Abstract
MalT is the central transcriptional activator of all mal genes in Escherichia coli. Its activity is controlled by the inducer maltotriose. It can be inhibited by the interaction with certain proteins, and its expression can be controlled. We report here a novel aspect of mal gene regulation: the effect of cytoplasmic glucose and glucokinase (Glk) on the activity and the expression of MalT. Amylomaltase (MalQ) is essential for the metabolism of maltose. It forms maltodextrins and glucose from maltose or maltodextrins. We found that glucose above a concentration of 0.1 mM blocked the activity of the enzyme. malQ mutants when grown in the absence of maltodextrins are endogenously induced by maltotriose that is derived from the degradation of glycogen. Therefore, the fact that glk malQ+ mutants showed elevated mal gene expression finds its explanation in the reduced ability to remove glucose from MalQ-catalyzed maltodextrin formation and is caused by a metabolically induced MalQ− phenotype. However, even in mutants lacking glycogen, Glk controls endogenous induction. We found that overexpressed Glk due to its structural similarity with Mlc, the repressor of malT, binds to the glucose transporter (PtsG), releasing Mlc and thus increasing malT repression. In addition, even in mutants lacking Mlc (and glycogen), the overexpression of glk leads to a reduction in mal gene expression. We interpret this repression by a direct interaction of Glk with MalT concomitant with MalT inhibition. This repression was dependent on the presence of either maltodextrin phosphorylase or amylomaltase and led to the inactivation of MalT.
The Escherichia coli maltose system (4, 52) is geared for the efficient utilization of maltose and maltodextrins. Ten mal genes encode proteins found in all compartments of the cell. The lambda receptor in the outer membrane (43, 49) facilitates the diffusion of maltodextrins into the periplasmic space, where they are taken up into the cytoplasm via a binding-protein-dependent ABC transporter (32, 55). There are two main enzymes catalyzing the degradation of maltose and maltodextrins to glucose and α-glucose-1-phosphate. Amylomaltase (MalQ) (29), a maltodextrin glucanotransferase (41, 59), forms from any maltodextrin, including maltose, larger maltodextrins, and glucose (16, 34, 60). Maltotetraose and longer maltodextrins are substrates of the maltodextrin phosphorylase (MalP) (53, 58), yielding by phosphorolysis α-glucose-1-phosphate and smaller maltodextrins. Two other enzymes are a periplasmic amylase (MalS) (20, 51) and a cytoplasmic maltodextrin glucosidase (MalZ) that are not essential for maltose or maltodextrin utilization (44, 51, 57). While MalS produces preferentially maltohexaose from longer maltodextrins in the periplasm, MalZ degrades longer maltodextrins by cleaving glucose from the reducing end of the dextrins in the cytoplasm. The smallest substrate of MalZ is maltotriose, producing maltose and glucose. All mal genes are under the positive control of MalT (45), which in turn is activated by the inducer maltotriose (42). In addition to being controlled by MalT, MalZ is also induced under osmoregulation even in a malT mutant (15). The control of mal gene expression is surprisingly complex. Aside from the basic inducer-dependent activation of MalT as a specific transcriptional activator for all mal genes, there are additional regulatory circuits at work. The Phosphotransferase (PTS)-mediated uptake of glucose, controlling the level of the cyclic AMP (cAMP)/CAP complex, subjects malT, as well as the genes encoding the ABC transporter, to catabolite repression (9, 10, 46); Mlc, a global repressor of sugar metabolism, also controls malT expression in a glucose transport-dependent fashion (14, 48). This mechanism is unusual since Mlc is inactivated as a repressor by sequestration to a transporting and dephosphorylated cytoplasmic domain of PtsG (EIIBGlc) (25, 30, 31, 39, 54, 56). The global regulators H-NS and StpA have also been reported to act on malT expression (21). The activity of MalT as a transcriptional activator can be modulated (reduced) by interaction with several proteins; this is dramatically seen when they are overproduced: a cytoplasmic esterase (Aes) (23, 36) and a cytoplasmic cysthathionase (MalY) (11, 50, 62). The physiological connection of these enzymes to the maltose system remains unclear. However, the most significant regulating protein is MalK, the ATP-hydrolyzing subunit of the maltodextrin ABC transporter (22, 35). The maltose transport system in nontransporting state binds, via its MalK subunit, MalT, leading to the inactivation of MalT activity (1, 2).
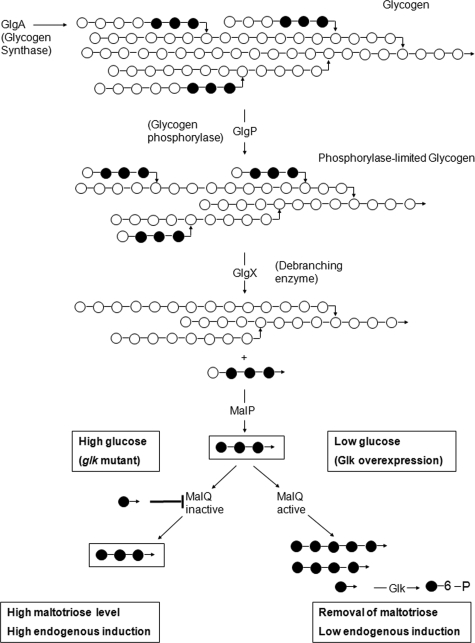
The mal system shows the phenomenon of endogenous induction. Thus, significant basal expression of the system does occur in the absence of external maltodextrins and is caused by endogenously produced inducer (13). The formation of the inducer maltotriose occurs by degradation of glycogen. Glycogen phosphorylase (GlgP) produces phosphorylase-limited-glycogen (see Fig. 6), harboring maltotetraose and maltotriose chains α(1-6) glucosidically linked to the main glycogen chain. Maltotetraosyl and maltotriosyl residues are cleaved by the glycogen-debranching enzyme (GlgX) to form linear maltotetraose and maltotriose. The former is converted to the inducer maltotriose by MalP, the maltodextrin phosphorylase. The level of maltotriose (and thus endogenous induction) is reduced by MalZ forming glucose and maltose from maltotriose (15). The role of MalQ in endogenous induction is more difficult to understand. On the one hand, it reduces the amount of maltotriose by repolymerizing it to higher maltodextrins plus glucose, which is removed by glucokinase-dependent phosphorylation. On the other hand, and particularly in the presence of MalZ when maltose is ultimately formed from glycogen, MalQ is able to reform maltotriose from maltose, maintaining sufficient levels of maltotriose for induction (15). This is also the mode by which exogenous maltose is transformed into maltotriose after entering the cell. However, when MalQ is lacking, glycogen-dependent endogenous induction becomes high (17), particularly in the absence of MalZ, demonstrating that glycogen-derived inducer is indeed maltotriose.
FIG. 6.
