Abstract
The S-layer protein SwmA is required for nonflagellar swimming in marine Synechococcus. An analysis of mutations in seven genes at two loci in the Synechococcus sp. strain WH8102 genome indicates that a multicomponent transporter and glycosyltransferases are required for the production and proper localization of SwmA.
The mechanism of nonflagellar motility by which certain strains of marine Synechococcus swim in the absence of any extracellular organelle remains mysterious. The cell surface itself is predicted to produce thrust (11), and to date, two cell surface proteins required for swimming have been characterized (7, 14). SwmA is a 130-kDa glycoprotein that forms a paracrystalline surface layer (S-layer) (16). Whether the S-layer plays a direct role in motility or a more indirect role, e.g., being required for the proper placement and functioning of other components of the motility apparatus, remains unclear. SwmB is a highly repetitive, 1.12-MDa protein which is also required for motility and is similarly localized near the cell surface where it is arranged in a punctate manner (14).
Transposon mutagenesis identified three separate chromosomal regions required for swimming motility in Synechococcus sp. strain WH8102 (15). In addition to the genes coding for SwmA and SwmB, two separate multicomponent ABC transporter genes, several putative glycosyltransferase genes, and various conserved and hypothetical genes of unknown function comprise the remaining genes present in these motility loci (15). We show here that mutations in several of these open reading frames (ORFs; SYNW0079, SYNW0087 to SYNW0089, and SYNW0192 to SYNW0195) affect the production and cellular localization of SwmA, and in the case of SYNW0087 and SYNW0195, that of a 70-kDa outer membrane protein (OMP).
Mutagenesis.
Directed mutagenesis of each putative motility gene identified through transposon mutagenesis reproduced the nonmotile phenotype of the transposon mutant (15) (Fig. 1). Three additional ORFs present in these loci, SYNW0089, SYNW0194, and SYNW0195, were inactivated by insertional mutagenesis for this work using gene fragments 91118 to 91367, 197115 to 197342, and 198491 to 198714, respectively (numbering according to genome sequence [18]), as previously described (6, 15) (Table 1). Inactivation of SYNW0089 did not affect motility, while mutations in SYNW0194 and SYNW0195 resulted in a complete loss of motility. As with previously reported mutants (15), neither of the two new motility mutants described here has been observed to rotate.
FIG. 1.
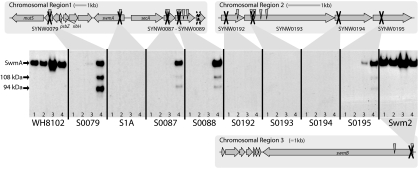
Chromosomal regions containing genes required for swimming motility (15) and anti-SwmA Western analysis of motility mutants. Transposon insertions which eliminate motility are indicated by arrowheads. Directed insertional inactivations are marked with an X; all directed inactivations except that in SYNW0089 (dashed X) abolish motility. Western analysis was conducted on whole cells (lane 1), insoluble OMPs (lane 2), soluble OMPs and periplasmic proteins (lane 3), and spent culture medium (lane 4) from the wild-type strain WH8102 and all nonmotile mutants. SwmA and additional bands with apparent molecular masses of 108 and 94 kDa are indicated on the left.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| MC1061 | Host for pRK24 and pRL528; used as a donor in pJM construction conjugations | 9 |
| Synechococcus | ||
| WH8102 | Wild-type, motile strain recipient in conjugations with pMUT100 constructions | J. Waterbury |
| S0079 | Nonmotile mutant, SYNW0079-directed knockout | 15 |
| S1A | Nonmotile mutant, swmA (SYNW0085)-directed knockout | 15 |
| S0087 | Nonmotile mutant, SYNW0087-directed knockout | 15 |
| S0088 | Nonmotile mutant, SYNW0088-directed knockout | 15 |
| S0089 | SYNW0089-directed knockout, wild-type motility | This work |
| S0192 | Nonmotile mutant, SYNW0192-directed knockout | 15 |
| S0193 | Nonmotile mutant, SYNW0193-directed knockout | 15 |
| S0194 | Nonmotile mutant, SYNW0194-directed knockout | This work |
| S0195 | Nonmotile mutant, SYNW0195-directed knockout | This work |
| Swm2 | Nonmotile mutant, swmB (SYNW0953)-directed knockout | 15 |
| Plasmids | ||
| pMUT100 | Kanr Tetr; suicide vector | 7 |
| pRK24 | Tcr Ampr; conjugal plasmid RK2 derivative | 7, 17 |
| pRL528 | Cmr; helper plasmid, carries mob | 7, 12 |
| pCR2.1-TOPO | Kanr Ampr; PCR cloning vector | Invitrogen |
| pJM19 | pMUT100 containing SYNW0089 nucleotide fragment from 91118-91367 | This work |
| pJM60 | pMUT100 containing SYNW0194 nucleotide fragment from 197115-197342 | This work |
| pJM61 | pMUT100 containing SYNW0195 nucleotide fragment from 198491-198714 | This work |
Nucleotide and ORF numbering as given in reference 18.
