaller than that elicited by mutations in the M site. Mutations in the D site had almost no discernible effect on the β-galactosidase activity. The effects of mutations in the RamA binding sites on the gapA promoter activity were not additive, in contrast to those on the binding of RamA (Fig. 5). The β-galactosidase activity of strain KT13PM, containing mutations in both the P and M sites, was higher than that of KT13M but lower than that of KT13P. The combination of the D site mutation with the mutations of other KT13 derivatives had no effect on β-galactosidase (Fig. 6B, KT13PD, KT13MD, and KT13PMD). Similar effects of mutations in the RamA binding sites on the gapA promoter activity were also observed in acetate-grown cells, although the activity was lower than that in glucose-grown cells (Fig. 6C). These results suggested that the gapA promoter activity is affected by the coordination of multiple RamAs binding to the M and P sites in the promoter under the conditions tested and that the binding of RamA to the gapA promoter activates the gene expression not only in glucose-grown cells but also in acetate-grown cells.
FIG. 6.
Effects of mutation of the RamA binding sites and deletion of the ramA and sugR genes on expression of the gapA promoter-lacZ translational fusion. (A) The gapA promoter-lacZ fusion constructs are indicated. The strains carrying the fusion constructs in their genome are indicated at the left side. The TSP of the gapA gene is indicated with +1. The three RamA binding sites (P, M, and D) without or with mutations are indicated with white or black boxes, respectively. The mutated gapA promoter-lacZ fusions, containing RamA binding sites mutated as shown in Fig. 3C, were introduced into the wild type, yielding KT13 derivatives (KT13P, KT13M, KT13D, KT13PM, KT13MD, KT13PD, and KT13PMD). The gapA promoter-lacZ fusion was also introduced into the wild-type strain (KT2), the ramA mutant (KT11), the sugR mutant (KT3), and the ramA-sugR double mutant (KT12). (B, C) β-Galactosidase activity in each strain grown in nutrient-rich A medium containing 1% glucose (B) or acetate (C) at the onset of the stationary phase. Mean values from at least three independent cultures are shown, with standard deviations.
To examine the roles of the two transcriptional regulators, RamA and SugR, in gapA expression, the gapA promoter activities in the ramA, sugR, and ramA-sugR mutants were determined (Fig. 6). In the presence of glucose, the β-galactosidase activities in the ramA (KT11) and ramA-sugR mutants (KT12) were decreased to the same level, which was lower than that in the wild type (KT2). In the presence of acetate, the β-galactosidase activity of the ramA mutant was the same as that of the wild-type strain. The gapA promoter activity of the ramA-sugR mutant was higher than that of the wild type and lower than that of the sugR mutant strain (KT3). These results suggested that RamA acts as a primary positive regulator of gapA expression regardless of the carbon source used, while SugR acts as a negative regulator of gapA expression in the absence of glucose.
RamA activates sugR expression.
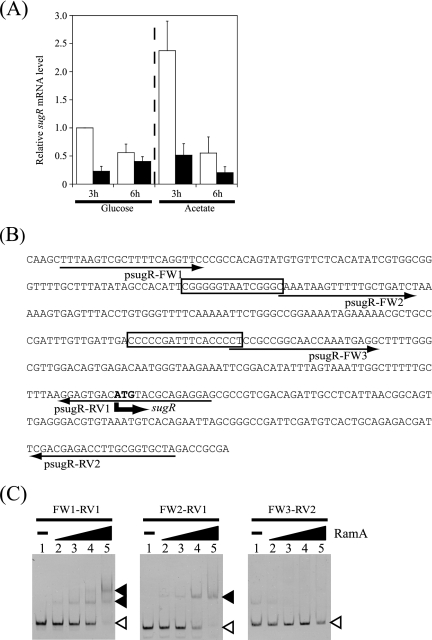
The PgapA-lacZ activity of the ramA mutant (KT11) was higher than that of the wild type harboring mutated RamA binding sites (KT13PMD) in the presence of acetate but similar in the presence of glucose (Fig. 6). This discrepancy might be attributed not only to differences in growth characteristics between the wild type and the ramA mutant but also to differences in the expression of other regulators binding to gapA promoters in the two strains. Thus, we compared the expression of the sugR gene in the wild type with that in the ramA mutant by qRT-PCR. The level of sugR mRNA in the wild-type strain grown on acetate was twice that of the glucose-grown cells at the exponential phase (Fig. 7A). At the onset of the stationary phase, the level of sugR mRNA in the wild-type strain grown on either glucose or acetate was lower than the level at the exponential phase. The level of sugR mRNA in the ramA mutant was less than 20% or 30% of that of the wild-type strain grown on glucose or acetate, respectively, at the exponential phase (Fig. 7A). At the onset of the stationary phase, the level of sugR mRNA of the ramA mutant grown on either glucose or acetate was lower than that of the wild type. These results suggested that RamA positively regulates sugR expression.