Effect of cytoplasmic glucose on the activity of MalQ and on glycogen-dependent endogenous induction. Shown is the pathway of glycogen degradation producing maltotriose, the inducer of MalT, the transcriptional activator of all mal genes. Glucosyl residues are indicated by small circles, horizontal lines between the circles indicate α(1-4) linkages, bent arrows indicate α(1-6) linkages. Unlinked arrows indicate the reducing end of the maltodextrins and glucose. Solid circles indicate the origin of maltotriose from glycogen and its further metabolism. (Lower left branch) In a glk mutant, glucose formed by the action of MalQ or by hydrolysis of glucose containing substrates (not shown) inhibit MalQ, preventing the removal of maltotriose, thus establishing high endogenous induction. (Lower right branch) Overexpression of Glk removes free glucose, allowing high MalQ activity. This leads to the removal of maltotriose by the formation of larger maltodextrins, thus causing the loss of MalT activation and a reduction in mal gene expression. Not shown here is the action of MalZ or the synthesis of glycogen.
We demonstrate here that MalQ is strongly feedback inhibited by low concentrations of glucose, pointing to the function of glucose in controlling the activity of MalQ and thus in glycogen-derived endogenous induction. At a high internal glucose concentration, for instance when glucokinase is lacking (as in a glk mutant), a malQ+ strain becomes phenotypically partially MalQ−, exhibiting increased endogenous induction. In contrast, the removal of glucose activates MalQ and will therefore reduce endogenous induction (see Fig. 6). However, endogenous induction is still observed in mutants lacking glycogen synthase (GlgA), MalP, MalQ, and MalZ (15). In the past we have proposed a mechanism in which glucose is involved in the formation of endogenous inducer, be it maltotriose or an alternative, as-yet-unknown inducer. This view was corroborated by the observation that overexpressed glucokinase (by removing glucose via ATP-dependent phosphorylation) would reduce mal gene expression, whereas the addition of glucose to a glucokinase mutant and after transport into the cytoplasm in an unphosphorylated form (in a PtsG mutant via the glucose/galactose ABC transporter) would induce mal gene expression (13, 27). We now have made the observation that, in a strain lacking glycogen, MalP, MalQ, and MalZ, the action of glucokinase was dependent on Mlc and acted by binding to PtsG releasing Mlc as transcriptional repressor for malT (see Fig. 8). In addition, we found that even in the absence of Mlc, overexpressed glucokinase was able to strongly reduce mal gene expression. This Mlc-independent repression by glucokinase could only be observed in the presence of an intact malP or an intact malQ gene. We propose that glucokinase, together with MalP or MalQ, forms a complex that inhibits MalT (see Fig. 7).
FIG. 8.
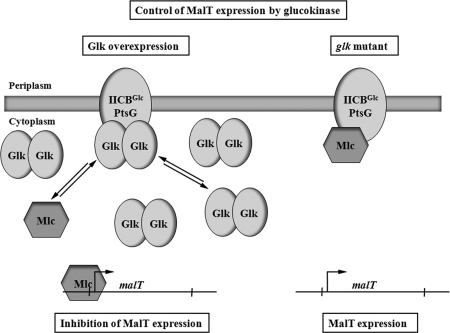
The competition between Mlc and glucokinase for PtsG binding affects the expression of malT. Two situations are shown. On the right-hand side, the “normal” situation is shown. A certain portion of Mlc, the repressor of malT, is bound by PtsG and is not available for the inhibition of malT transcription. On the left-hand side, the overexpression of Glk is shown. The high concentration of Glk replaces Mlc on PtsG. The increased concentration of Mlc leads to the inhibition of malT transcription and therefore to a reduced mal gene expression. Not reflected in this scheme is the dependence of malT expression on the cAMP/CAP complex and the state of PtsG phosphorylation on binding Mlc (or Glk).
FIG. 7.
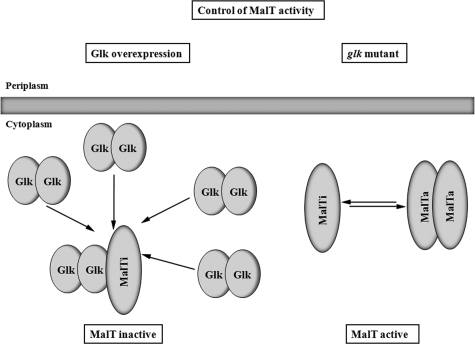
Binding of Glk to MalT inhibits MalT activity. Two situations are shown. On the right-hand side, the “normal” situation is shown. MalT exists in an equilibrium of the inactive monomer and the active dimer (multimer). On the left-hand side, the overproduction of glucokinase is shown. In the proposed model, high concentrations of Glk result in the binding of Glk dimers to monomeric and inactive MalT, preventing mal gene expression. Not shown in this scheme is the essential role of MalP or MalQ in Glk-dependent MalT inhibition. Also not shown is the role of maltotriose in preventing the inhibition by Glk. The established inhibition of MalT by other proteins such as Aes, MalK, or MalY is not shown but was the basis for this model. Also novel is the proposal that even in the absence of the inducer maltotriose MalT can form a transcriptionally active species.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. They were grown under aerobic conditions in tryptone broth (TB) or minimal medium A (MMA) supplemented with 1% (wt/vol) Casamino Acids (CAA) (28). When the activity of lacZ fusions was assayed, precultures were grown in TB and subcultured into minimal medium for assays. Maintenance of plasmids was ensured by the addition of the appropriate antibiotics (ampicillin, 100 μg ml−1; kanamycin, 25 μg ml−1).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or descriptiona | Source or reference |
|---|---|---|
| E. coli strains | ||
| AS40 | MC4100 ΔmalB107 trp::[Kanr-malEΔp92-lacZ]opmalT+opmR::Tn10 | 50 |
| AS54 | MC4100 ΔmalB107 trp::[Kanr-malEΔp92-lacZ]opmalT(T38R) opmR::Tn10 | 50 |
| AS53 | MC4100 ΔmalB107 trp::[Kanr-malEΔp92-lacZ]opmalT(D65E) opmR::Tn10 | 50 |
| BRE1162 | MC4100 φ(malK-lacZ)1113(λplacMu50) | 5 |
| CL19 | Bre1162 ΔglgA::frtglk15::Tn10 (Cam) mlc::tet | This study |
| CL27 | Bre1162 glgA::Tn10 ΔmalZ::frt Δglk::kan | This study |
| CL28 | Bre1162 ΔmalZ::frt Δglk::kan | This study |
| GW11 | Bre1162 ΔmalZ::frtglgA::Tn10 Δ(malQ malP)::frt glk15::Tn10 (Cam) | This study |
| GW12 | Bre1162 ΔmalZ::frtglgA::Tn10 Δ(malQ malP)::frt glk15::Tn10 (Cam) mlc::kan | This study |