Cellular fractionation.
Outer membranes and cell surface proteins were released by incubation with EDTA and then separated into two fractions by ultracentrifugation as previously described (7, 14), resulting in an insoluble outer membrane fraction and a fraction containing both soluble periplasmic proteins and nonintegral outer membrane proteins. These fractions, along with proteins concentrated from spent medium as well as whole cells, were separated on either Novex Tricine 10 to 20% gradient gels (Invitrogen, Carlsbad, CA) for staining with SYPRO Ruby (Sigma, St. Louis, MO) or Nu-PAGE Novex Tris-acetate 3 to 8% gradient gels (Invitrogen) for Western blotting using antisera raised against SwmA as described previously (14).
Two classes of mutations affect SwmA.
All of the motility mutants, with the exception of swmB mutant Swm2, have some defect in either SwmA production or localization. These defects can be grouped into one of the following two classes: mutations that result in no detectable SwmA and mutations that result in altered localization of SwmA. In wild-type strain WH8102, SwmA forms a S-layer, which is released along with the outer membrane by treatment with EDTA and is found predominantly in the soluble fraction. Mutations in ORFs SYNW0192, SYNW0193, and SYNW0194 result in no detectable SwmA in whole cells, any of the membrane fractions, or the spent medium (Fig. 1). These ORFs are carried in chromosomal region two, and intergenic spacing (distances between ORFs SYNW0192, SYNW0193, SYNW0194, and SYNW0195, which are −1, 2, and 5 bp, respectively) suggests that they, along with SYNW0195, are likely to be cotranscribed (15) (Fig. 1). ORFs SYNW0193 and SYNW0194 are predicted to encode two of the three components of a type I secretion system. Type I secretion systems consist of an ABC transporter, an accessory membrane fusion protein (MFP), and an OMP, which often function with multiple transport systems and are not always linked on the chromosome (1, 13). SYNW0193 is predicted to encode an ABC transporter (most similar to the protein 1 exporter family 3.A.1.109) (19), and SYNW0194 is predicted to encode an MFP. Type I secretion systems have been shown to transport allocrites (the molecule or compound transported) possessing a glycine- and aspartic acid-rich repeat motif (3, 5, 10), which is present in multiple copies in SwmA (7). Moreover, a number of other S-layer proteins have been shown to be exported by similar type I transport systems (2, 22). These results suggest that SYNW0193 and SYNW0194 comprise the ABC transporter and MFP, respectively, of a type I secretion system dedicated to exporting SwmA. The observation that no SwmA is found in the spent medium from these strains supports this assertion. It appears that without this transporter, SwmA is not produced at all, as it is not detected in whole cells. It is possible that due to mislocalization or the loss of an interacting or modifying protein, SwmA is produced but is unstable and consequently not detected. Inactivation of SYNW0192 also results in the total absence of SwmA in strain S0192. While this may be due to polar effects on downstream ORFs, the encoded product of SYNW0192 has weak similarity to peptidyl-prolyl isomerases (15), which have been implicated in maturation of OMPs (4) and are themselves important components of type I secretion systems. Mutant complementation experiments are not yet possible for Synechococcus sp. strain WH8102, and hence, at this point it is difficult to separate the requirement for each gene from polar effects. Nevertheless, given that the phenotypes of S0194 and S0195 (see below and Fig. 1) are distinct, it appears that S0194 is required for SwmA production and S0195 for localization.