FIG. 7.
RamA directly regulates sugR expression. (A) The levels of sugR mRNA in the wild-type R (white) and the ramA mutant KT6 (black) during growth in nutrient-rich A medium with 1% glucose or acetate at the exponential (3 h) and the stationary (6 h) phases were analyzed by qRT-PCR, and the levels relative to that in the wild type grown in the presence of glucose at 3 h were determined. Mean values from three independent cultures are shown, with standard deviations. (B) DNA sequence of the upstream region of sugR of C. glutamicum. The proposed RamA binding sites are boxed. The start codon of sugR is indicated by a bold arrow and bold letters. Thin arrows indicate the primers used to prepare probes for EMSAs. (C) The sugR promoter fragments prepared by PCR using primers indicated at the top of the gels were incubated with different concentrations of RamA: lane 1, no protein; lane 2, 200 nM; lane 3, 400 nM; lane 4, 800 nM; lane 5, 1,600 nM. Probe DNA and DNA-RamA complexes are indicated with white and black arrowheads, respectively.
To examine whether RamA regulates sugR expression directly or indirectly, we performed EMSA using the upstream region of the sugR gene, which was amplified with the primers indicated in Fig. 7B. When using the DNA fragment amplified with primer pair psugR FW1/psugR RV1 (Fig. 7B), two shifted bands were observed in the presence of RamA, suggesting that RamA is able to bind to the upstream region of sugR and that at least two RamA binding sites are located in the promoter (Fig. 7C). We found two putative RamA binding sites comprised of tandem GC-rich stretches at positions −110 and −222 with respect to the translational start point of the sugR gene (Fig. 7B). When using the sugR promoter fragment containing one or no RamA binding site, one or no shifted band was observed, respectively (Fig. 7C), confirming that RamA bound to the two RamA binding sites. These results demonstrated that RamA directly activates sugR expression.
DISCUSSION
In this study, we identified the LuxR-type transcriptional regulator, RamA, as a protein binding to the gapA promoter and showed that RamA positively regulates gapA expression in C. glutamicum. RamA was first identified as a positive transcriptional regulator of the expression of genes involved in acetate metabolism, including the pta-ack operon, aceA, and aceB, encoding phosphotransacetylase and acetate kinase, isocitrate lyase, and malate synthase, respectively, and the ramA mutant is incapable of growing on acetate as the sole carbon source (6). RamA is also involved in the regulation of genes encoding PS2 protein (cspB), a component of the surface layer of C. glutamicum cells (16); RamB (ramB), a repressor of genes involved in acetate metabolism (4, 14); alcohol dehydrogenase (adhA) (1); and resuscitation promoting factor 2 (rpf2) (23). Thus, RamA is a global regulator rather than an acetate-specific regulator. Our current study expanded the RamA regulon to sugar metabolism. It has been previously implied, by the fact that the final cell density of the ramA mutant was lower than that of the wild type during growth on glucose, that RamA is involved in glucose metabolism (6). As the gapA gene constitutes an operon with the pgk and tpi genes, encoding phosphoglycerate kinase and triosephosphate isomerase, respectively (38), these glycolytic genes are under the control of the gapA promoter. The negative effect of ramA inactivation on growth on glucose might be attributed in part to the decrease in expression of the gapA operon.
The results of EMSAs and DNase I footprint analyses showed that RamA binds to three sites containing a single or tandem putative consensus sequences, a stretch of four to five G or C residues flanked by A, C, or T (6), in the gapA promoter. The three RamA binding sites in the gapA promoter were centered at positions −84 (P), −168 (M), and −198 (D) with respect to the TSP of the gapA gene (Fig. 3C). The RamA binding site varies in location and number in the promoter of its target genes, with one common feature being that almost all sites are located upstream of the −35 region where RNA polymerase binds; one to four sites are located in the region between position −50 and −247 relative to the TSP of the target genes (1, 2, 4, 6, 14, 16). The fact that a transcriptional regulator binding to a region upstream of −35 in the target promoter generally functions as an activator is consistent with the role of RamA in regulation of gapA expression (31). However, it should be noted that the location of the binding sites of RamA in the promoter of target genes, e.g., −247 and −176 with respect to the TSP of ramB and cspB, respectively, seems to be more upstream than the sites of typical bacterial transcriptional activators.