| GW15 | Bre1162 ΔmalZ::frtglgA::Tn10 ΔmalP::kan glk15::Tn10 (Cam) | This study |
| GW26 | MC4100 ΔmalB107 trp::[Kanr-malEΔp92-lacZ]opmalT+opmR::Tn10 ΔglgA::cam | This study |
| GW27 | MC4100 ΔmalB107 trp::[Kanr-malEΔp92-lacZ]opmalT(T38R) opmR::Tn10 ΔglgA::cam | This study |
| GW28 | MC4100 ΔmalB107 trp::[Kanr-malEΔp92-lacZ]opmalT(D65E) opmR::Tn10 ΔglgA::cam | This study |
| JM101 | Δ(lac-proAB) thi-1supE F′[traD36proAB+lacIqlacZΔM15] | 37 |
| JM-G77 | JM101 ΔptsG::cat ΔptsHI crr::kan ptsG-lacZ in the λ attachment site | 37 |
| JW2385 | Δglk::kan, derivative of MG1655 | NBRP (NIG, Japan): E. coli |
| M15 | F−nalS strS rifS thi lac mtl | Qiagen |
| MAD103 | Bre1162 ΔmalZ::frt glgA::Tn10 | This study |
| MAD104 | Bre1162 ΔmalZ::frtglk15::Tn10 (Cam) | This study |
| MAD106 | Bre1162 ΔmalZ::frtglgA::Tn10 glk15::Tn10 (Cam) | This study |
| MAD107 | Bre1162 ΔmalZ::frtglk15::Tn10 (Cam) ΔmalQ::frt | This study |
| MAD108 | Bre1162 ΔmalZ::frtglgA::Tn10 glk15::Tn10 (Cam) ΔmalQ::kan | This study |
| MC4100 | araD139 deoC1 flbB5301 ptsF25 rbsR relA1 rpsL150 Δ(argF-lac)U169 | 8 |
| MG1655 | Laboratory wild-type strain | |
| RD38 | Bre1162 ΔmalZ::kanglgA::Tn10 | 15 |
| RD103 | Bre1162 ΔmalZ::kan ΔglgA::frt malP::Tn10 | This study |
| RD105 | Bre1162 ΔmalZ::kan ΔglgA::frt glk15::Tn10 (Cam) malP::Tn10 | This study |
| RD126 | Bre1162 ΔmalZ::frtglgA::Tn10 Δ(malQ malP)::kan | This study |
| RD129 | Bre1162 ΔmalZ::frt Δ(malQ malP)::kan | This study |
| TB04 | Bre1162 ΔmalZ::kan | 15 |
| TB48 | Bre1162 ΔglgA::cammalT::Tn5 | This study |
| Plasmids | ||
| pREP4 | pACYC derivative containing the p15A replicon and lacIq; Kanr | Qiagen |
| pMAD145 | pQE70 (Qiagen) C-terminal His6-tagged MalQ | This study |
| pBK1 | Derivative of pGDR11 lacIq N-terminal His6 tag Glk; Ampr | Laboratory collection |
| pGDR11 | pQE31 (Qiagen) lacIq N-terminal His6 tag vector; Ampr | 36 |
| pTSG11 | Expresses ptsG under lacIq and under its own promoter | 6 |
| pCSF2 | Derivative of pACYC, glk+ under natural promoter; Kanr | 27 |
| pCSF34 | Derivative of pACYC, glk15 (Δ305-321) under natural promoter; Kanr | 27 |
| pIA1 | TTC1688 (encoding glucokinase of T. thermophilus) cloned into pGDR11; Ampr | This study |
Ampr, ampicillin resistance; Kanr, kanamycin resistance.
Strain constructions.
The strains of the present study were constructed via P1 transduction as described by Miller (28). Deletions in malP, malQ, and malZ were done by the method of Datsenko and Wanner (12) in combination with the heat-inducible λRED recombination system in strain DY330 (61). For multiple deletions, the antibiotic resistance cassette was removed with plasmid pCP20 harboring the Saccharomyces cerevisiae FLP recombinase (12). All strains were derivatives of Bre1162 (5), which in turn is a derivative of the standard laboratory lac deletion strain MC4100 (8). Bre1162 carries a transcriptional malK-lacZ fusion. All mal gene expression data were obtained with this malK-lacZ fusion. The mutation in glgA was a glgA::Tn10 insertion. Strains carrying this insertion lack glycogen. The mutation in glk initially routinely used for a standard Glk− phenotype was an insertion of the chloramphenicol cassette, Tn10(cam), after amino acid 305 in Glk (27). In the course of this project it became clear that this mutation had resulted in the loss of glucokinase activity, but the C-terminally truncated protein still showed the ability to interact with PtsG. We termed this mutation glk15::Tn10 (Cam) (27). A complete glk deletion was subsequently obtained by the Datsenko-Wanner technique. The strains GW26, GW27, and GW28 were obtained by transducing a P1 lysate of strain TB48 (ΔglgA::cam malT::Tn5) into AS40, AS54, and AS53, selecting for chloramphenicol resistance and screening for the loss of kanamycin resistance and dark blue color on X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) plates. This assured the presence of the malT alleles from AS40, AS53, and AS54. The strains retained the opmR::Tn10 marker and were auxotrophic for tryptophan.
Cloning of malQ (pMAD145).
Plasmid pMAD145 carries the malQ gene fused C-terminally to a His6 tag encoding sequence under the control of the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible T5 promoter-lac operator system. It was constructed by ligating a BamHI/SphI-restricted 2,319-bp DNA fragment into BamHI/SphI-restricted plasmid pQE-70. The fragment was obtained by PCR using the primers malQ_7up (5′-CAC CGG GCT ACT ACC TGG CGA AGA AT-3′) and malQ_BamHI (5′-CAA CCG CGG ATC CCT TCT TCT TCG CTG CAG-3′), introducing a BamHI site instead of the malQ stop codon. Chromosomal DNA of E. coli MC4100 was used as a template. Final sequencing revealed a silent mutation in the malQ-His6 open reading frame (cytosine at position 1692 was exchanged by thymine). Strain M15 carrying plasmid pREP4 (harboring lacIq) was used for the transformation with pMAD145 and IPTG-inducible expression of malQ-His6.
Cloning of TTC1688 of Thermus thermophilus encoding glucokinase (GlKTth protein) yielding pIA1.
pIA1 was constructed by ligating a HindIII/SphI-restricted DNA fragment into HindIII/SphI-restricted plasmid pGDR11. The fragment was obtained by PCR using primers Tth_Glk_fw (5′-GGACGGCATGCGAAGGTGGTGGGGCCTGGACCTGGG-3′) and Tth_Glk_rv (5′-GACCAAGCTTTTACCCGCTTCCATCCTTCACCTCCAGGTAGGCGGTG-3′). Chromosomal DNA of T. thermophilus HB27 was used as a template. Cloned in pGRD11 GlKTth can be heterologously expressed in E. coli in soluble form. It carries an N-terminal His6 tag. The protein can be induced by IPTG. Even though derived from a thermophilic bacterium, the purified enzyme still showed considerable activity at 37°C and phosphorylated glucose and mannose, with a Km of 0.16 mM.
Overexpression and purification of MalQ.