A second class of mutations (in ORFs SYNW0079, SYNW0087, SYNW0088, and SYNW0195) affects SwmA localization. Strains with these mutations still produce SwmA, although it is largely undetectable in whole cells or the membrane fractions. Instead, SwmA accumulates in the medium at concentrations similar to those of the wild type. Moreover, using a polyclonal anti-SwmA antibody, Western analyses of these four strains reveal both an anti-SwmA reactive band of wild-type size as well as two smaller bands (approximately 108 kDa and 94 kDa) (Fig. 1). These smaller bands are never seen in Western analysis of the wild-type strain or SwmB mutant strain Swm2, indicating that they are unlikely to be nonspecific proteolytic products. Three of the four mutations resulting in this phenotype are in genes predicted to encode glycosyltransferases (SYNW0087, SYNW0088, and SYNW0195; SYNW0079 is a conserved hypothetical), suggesting a role in the modification of SwmA, a protein which is known to be glycosylated (7). The predicted molecular mass of SwmA is 81.3 kDa, while the mature, posttranslationally modified protein has an apparent molecular mass of 130 kDa (7). The two additional bands detected by Western analysis may represent either unglycosylated or partially glycosylated forms of SwmA. Although these predicted glycosyltransferases can be assigned to glycosyltransferase protein families 2 and 4 based on sequence homology (8), these families are general classifications and include many enzymes involved in cell envelope biogenesis. Thus, determining whether any would act directly on SwmA is not possible based solely on sequence information. Moreover, glycosylation of bacterial proteins, and particularly the well-studied S-layer glycoproteins, is highly diverse in terms of glycan type, linkage, and sugar composition (20, 21). Nevertheless, these strains are still able to produce SwmA positive for glycosylation by periodic acid-Schiff staining (data not shown) and with electrophoretic mobility equivalent to that of the wild type. While SwmA appears to still be glycosylated in these mutants, it is possible that some undetermined defect in this posttranslational modification results in aberrant attachment of the S-layer to the cell and a loss of motility. Future experiments analyzing the carbohydrate composition of SwmA from the wild type and mutants should provide insights into this question.
Mutations affecting other cell surface proteins.
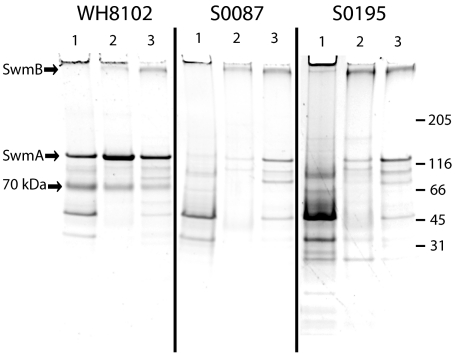
In addition to the effects on SwmA, one other abundant component of the outer membrane appears greatly reduced or absent in two mutants. While all of the mutants analyzed here continue to produce the other major cell surface protein implicated in swimming motility, SwmB (14 and data not shown), a previously identified 70-kDa glycoprotein which is found in abundance in both cell surface fractions and spent medium of wild-type cultures (7, 14), appears absent in strains SYNW0087 and SYNW0195 (Fig. 2). As mentioned above, ORFs SYNW0087 and SYNW0195 are both predicted to encode glycosyltransferases. It is tempting to speculate that the proteins encoded by these genes participate in the glycosylation of the 70-kDa glycoprotein. Perhaps the 70-kDa protein is not present at all in these cellular fractions or is not glycosylated, as it is in wild-type cells, resulting in a shift of electrophoretic mobility. SwmA also appears to have defective glycosylation in these strains, and perhaps sugar moieties are transferred to both proteins by these predicted glycosyltransferases. Alternatively, the 70-kDa glycoprotein may require other components of the cell to be properly modified before it can be properly localized itself. Whether the 70-kDa protein is required for motility remains to be determined, as efforts to inactivate the gene encoding it have so far been unsuccessful, suggesting it may be essential.
FIG. 2.
SYPRO-stained 10 to 20% Tricine gels of proteins from the insoluble outer membrane fraction (lane 1), soluble outer membrane and periplasmic fraction (lane 2), and spent culture medium (lane 3). SwmA and SwmB are indicated by arrows, as well as a 70-kDa band that is absent in nonmotile strains SYNW0087 and SYNW0195. The migration of molecular mass standards is indicated on the right in kilodaltons.
Acknowledgments
This work was supported by DOE grant no. DE-FG03-01ER63148, with partial support for J.M. through the Scripps Institution of Oceanography Graduate Student Office. The Marine Biology Research Division of the Scripps Institution of Oceanography supported publication costs.
Footnotes
Published ahead of print on 5 December 2008.