The mutations in the M binding site in the gapA promoter reduced the promoter activity to 30% of that of the native one, while the mutations in all three sites decreased the activity to 70% of that of the native one, as observed for deletion of the ramA gene (Fig. 6, strains KT2, KT13M, KT13PMD, and KT11). This suggests that the M site is the most important for RamA to activate the gapA promoter activity. The reason why the activity in KT13PM was higher than that in KT13M is not clear so far. Since RamA has the highest affinity for the M site (Fig. 4 and 5), RamA probably binds to the P and/or D sites after binding to the M site. When the M site is occupied by RamA, binding of RamA to the P site is likely to have a positive effect on the gapA promoter. On the other hand, when the M site is not occupied (which could not occur in vivo except in strains KT13M and KT13MD), binding of RamA to the P site would have a negative effect on the gapA promoter (Fig. 6, KT13M and KT13MD). In fact, PgapA-lacZ with mutations in the M site had higher activity in the ramA mutant background than in the wild type (data not shown). The mutations in the P site had little effect on the binding of RamA and a small effect on the gapA promoter activity (Fig. 5 and 6, KT13P), suggesting that the P site may function properly only in combination with the M site to let RamA alter the gapA promoter conformation and activate gapA expression. The binding of RamA to the D site does not seem to affect the promoter activity, at least under the conditions tested. The binding sites of RamA are almost perfectly conserved in the gapA promoter region of C. glutamicum ATCC 13032 (data not shown), indicating that RamA also binds to the same regions in this strain and the importance of the alignment of the RamA binding sites. As far as we know, this is the first instance in which multiple binding sites of RamA in the target promoter were separately investigated for in vivo functions. However, it remains to be investigated how RamA activates transcription of the target genes.
It is still unknown what environmental conditions and physiological signals RamA senses (2, 6). The genes regulated by RamA are involved in acetate metabolism, whose gene expression in the presence of glucose is downregulated relative to that in the presence of acetate. gapA expression, on the other hand, is upregulated in the presence of glucose compared with that in the presence of acetate (15, 17, 33). These findings suggest that effector molecules, which control RamA activity, may be present in both glucose- and acetate-grown cells. The expression of the glyoxylate shunt genes, aceA and aceB, and that of the alcohol dehydrogenase gene, adhA, is increased in response to the presence of inducer substrates, i.e., acetate and ethanol, respectively, but the expression levels of these genes in the ramA mutant are lower than those in the wild type regardless of the carbon source used (1, 6). Similarly, the activity of the gapA promoter with mutations in the RamA binding sites was lower than that of native promoter in both glucose- and acetate-grown cells (Fig. 6). These findings suggest that RamA is required for basal upregulation of the expression of the target genes rather than carbon source-dependent regulation. This would enable the cell to maintain gapA expression at a high level. gapA expression is controlled by SugR in response to the carbon source used. Further studies are required to address an effector regulating RamA activity.
We showed that RamA directly activates the expression of sugR, suggesting that gapA expression is under the control of a complex transcriptional regulatory network. The expression of the gapA gene in E. coli and B. subtilis is negatively regulated by Cra and CggR, respectively (7, 29, 39). A component of PTS is required for upregulation of gapA in E. coli (3), while CcpA is indirectly involved in upregulation of gapA in B. subtilis (11, 30). As CcpA is required for upregulation of PTS in B. subtilis, the uptake of sugar is inhibited in the ccpA mutant (28). Repression of gapA expression by Cra and CggR is relieved in the presence of sugar phosphate involving fructose-1,6-bisphosphate, which is generated upon the uptake of sugar. In C. glutamicum, gapA expression is repressed by SugR in the absence of sugar, and the repression is also relieved in the presence of fructose-1-phosphate and fructose-1,6-bisphosphate (43). Taken together, derepression of gapA expression in these organisms requires functional PTS and efficient uptake of sugar. On the other hand, it is noteworthy that in C. glutamicum a different type of global regulator, RamA, which regulates the expression of the sugar-sensing global regulator SugR, is involved in complex regulation of the glycolytic gene. This complex regulatory mechanism may be advantageous for C. glutamicum to control gapA expression, a key element of the glycolytic pathway, in response to changes in physiological conditions. Very recently it was shown that SugR also represses genes encoding glycolytic enzymes 6-phosphofructokinase, fructose-1,6-bisphosphate aldolase, enolase, and pyruvate kinase (9).