E. coli strain M15 harboring plasmids pREP4 and pMAD145 was grown in NZA medium (0.5% [wt/vol] yeast extract, 0.75% [wt/vol] NaCl, 1% [wt/vol] N-Z-Amine A [Sigma Aldrich, Munich, Germany]) containing 100 μg of ampicillin/ml and 25 μg of kanamycin/ml at 37°C. When the cells reached an optical density at 578 nm of 0.8, IPTG was added to a final concentration of 1 mM. Cells were grown for an additional 1.5 h and then harvested by centrifugation. The pellet was resuspended in 3 ml of buffer containing 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, and 17 mM mercaptoethanol. Cells were disrupted by passing them three times at 12,000 lb/in2 through a French pressure cell, followed by centrifugation at 18,000 × g for 45 min at 4°C. The supernatant was loaded onto a Ni affinity column (HiTrap chelating HP 1-ml column) equilibrated with buffer. Bound protein was eluted with a linear gradient of 0 to 500 mM imidazole within 20 column volumes. The protein was extensively dialyzed against 50 mM Tris-HCl (pH 7.5) containing 10 mM MgCl2. The solution was sterilized by filtration and stored at 4°C.
Thin-layer chromatography.
[14C]maltose and [14C]glucose were purchased from Amersham with specific radioactivities of 630 and 268 mCi mmol−1, respectively. Both sugars were uniformly labeled. [14C]maltotriose was synthesized and purified as described previously (16, 33). Thin-layer chromatography (silica gel 60 from Merck of 0.25-mm thickness on glass support) was developed with butanol-ethanol-water (5:3:2) and visualized by autoradiography.
β-Galactosidase assay.
β-Galactosidase was assayed as described by Miller (28) with the following alterations. We omitted mercaptoethanol from the Z-buffer. Hydrolysis of ortho-nitrophenyl-β-galactoside (ONPGal) was done at a constant temperature of 28°C. After stopping the reaction with sodium carbonate, we clarified the suspension by centrifugation before measuring the optical density at 405 nm. We used an extinction coefficient of 4,860 M−1 cm−1. The specific activity is given in micromoles of ONPGal hydrolyzed per minute per milligram of protein at 28°C. The protein concentration was taken from the optical density at 578 nm of the bacterial culture. For the correlation, it was assumed that the optical density at 578 nm of 1.0 is equivalent to 107 μg of protein per ml (28). A specific activity of 1 corresponds to about 1,000 Miller units.
Membrane preparation and binding of Glk to PtsG.
The protocol described by Lee et al. (25) was followed with several alterations. JM-G77 (lacking ptsG) and JM-G77 transformed with ptsG11 (encoding EIIBCGlc) were used for membrane preparations. JM-G77 carries ΔptsG::cam and ΔptsHI crr::kan insertions that eliminate all PTS phosphorylation (37, 38). Both strains were grown overnight in 500 ml of MMA with 0.4% (vol/vol) glycerol as the carbon source. The cells were harvested by centrifugation, washed twice with 0.9% (wt/vol) NaCl, and resuspended in 3 ml of binding buffer (20 mM HEPES, 0.8 mM Na2HPO4, 5 mM KCl, 137 mM NaCl [pH 7.0]). Cells were broken in a French pressure cell at 16,000 lb/in2. Unbroken cell fragments were removed by centrifugation (at 25,000 × g for 15 min at 4°C), and the supernatant was kept on ice (crude cellular extract). The protein concentration was determined. A volume containing 2 mg of total cellular protein was centrifuged at 100,000 × g for 15 min. The supernatant was discarded. The pellet (membrane fraction) was resuspended in 100 μl of binding buffer. Then, 2 μg of purified Glk-His6 was added, followed by incubation at 37°C for 20 min. The membrane suspension was centrifuged for 15 min at 100,000 × g at 4°C. The supernatant was removed and designated SN1 (50 μl was used for further sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] analysis). The pellet resuspended in 100 μl of binding buffer and washed once was designated P1 (50 μl was used for further SDS-PAGE analysis). The remaining 50 μl of P1 was centrifuged again at 100,000 × g at 4°C for 15 min. The supernatant was removed and used for further SDS-PAGE analysis, and this was named SN2. The pellet was resuspended in 50 μl of binding buffer named P2. Equal samples of supernatant and resuspended membranes were subjected to SDS-PAGE analysis, followed by Western blotting with anti-His tag antibodies. The antibodies were obtained from Qiagen. Western blotting was done according to the supplier's protocol. Detection was performed with alkaline phosphatase.
Comparison of the amounts of Glk in different strains.
Strains were grown in 100 ml of LB medium at 37°C overnight. Cells were harvested by centrifugation, washed once in phosphate-buffered saline containing 10 mM MgSO4, and resuspended in 2 ml of the same buffer. After sonication (three times for 30 s) unbroken cells were removed by centrifugation (17,000 × g, 4°C, 1 h), and the supernatant was finally centrifuged for 1 h at 100,000 × g and 4°C. The protein concentration of the supernatant was determined, and a volume containing 90 μg of total cellular protein was precipitated with 7.5% (vol/vol) trichloroacetic acid on ice for 20 min. The precipitate was washed once with ice-cold acetone, and 50 μg was analyzed by SDS-PAGE, followed by Western blotting with anti-Glk antibodies. Western blotting was done according to the supplier's protocol. Detection was performed with alkaline phosphatase.
RESULTS
Overexpression and purification of MalQ.
The malQ gene was amplified by PCR from the chromosome of MC4100 and cloned as a C-terminal His6 version in an IPTG-inducible expression vector. The encoded protein was well expressed and could readily be detected in crude cellular extracts by SDS-PAGE as a prominent band of about 75 kDa (apparent molecular mass). Induction of the enzyme by 1 mM IPTG caused retardation of growth and cessation of growth after 2 h of induction. Nevertheless, the enzyme remained soluble in extracts of overexpressing cells and could easily be purified to apparent homogeneity by nickel-NTA affinity chromatography. The purified enzyme was extensively dialyzed against 50 mM Tris-HCl (pH 7.6) containing 10 mM MgCl2. The solution was sterilized by filtration and kept at 4°C. From a 1-liter culture more than 5 mg of purified MalQ was routinely obtained.
Inhibition of MalQ activity by glucose.