REFERENCES
- 1.Andersen, C. 2003. Channel-tunnels: outer membrane components of type I secretion systems and multidrug efflux pumps of Gram-negative bacteria. Rev. Physiol. Biochem. Pharmacol. 147122-165. [DOI] [PubMed] [Google Scholar]
- 2.Awram, P., and J. Smit. 1998. The Caulobacter crescentus paracrystalline S-layer protein is secreted by an ABC transporter (type I) secretion apparatus. J. Bacteriol. 1803062-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann, U., S. Wu, K. M. Flaherty, and D. B. McKay. 1993. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 123357-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrens, S., R. Maier, H. de Cock, F. X. Schmid, and C. A. Gross. 2001. The SurA periplasmic PPIase lacking its parvulin domains functions in vivo and has chaperone activity. EMBO J. 20285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binet, R., S. Letoffe, J. M. Ghigo, P. Delepelaire, and C. Wandersman. 1997. Protein secretion by Gram-negative bacterial ABC exporters—a review. Gene 1927-11. [DOI] [PubMed] [Google Scholar]
- 6.Brahamsha, B. 1996. A genetic manipulation system for oceanic cyanobacteria of the genus Synechococcus. Appl. Environ. Microbiol. 621747-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahamsha, B. 1996. An abundant cell-surface polypeptide is required for swimming by the nonflagellated marine cyanobacterium Synechococcus. Proc. Natl. Acad. Sci. USA 936504-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell, J. A., G. J. Davies, V. Bulone, and B. Henrissat. 1997. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem. J. 326929-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadaban, M., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138179-207. [DOI] [PubMed] [Google Scholar]
- 10.Delepelaire, P. 2004. Type I secretion in gram-negative bacteria. Biochim. Biophys. Acta 1694149-161. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers, K. M., A. D. T. Samuel, H. C. Berg, and R. Montgomery. 1996. Do cyanobacteria swim using traveling surface waves? Proc. Natl. Acad. Sci. USA 938340-8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhai, J., and C. P. Wolk. 1988. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 167747-754. [DOI] [PubMed] [Google Scholar]
- 13.Holland, I. B., L. Schmitt, and J. Young. 2005. Type 1 protein secretion in bacteria, the ABC-transporter dependent pathway. Mol. Membr. Biol. 2229-39. [DOI] [PubMed] [Google Scholar]
- 14.McCarren, J., and B. Brahamsha. 2007. SwmB, a 1.12-megadalton protein that is required for nonflagellar swimming motility in Synechococcus. J. Bacteriol. 1891158-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarren, J., and B. Brahamsha. 2005. Transposon mutagenesis in a marine Synechococcus strain: isolation of swimming motility mutants. J. Bacteriol. 1874457-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarren, J., J. Heuser, R. Roth, N. Yamada, M. Martone, and B. Brahamsha. 2005. Inactivation of swmA results in the loss of an outer cell layer in a swimming Synechococcus strain. J. Bacteriol. 187224-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer, R., D. Figurski, and D. R. Helinski. 1977. Physical and genetic studies with restriction endonucleases on the broad host range plasmid RK2. Mol. Gen. Genet. 152129-135. [DOI] [PubMed] [Google Scholar]
- 18.Palenik, B., B. Brahamsha, F. W. Larimer, M. Land, L. Hauser, P. Chain, J. Lamerdin, W. Regala, E. E. Allen, J. McCarren, I. Paulsen, A. Dufresne, F. Partensky, E. A. Webb, and J. Waterbury. 2003. The genome of a motile marine Synechococcus. Nature 4241037-1042. [DOI] [PubMed] [Google Scholar]
- 19.Saier, M. H. 1999. A functional-phylogenetic system for the classification of transport proteins. J. Cell. Biochem. 3384-94. [DOI] [PubMed] [Google Scholar]
- 20.Schäffer, C., M. Graninger, and P. Messner. 2001. Prokaryotic glycosylation. Proteomics 1248-261. [DOI] [PubMed] [Google Scholar]
- 21.Schäffer, C., and P. Messner. 2004. Surface-layer glycoproteins: an example for the diversity of bacterial glycosylation with promising impacts on nanobiotechnology. Glycobiology 1431R-42R. [DOI] [PubMed] [Google Scholar]
- 22.Thompson, S. A., O. L. Shedd, K. C. Ray, M. H. Beins, J. P. Jorgensen, and M. J. Blaser. 1998. Campylobacter fetus surface layer proteins are transported by a type I secretion system. J. Bacteriol. 1806450-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]