In summary, we identified transcriptional regulators involved in gapA expression. C. glutamicum gapA expression is negatively regulated by SugR during growth in the absence of sugar and positively regulated by RamA regardless of the carbon source used. We obtained other transcriptional regulators binding to the gapA promoter in this study (Fig. 1). Although deletion of gntR1 or cgR2877 had no effect on gapA expression under the conditions tested, these regulators may be involved in control of gapA expression under other conditions. It should be noted that GntR1 is involved in upregulation of the ptsG gene encoding a glucose-specific component of PTS, EIIGlc (12). GlxR has been reported to act as a repressor for various genes, including aceB, gntP, and gntV (23, 24, 28). The whole binding sites of GlxR on the genome were predicted by in silico analysis and confirmed by EMSAs, suggesting that GlxR is involved in the activation and repression of the expression of numerous genes (26). The binding site of GlxR in the gapA promoter region, centered at position −245 with respect to the TSP (15), seems to be far upstream with respect to the TSP to repress gapA expression, suggesting that GlxR may positively regulate gapA expression. A study to identify a function of these regulators is under way in our laboratory.
Supplementary Material
Acknowledgments
We thank Crispinus A. Omumasaba (RITE) for critical reading of the manuscript.
This work was partially supported by a grant from the New Energy and Industrial Technology Development Organization (NEDO).
Footnotes
Published ahead of print on 1 December 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Arndt, A., and B. J. Eikmanns. 2007. The alcohol dehydrogenase gene adhA in Corynebacterium glutamicum is subject to carbon catabolite repression. J. Bacteriol. 1897408-7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bott, M. 2007. Offering surprises: TCA cycle regulation in Corynebacterium glutamicum. Trends Microbiol. 15417-425. [DOI] [PubMed] [Google Scholar]
- 3.Charpentier, B., V. Bardey, N. Robas, and C. Branlant. 1998. The EIIGlc protein is involved in glucose-mediated activation of Escherichia coli gapA and gapB-pgk transcription. J. Bacteriol. 1806476-6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer, A., M. Auchter, J. Frunzke, M. Bott, and B. J. Eikmanns. 2007. RamB, the transcriptional regulator of acetate metabolism in Corynebacterium glutamicum, is subject to regulation by RamA and RamB. J. Bacteriol. 1891145-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer, A., and B. J. Eikmanns. 2007. RamA, the transcriptional regulator of acetate metabolism in Corynebacterium glutamicum, is subject to negative autoregulation. J. Mol. Microbiol. Biotechnol. 1251-59. [DOI] [PubMed] [Google Scholar]
- 6.Cramer, A., R. Gerstmeir, S. Schaffer, M. Bott, and B. J. Eikmanns. 2006. Identification of RamA, a novel LuxR-type transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J. Bacteriol. 1882554-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doan, T., and S. Aymerich. 2003. Regulation of the central glycolytic genes in Bacillus subtilis: binding of the repressor CggR to its single DNA target sequence is modulated by fructose-1,6-bisphosphate. Mol. Microbiol. 471709-1721. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez, H., C. Rollin, A. Guyonvarch, J. L. Guerquin-Kern, M. Cocaign-Bousquet, and N. D. Lindley. 1998. Carbon-flux distribution in the central metabolic pathways of Corynebacterium glutamicum during growth on fructose. Eur. J. Biochem. 25496-102. [DOI] [PubMed] [Google Scholar]