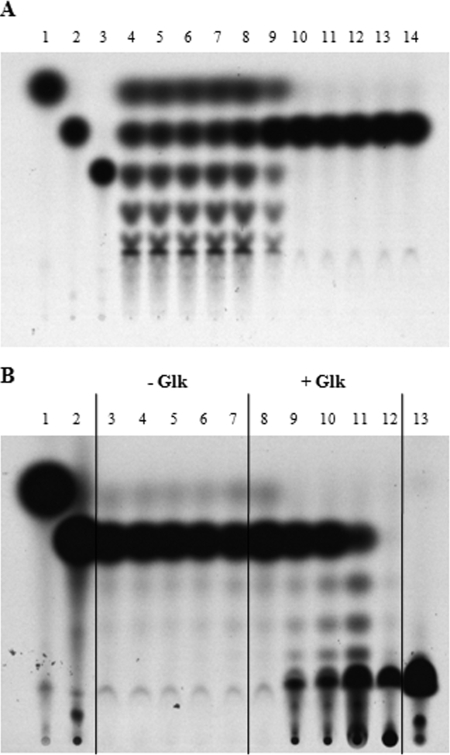
MalQ is able to act on maltose, forming glucose and a series of maltodextrins. We used uniformly 14C-labeled maltose at 75 μM as a substrate and analyzed the formation of labeled maltodextrins (and glucose) after 10 min of incubation at 30°C by thin-layer chromatography, followed by autoradiography (Fig. 1A). The presence of unlabeled glucose slowed the reaction visibly at concentrations higher than 20 μM; at 100 μM unlabeled glucose a reaction product was no longer formed (Fig. 1A). When glucokinase plus 24 mM ATP and 50 mM MgCl2 was added to a reaction mixture that was blocked by 100 μM unlabeled glucose, the activity of MalQ resumed. After 1 min the time-dependent formation of labeled maltodextrins plus glucose-6-phosphate was again observed. After 20 min, all maltodextrins had been transformed into long nonmigrating oligosaccharides and glucose-6-phosphate (Fig. 1B). This is caused by the effective removal of glucose from the reaction mixture. The MalQ inhibiting effect of glucose is counteracted by increasing amounts of maltose. These findings demonstrate that under in vivo conditions where free glucose is continuously removed, MalQ will remain highly active and will lead to the polymerization of maltodextrins (including the inducer maltotriose) to long oligosaccharides, resulting in the loss of induction.
FIG. 1.
In vitro MalQ activity with maltose as substrate. (A) The reaction was performed with 4.4 μg of C-terminally His6-tagged MalQ in a total volume of 16 μl containing 250 mM Tris-HCl (pH 7.5), 10 mM MgCl2, and different concentrations of unlabeled glucose. After 10 min of incubation at room temperature, [14C]maltose (final concentration, 75 μM) was added to the mixture, followed by incubation for another 20 min at 30°C. Portions (5 μl) of the reaction were spotted. The figure represents the autoradiogram of the TLC plate. The standards were as follows: lane 1, [14C]glucose; lane 2, [14C]maltose; lane 3, [14C]maltotriose. Glucose additions to the enzymatic assays were as follows: lane 4, no glucose; lane 5, 0.0002 mM; lane 6, 0.001 mM; lane 7, 0.002 mM; lane 8, 0.01 mM; lane 9, 0.02 mM; lane 10, 0.1 mM; lane 11, 0.2 mM; lane 12, 1.0 mM; lane 13, 2.0 mM; lane 14, 10 mM. (B) The reaction was performed as in panel A with the additional 50 mM MgCl2, 25 mM ATP, and 100 μM glucose without (lane 3 to 7) and with 1.5 μg of Glk (lanes 8 to 12). After 0, 1, 2, 5, and 20 min, 4 μl of the reaction was spotted. The standards were as follows: lane 1, [14C]glucose; lane 2, [14C]maltose; lane 13, [14C]glucose-6-phosphate.
Effect of glucose on the endogenous induction of mal gene expression in strains synthesizing glycogen.
As a measure of mal gene expression, we used a transcriptional lacZ fusion to malK encoding the ATP-hydrolyzing subunit of the maltose/maltodextrin ABC transporter. Thus, the tester strains lacked maltose/maltodextrin transport and, due to the lack of MalK, they contained MalT, the transcriptional regulator, in a MalK-uninhibited state (24). In addition, we deleted the malZ gene to avoid complications due to the degradation of the inducer maltotriose by MalZ (15, 57). As a carbon source we used 1% (wt/vol) CAA in MMA. The use of glycerol was avoided since glycerol exerts a considerable catabolite repression on the maltose system (19). The expression of uninduced maltose transport of cells grown on glycerol is about 10 times lower than when grown on CAA (16). We used cells that had been grown overnight. The specific galactosidase activity of a malK-lacZ fusion (in strain GW12) decreased after inoculation about twofold in the early logarithmic growth phase. It increased and peaked (30% higher than the overnight culture) in the late log phase and leveled off in stationary phase. Thus, the most reproducible values were obtained in stationary-phase cells. Therefore, expression assays were done in stationary cultures. They were repeated at least four times. They showed routinely a variation of ±5%.
In the first set of experiments we tested malK-lacZ expression in a glgA+ (glycogen synthase) malQ+ strain in the presence or absence of chromosomally or plasmid-encoded and overexpressed glucokinase. Table 2 shows that the presence of chromosomally encoded glucokinase reduced the expression in comparison to the strain lacking glucokinase activity. When glucokinase was overproduced, the expression dropped to near zero. In contrast, in a strain lacking MalQ or both MalQ and MalP (but still producing glycogen and thus maltotriose) the overproduction of glucokinase was less dramatic than in a malQ+ strain but was still recognizable (Table 2). We conclude that one part of the “glucose effect” on mal gene expression is that with reduced glucose phosphorylating capabilities, MalQ becomes increasingly inhibited by glucose, leading to a partial MalQ minus phenotype, which in turn increases mal gene expression by the glycogen-derived formation of maltotriose (that is no longer effectively removed by MalQ). However, it became clear that, in addition to controlling MalQ activity, the overproduction of glucokinase must have additional effects on mal gene expression that would reveal themselves more clearly only in the absence of glycogen synthesis.
TABLE 2.
Role of MalQ in controlling endogenous glycogen-derived maltotriose-dependent mal gene expression
| Straina | Additional genotype | MalK-LacZ activity |
|---|---|---|
| TB04 | malZ | 2.51 |
| MAD104 | malZ glk15::Tn10 (Cam) vector | 4.21 |
| CL28 | malZ Δglk | 4.17 |
| MAD104 | malZ glk15::Tn10 (Cam) pglk+ | 0.08 |
| CL28 | malZ Δglk vector | 4.35 |
| CL28 | malZ Δglk pglk+ | 0.07 |
| MAD107 | malZ glk15::Tn10 (Cam) malQ vector | 6.73 |
| MAD107 | malZ glk15::Tn10 (Cam) malQ pglk+ | 4.18 |
| RD129 | malZ malP malQ vector | 6.90 |
| RD129 | malZ malP malQpglk+ | 3.39 |
All strains are derivatives of BRE1162 (malK-lacZ malQ+ malP+ glgA+). The strains were grown in MMA containing 1% (wt/vol) CAA.
Role of glucokinase for endogenous induction in the absence of glycogen.
As demonstrated above, the presence of glucose controls glycogen-derived endogenous induction by affecting the enzymatic activity of MalQ. According to Fig. 1, by removing glucose via Glk, MalQ is no longer inhibited and repolymerizes the glycogen-derived inducer maltotriose to larger dextrins. These are substrates of maltodextrin phosphorylase (MalP), which in turn forms α-glucose-1-phosphate that enters glycolysis via phosphoglucomutase. However, even in the absence of glycogen- and maltodextrin-metabolizing enzymes, basal mal gene expression can still be observed. The origin of this induction, in particular the nature of the “glycogen-independent” inducer, remained unclear. Previously, we had argued that glucose was involved in the formation of an as-yet-unidentified sugar with properties similar to those of maltotriose that would activate MalT. Here, we demonstrate that glycogen-independent induction is due to two mechanisms: one controls the activity of MalT, and the other controls, via Mlc, the basal expression of malT. Both mechanisms do not require the existence of an alternative inducer.
Glucokinase interacts with MalT in the presence of MalP or MalQ.
The second set of experiments probing malK-lacZ expression were done in strains lacking glycogen (glgA mutation), as well as either malP or malQ alone or both malQ and malP. Table 3 shows that the basal mal gene expression in a malP+ malQ+ background is not noticeably altered by the absence of glucokinase (RD38 or MAD103 versus MAD106). However, it can be reduced 20-fold by the overproduction of glucokinase. This dramatic reduction in mal gene expression is dependent on the additional presence of MalP or MalQ. Strains lacking MalP (RD105 and GW15) or MalQ (MAD108) still showed strong reduction of mal gene expression upon overexpression of glucokinase, whereas a strain lacking both malQ and malP (GW11) only showed a twofold reduction upon overexpression of glucokinase (Table 3). The strong reduction in mal gene expression by overproduced glucokinase in the presence of MalP and MalQ is also observed in a strain lacking Mlc (Table 3, strain CL19), where the expression of malT is increased due to the loss of the repressor for malT (14).
TABLE 3.
Role of glucokinase in controlling MalT activity
| Straina | Additional genotype | MalK-LacZ activity |
|---|---|---|
| RD38 | glk+ | 1.97 |
| MAD103 | glk+ | 2.16 |
| MAD106 | glk15::Tn10 (Cam) | 2.16 |
| MAD106 | glk15::Tn10 (Cam) vector | 2.28 |
| MAD106 | glk15::Tn10 (Cam) pglk+ | 0.09 |
| CL27 | Δglk::kan | 2.20 |
| CL27 | Δglk vector | 1.92 |
| CL27 | Δglk pglk+ | 0.05 |
| MAD 108 | glk15::Tn10 (Cam) malQ | 2.12 |
| MAD108 | glk15::Tn10 (Cam) malQ vector | 2.0 |
| MAD108 | glk15::Tn10 (Cam) malQ pglk+ | 0.13 |
| RD103 | malP | 2.19 |
| RD105 | glk15::Tn10 (Cam) malP | 1.95 |
| RD105 | glk15::Tn10 (Cam) malP vector | 2.02 |
| RD105 | glk15::Tn10 (Cam) malP pglk+ | 0.09 |
| GW15 | malP glk15::Tn10 (Cam) vector | 1.97 |
| GW15 | malP glk15::Tn10 (Cam) pglk+ | 0.06 |
| RD126 | malP malQ | 1.87 |
| GW11 | glk15::Tn10 (Cam) malP malQ | 1.82 |
| GW11 | glk15::Tn10 (Cam) malP malQ vector | 1.87 |
| GW11 | glk15::Tn10 (Cam) malP malQ pglk+ | 0.85 |
| CL19 (malZ+) | glk15::Tn10 (Cam) mlc vector | 5.90 |
| CL19 (malZ+) | glk15::Tn10 (Cam) mlc pglk+ | 0.46 |
All strains harbor malK-lacZ and lack glgA and malZ.
In a background of glgA+ (Table 2), this MalP/MalQ-dependent reduction of mal gene expression by glucokinase overproduction can only be observed in a strain that is MalP+ and MalQ+. There, the MalP/MalQ/glucokinase effect is compounded by the effect of the high activity of MalQ in the presence of glucokinase that increases repression by the removal of the inducer maltotriose. The MalP/glucokinase-dependent repression in a strain containing glycogen is not observed when MalQ is lacking (Table 2, strain MAD107). The explanation for this difference is the production of large amounts of the inducer maltotriose from glycogen (that is not curbed in the absence of MalQ). Thus, maltotriose apparently counteracts the combined effect of MalP and glucokinase. We propose that the combination of MalP/MalQ and glucokinase acts directly on MalT competing with maltotriose, similar to what has been observed with MalK (22), Aes (36), or MalY (62), proteins that interact directly with MalT (23). Thus, the simplest explanation for this MalP/MalQ/glucokinase-dependent repression would likewise be a direct interaction between MalT and glucokinase that is stimulated by MalP or MalQ.
Evidence for MalT being the target for the inhibition by Glk.
In previous work studying the interaction of MalK, MalY, and Aes with MalT (50), we had tested 26 MalT mutants that exhibited a constitutive phenotype being partially independent of the inducer maltotriose. These mutants show elevated mal gene expression as tested by a malE-lacZ fusion in a ΔmalK background. All of the mutations are in the first third of MalT. None of the mutants are resistant against all MalT inhibitors, but some are nearly resistant to Aes, indicating the interaction site in MalT with Aes. We transduced the ΔglgA::cam allele into three of these constructs harboring the wild type and the T38R and D65E mutations in MalT and tested the resulting strains for their malE-lacZ expression in the presence or absence of plasmid-encoded Glk (Table 4). In this setup the overexpression of Glk reduced the malE expression in a malT+ background threefold (GW26), a result clearly less dramatic than the malK expression in a similar background (MAD106, Table 3). Nevertheless, this value allowed us to test whether the two mutations in MalT have altered sensitivity toward Glk. GW27 harboring the T38R mutation showed a twofold increase in basal activity in respect to the wild type and nearly the same reduction by overexpressed Glk (Table 4). However, strain GW28 harboring the D65E mutation was entirely resistant to overproduction of Glk and showed nearly tenfold increase in basal activity compared to the wild type (Table 4). This demonstrates that MalT is the target for the interaction with Glk.
TABLE 4.
The D65E mutation in MalT is resistant to inhibition by Glk
| Straina | Additional genotype | MalE-LacZ activity |
|---|---|---|
| GW26 | malT+ | 0.38 |
| GW26 | malT+pglk+ | 0.11 |
| GW27 | malT(T38R) | 0.64 |
| GW27 | malT(T38R) pglk+ | 0.27 |
| GW28 | malT(D65E) | 2.60 |
| GW28 | malT(D65E) pglk+ | 2.65 |
All strains lack malK, are ΔglgA ompR::Tn10 harboring malE-lacZ in the tryp gene cluster.
Glucokinase regulates the expression of malT via the release of Mlc from PtsG.