- 9.Engels, V., S. N. Lindner, and V. F. Wendisch. 2008. The global repressor SugR controls expression of genes of glycolysis and of the l-lactate dehydrogenase LdhA in Corynebacterium glutamicum. J. Bacteriol. 1908033-8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engels, V., and V. F. Wendisch. 2007. The DeoR-type regulator SugR represses expression of ptsG in Corynebacterium glutamicum. J. Bacteriol. 1892955-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fillinger, S., S. Boschi-Muller, S. Azza, E. Dervyn, G. Branlant, and S. Aymerich. 2000. Two glyceraldehyde-3-phosphate dehydrogenases with opposite physiological roles in a nonphotosynthetic bacterium. J. Biol. Chem. 27514031-14037. [DOI] [PubMed] [Google Scholar]
- 12.Frunzke, J., V. Engels, S. Hasenbein, C. Gätgens, and M. Bott. 2008. Co-ordinated regulation of gluconate catabolism and glucose uptake in Corynebacterium glutamicum by two functionally equivalent transcriptional regulators, GntR1 and GntR2. Mol. Microbiol. 67305-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaigalat, L., J. P. Schlueter, M. Hartmann, S. Mormann, A. Tauch, A. Püehler, and J. Kalinowski. 2007. The DeoR-type transcriptional regulator SugR acts as a repressor for genes encoding the phosphoenolpyruvate:sugar phosphotransferase system (PTS) in Corynebacterium glutamicum. BMC Mol. Biol. 8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerstmeir, R., A. Cramer, P. Dangel, S. Schaffer, and B. J. Eikmanns. 2004. RamB, a novel transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J. Bacteriol. 1862798-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han, S. O., M. Inui, and H. Yukawa. 2007. Expression of Corynebacterium glutamicum glycolytic genes varies with carbon source and growth phase. Microbiology 1532190-2202. [DOI] [PubMed] [Google Scholar]
- 16.Hansmeier, N., A. Albersmeier, A. Tauch, T. Damberg, R. Ros, D. Anselmetti, A. Pühler, and J. Kalinowski. 2006. The surface (S)-layer gene cspB of Corynebacterium glutamicum is transcriptionally activated by a LuxR-type regulator and located on a 6 kb genomic island absent from the type strain ATCC 13032. Microbiology 152923-935. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi, M., H. Mizoguchi, N. Shiraishi, M. Obayashi, S. Nakagawa, J. Imai, S. Watanabe, T. Ota, and M. Ikeda. 2002. Transcriptome analysis of acetate metabolism in Corynebacterium glutamicum using a newly developed metabolic array. Biosci. Biotechnol. Biochem. 661337-1344. [DOI] [PubMed] [Google Scholar]
- 18.Hermann, T. 2003. Industrial production of amino acids by coryneform bacteria. J. Biotechnol. 104155-172. [DOI] [PubMed] [Google Scholar]
- 19.Inui, M., H. Kawaguchi, S. Murakami, A. A. Vertès, and H. Yukawa. 2004. Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. J. Mol. Microbiol. Biotechnol. 8243-254. [DOI] [PubMed] [Google Scholar]
- 20.Inui, M., S. Murakami, S. Okino, H. Kawaguchi, A. A. Vertès, and H. Yukawa. 2004. Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J. Mol. Microbiol. Biotechnol. 7182-196. [DOI] [PubMed] [Google Scholar]
- 21.Inui, M., M. Suda, S. Okino, H. Nonaka, L. G. Puskás, A. A. Vertès, and H. Yukawa. 2007. Transcriptional profiling of Corynebacterium glutamicum metabolism during organic acid production under oxygen deprivation conditions. Microbiology 1532491-2504. [DOI] [PubMed] [Google Scholar]
- 22.Inui, M., M. Terasawa, and H. Yukawa. 1999. Encyclopedia of bioprocess technology: fermentation, biocatalysis, and bioseparation. Wiley, New York, NY.