Even in the absence of MalP or MalQ (conditions where the interaction of Glk with MalT is minimal) and with no glycogen present, endogenous induction (or basal mal gene expression) is still observed and significantly (>2-fold) reduced by overproduction of glucokinase (Table 5, strain GW11). We observed that the presence of Mlc was required for this reduction. Strain GW12 lacking Mlc in addition to glycogen, MalP, and MalQ, showed, as expected, high mal gene expression that was only insignificantly reduced by glucokinase overproduction (Table 5). Thus, it was likely that Mlc is involved in this MalP/MalQ-independent effect of glucokinase. Since glucokinase shows a structure (26) that is very similar to the structure of Mlc (48), we concluded that glucokinase at high concentrations will compete with Mlc for binding PtsG. Mlc released by glucokinase from PtsG would then be more effective in repressing malT transcription.
TABLE 5.
Role of glucokinase in controlling the expression of malT
| Straina | Additional genotype | MalK-LacZ activity |
|---|---|---|
| GW11 | glk15::Tn10 (Cam) malP malQ | 1.82 |
| GW11 | glk15::Tn10 (Cam) malP malQ vector | 1.87 |
| GW11 | glk15::Tn10 (Cam) malP malQ pglk+ | 0.85 |
| GW11 | glk15::Tn10 (Cam) malP malQ pptsG+ | 3.75 |
| GW12 | glk15::Tn10 (Cam) malP malQ mlc vector | 4.27 |
| GW12 | glk15::Tn10 (Cam) malP malQ mlcpglk+ | 3.94 |
| GW12 | glk15::Tn10 (Cam) malP malQ mlcpptsG+ | 4.25 |
| CL19 (malZ+) | glk15::Tn10 (Cam) mlc vector | 5.90 |
| CL19 (malZ+) | glk15::Tn10 (Cam) mlc pglk+ | 0.72 |
All strains harbor malK-lacZ and lack glgA and malZ.
One may argue that in the absence of glucose transport the EIIB domain of PtsG should be predominantly present in its phosphorylated form, preventing the binding of Mlc or of glucokinase for that matter. However, Mlc can obviously be bound and inactivated as a repressor even in the absence of glucose transport. This can be seen by the effect of overproduction of PtsG on mal gene expression (Table 5). The overproduction of PtsG in an mlc+ background (GW11) in contrast to its mlc derivative (GW12) titrates Mlc and increases mal gene expression even though no glucose transport occurs and the EIIB domain of PtsG should be predominantly in the phosphorylated state (Table 5). Thus, competition of Mlc binding to PtsG by glucokinase is a valid proposition for a mechanism in which glucokinase can affect glycogen-independent endogenous mal gene expression.
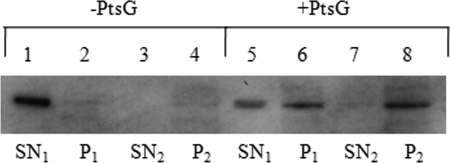
To demonstrate that glucokinase is indeed able to interact with PtsG, we prepared inverted membrane vesicles of a pstG deletion strain producing plasmid-encoded and IPTG-induced PtsG. In this setup, the EIIB domain of PtsG is exposed on the outside of the inverted vesicles. PtsG-dependent binding of glucokinase was demonstrated by ultracentrifugation and recovery of glucokinase in the washed pellet fraction. In contrast, the membranes of the same ptsG deletion strain without PtsG-encoding plasmid did not bind glucokinase (Fig. 2).
FIG. 2.
Interaction of Glk with PtsG. Portions (50 μg) of crude extract of strain JMG77(ΔptsG) (lanes 1 to 4) and JMG77 harboring plasmid-encoded PtsG (lanes 5 to 8) were analyzed by SDS-PAGE followed by Western blotting with anti-His tag antibodies to detect Glk. Lanes: 1 and 5, first membrane-free supernatants (SN1); lanes 2 and 6, first pellets (P1); lanes 3 and 7, second supernatants (SN2); lanes 4 and 8, second pellets (P2).
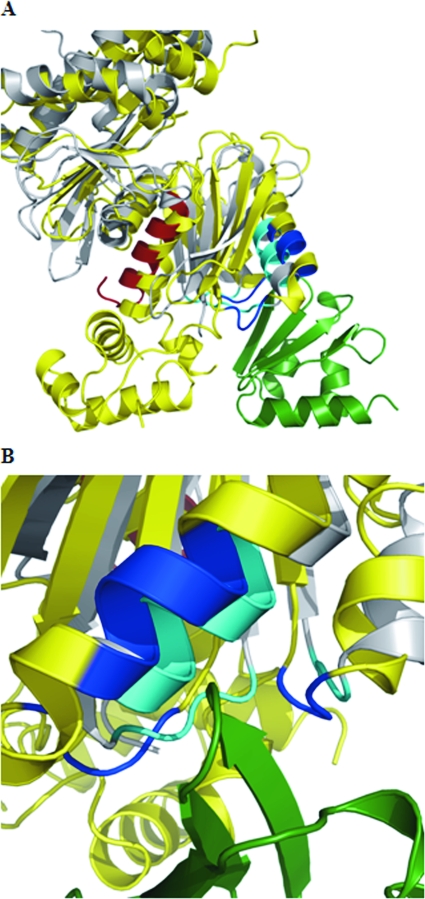
Recently, the crystal structure of Mlc in complex with the EIIB domain of PtsG has been determined (31). Since the crystal structure of glucokinase (aside from the lacking helix-turn-helix motif) is very similar to the Mlc structure (48), we tried to manually superimpose the glucokinase structure with the Mlc structure when in complex with EIIB (Fig. 3). Glucokinase could easily be brought into a position where the contact sites to EIIB are nearly identical to the contact sites between Mlc and EIIB. This suggests that both Mlc and glucokinase can compete at the same position of EIIBGlc.
FIG. 3.
Superimposition of GlK on interaction between Mlc and PtsG. (A) Partial view of Mlc (yellow) in its interaction with the EIIB domain of PtsG (green) (31). The interacting amino acids between the two proteins are shown in blue. Glk (in gray) (26) has been superimposed onto the Mlc structure (48) by using the Coot program (http://www.ysbl.york.ac.uk/∼emsley/coot/). The amino acids contacting the EIIB domain are in cyan. The C-terminal helix harboring the 16 amino acids lacking in Glk15 (Δ305-321) are shown in red. The helix-turn-helix domain of Mlc (lacking in Glk) is on the lower left-hand corner. (B) Detailed view of the interaction site seen from the side opposite that shown in panel A.
Differentiating the three functions of glucokinase by using glk deletions and glucokinase from T. thermophilus.