- 23.Jungwirth, B., D. Emer, I. Brune, N. Hansmeier, A. Pühler, B. J. Eikmanns, and A. Tauch. 2008. Triple transcriptional control of the resuscitation promoting factor 2 (rpf2) gene of Corynebacterium glutamicum by the regulators of acetate metabolism RamA and RamB and the cAMP-dependent regulator GlxR. FEMS Microbiol. Lett. 281190-197. [DOI] [PubMed] [Google Scholar]
- 24.Kim, H. J., T. H. Kim, Y. Kim, and H. S. Lee. 2004. Identification and characterization of glxR, a gene involved in regulation of glyoxylate bypass in Corynebacterium glutamicum. J. Bacteriol. 1863453-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita, S., S. Udaka, and M. Shimono. 1957. Studies on the amino acid fermentation. Part 1. Production of L-glutamic acid by various microorganisms. J. Gen. Appl. Microbiol. 3193-205. [PubMed] [Google Scholar]
- 26.Kohl, T. A., J. Baumbach, B. Jungwirth, A. Pühler, and A. Tauch. 2008. The GlxR regulon of the amino acid producer Corynebacterium glutamicum: in silico and in vitro detection of DNA binding sites of a global transcription regulator. J. Biotechnol. 135340-350. [DOI] [PubMed] [Google Scholar]
- 27.Kumagai, H. 2000. Microbial production of amino acids in Japan. Adv. Biochem. Eng. Biotechnol. 6971-85. [DOI] [PubMed] [Google Scholar]
- 28.Letek, M., N. Valbuena, A. Ramos, E. Ordonez, J. A. Gil, and L. M. Mateos. 2006. Characterization and use of catabolite-repressed promoters from gluconate genes in Corynebacterium glutamicum. J. Bacteriol. 188409-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludwig, H., G. Homuth, M. Schmalisch, F. M. Dyka, M. Hecker, and J. Stülke. 2001. Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Mol. Microbiol. 41409-422. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig, H., N. Rebhan, H.-M. Blencke, M. Merzbacher, and J. Stülke. 2002. Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation. Mol. Microbiol. 45543-553. [DOI] [PubMed] [Google Scholar]
- 31.Madan Babu, M., and S. A. Teichmann. 2003. Functional determinants of transcription factors in Escherichia coli: protein families and binding sites. Trends Genet. 1975-79. [DOI] [PubMed] [Google Scholar]
- 32.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 33.Muffler, A., S. Bettermann, M. Haushalter, A. Horlein, U. Neveling, M. Schramm, and O. Sorgenfrei. 2002. Genome-wide transcription profiling of Corynebacterium glutamicum after heat shock and during growth on acetate and glucose. J. Biotechnol. 98255-268. [DOI] [PubMed] [Google Scholar]
- 34.Okino, S., M. Inui, and H. Yukawa. 2005. Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl. Microbiol. Biotechnol. 68475-480. [DOI] [PubMed] [Google Scholar]
- 35.Omumasaba, C. A., N. Okai, M. Inui, and H. Yukawa. 2004. Corynebacterium glutamicum glyceraldehyde-3-phosphate dehydrogenase isoforms with opposite, ATP-dependent regulation. J. Mol. Microbiol. Biotechnol. 891-103. [DOI] [PubMed] [Google Scholar]
- 36.Saier, M. H., Jr., and T. M. Ramseier. 1996. The catabolite repressor/activator (Cra) protein of enteric bacteria. J. Bacteriol. 1783411-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 38.Schwinde, J. W., N. Thum-Schmitz, B. J. Eikmanns, and H. Sahm. 1993. Transcriptional analysis of the gap-pgk-tpi-ppc gene cluster of Corynebacterium glutamicum. J. Bacteriol. 1753905-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada, T., N. Fujita, M. Maeda, and A. Ishihama. 2005. Systematic search for the Cra-binding promoters using genomic SELEX system. Genes Cells 10907-918. [DOI] [PubMed] [Google Scholar]
- 40.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189113-130. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka, Y., H. Teramoto, M. Inui, and H. Yukawa. 2008. Regulation of expression of general components of the phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS) by the global regulator SugR in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 78309-318. [DOI] [PubMed] [Google Scholar]
- 42.Terasawa, M., H. Yukawa, and Y. Takayama. 1985. Production of L-aspartic acid from Brevibacterium by the cell re-using process. Process Biochem. 1124-128. [Google Scholar]
- 43.Toyoda, K., H. Teramoto, M. Inui, and H. Yukawa. 2008. Expression of the gapA gene encoding glyceraldehyde-3-phosphate dehydrogenase of Corynebacterium glutamicum is regulated by the global regulator SugR. Appl. Microbiol. Biotechnol. 81291-301. [DOI] [PubMed] [Google Scholar]
- 44.Vertès, A. A., M. Inui, M. Kobayashi, Y. Kurusu, and H. Yukawa. 1993. Presence of mrr- and mcr-like restriction systems in coryneform bacteria. Res. Microbiol. 144181-185. [DOI] [PubMed] [Google Scholar]
- 45.Yukawa, H., C. A. Omumasaba, H. Nonaka, P. Kós, N. Okai, N. Suzuki, M. Suda, Y. Tsuge, J. Watanabe, Y. Ikeda, A. A. Vertès, and M. Inui. 2007. Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 1531042-1058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.