Throughout the construction of strains used in the present study we had considered the glk::Tn10(cam) mutation as a null mutation of glk because of its complete loss in glucokinase activity (27). Since we had this mutation also present on plasmid pCSF34, where glk::Tn10(cam) is under its natural promoter control, we wanted to use it as a control for the effect of glk-overexpressing plasmids. To our surprise, this plasmid had the same effect on mal gene expression in MAD106 as had pCSF2, a corresponding plasmid expressing the wild-type glk under natural promoter control (Table 6). Since the protein encoded by pCSF34 did not show glucokinase activity, it could not act on the level of controlling MalQ activity (via removal of glucose). However, the C-terminally truncated protein (16 C-terminal amino acids are missing) could be interacting with PtsG (releasing Mlc and reducing malT expression) or with MalT (affecting MalT activity) or both. Looking at the structure of glucokinase, one can recognize that the 16 C-terminal amino acids form a helix that is not part of the interaction site with EIIB of PtsG (Fig. 3). We tested for the presence of such a truncated protein in the 100,000 × g supernatant of cellular extracts. Indeed, pCSF34 caused the synthesis of a protein not present in a strain with glk deleted (Fig. 4). Thus, an interaction of this truncated protein at least with PtsG appeared possible. To differentiate between an effect of the truncated Glk on PtsG and MalT, we used strain CL19. This strain lacks GlgA and Mlc, and therefore any reduction in mal gene expression by pCSF34 could only be due to the interaction with MalT. However, there was no significant reduction of mal gene expression in strain CL19 by pCSF34 (glk15 Δ306-321) (Table 6). Thus, Glk lacking the 16 C-terminal amino acids was able to interact with PtsG but not with MalT. In Fig. 5, the structure of Glk is shown. The C-terminal 16 amino acids form a prominent alpha-helix which must represent the interaction site with MalT. It is clearly separated from the interaction site with PtsG.
TABLE 6.
Differentiating the function of Glk in controlling mal gene expression
| Straina | Additional genotype | MalK-LacZ activity |
|---|---|---|
| MAD104 (glgA+) | glk15::Tn10 (Cam) vector | 4.21 |
| MAD104 (glgA+) | glk15::Tn10 (Cam) pglk+ | 0.08 |
| MAD104 (glgA+) | glk15::Tn10 (Cam) pTtglk+ | 1.59 |
| MAD106 | glk15::Tn10 (Cam) | 2.16 |
| MAD106 | glk15::Tn10 (Cam) pglk+ | 0.09 |
| MAD106 | glk15::Tn10 (Cam) pCSF34 (glk15 Δ306-321) | 0.88 |
| MAD106 | glk15::Tn10 (Cam) pCSF2glk+ | 0.58 |
| MAD106 | glk15::Tn10 (Cam) pTtglk+ | 1.20 |
| RD105 | glk15::Tn10 (Cam) malP | 1.95 |
| RD105 | glk15::Tn10 (Cam) malP vector | 2.02 |
| RD105 | glk15::Tn10 (Cam) malP pglk+ | 0.09 |
| RD105 | glk15::Tn10 (Cam) malP pTtglk+ | 1.05 |
| GW12 | glk15::Tn10 (Cam) malP malQ mlc vector | 4.27 |
| GW12 | glk15::Tn10 (Cam) malP malQ mlcpglk+ | 3.94 |
| GW12 | glk15::Tn10 (Cam) malP malQ mlcpTtglk+ | 4.39 |
| CL19 (malZ+) | glk15::Tn10 (Cam) mlc vector | 6.30 |
| CL19 (malZ+) | glk15::Tn10 (Cam) mlc pglk+ | 0.71 |
| CL19 (malZ+) | glk15::Tn10 (Cam) mlc pCFS2 (glk+) | 3.90 |
| CL19 (malZ+) | glk15::Tn10 (Cam) mlc pCSF34 (glk15 Δ306-321) | 4.75 |
| CL19 (malZ+) | glk15::Tn10 (Cam) mlc pTtglk+ | 6.37 |
All strains harbor malK-lacZ and lack glgA and malZ unless specified otherwise.
FIG. 4.
Size of the truncated version of Glk. Proteins were analyzed by SDS-PAGE, followed by Western blotting with anti-Glk antibodies. Lane 1, wild-type MG1655; lane 2, JW2385 (Δglk); lane 3, JW2385 harboring glk15 (Δ305-321); lane 4, JW2385 harboring plasmid-encoded N-terminal His6-tagged wild-type Glk under IPTG induction.
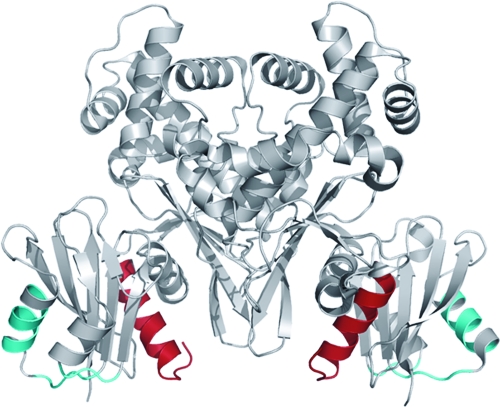
FIG. 5.
Structure of Glk. The structure of the Glk dimer (gray) (26) is shown. The C-terminal helix harboring the 16 amino acids lacking in Glk15 (Δ305-321) are shown in red. They most likely constitute the interaction domain with MalT. The putative interaction site with EIIB of PtsG is indicated in cyan.
The amount of chromosomally glk15::Tn10 (Cam)-encoded truncated glucokinase just as the wild-type Glk had no significant effect on the activity of MalT or PtsG. This is clear from the data in Table 3. The introduction of a complete deletion in glk (strain CL27) did not increase mal gene expression in the glgA malP+ malQ+ background in comparison to the glk15::Tn10 (Cam) mutation. Thus, where the amount of chromosomally encoded Glk endowed with glucokinase activity is concerned it makes itself apparent on mal gene expression only in a background of glgA+ malP+ malQ+ (Table 2, compare TB4 to MAD104).
As another control for the function of overexpressed E. coli glucokinase, we cloned TTC1688, a gene that is annotated to encode glucokinase from T. thermophilus, heterologously expressed it in E. coli, and purified it. The protein, GlKTth, consists of a 303-amino-acid polypeptide chain with only 22% sequence identity with E. coli Glk. It is able to phosphorylate glucose and mannose in a ATP-dependent fashion with a Km of 0.16 mM. At ambient temperature it represents an equilibrium of dimeric and tetrameric forms, as tested by molecular sieve chromatography. Even though the protein has a temperature optimum of 70°C, it is still active at 37°C. We noticed that, during the purification of GlKTth from E. coli extracts, the protein formed a tight complex with MalP from E. coli that could be dissociated by 1.5 M NaCl. E. coli Glk does not show this tight complex formation.
When the overexpression of pTtglk+ was tested in strain MAD104, a strain that produces glycogen (GlgA+) and does contain MalP and MalQ, mal gene expression was reduced 2.6-fold (Table 6). It was much less effective than the overproduction of E. coli Glk. Since GlKTth is still able to phosphorylate glucose, a controlling effect on MalQ activity (reducing maltotriose levels) was expected. When expressed in MAD106 or RD105 (lacking glycogen and therefore no longer producing endogenous maltotriose) the reduction in mal gene expression was <2-fold (Table 6). Thus, GlKTth not only affected MalQ activity but may also be able to interact with PtsG or with MalT or both. However, the overexpression of GlKTth in strains GW12 or CL19, strains lacking Mlc in addition to GlgA, there was no longer an effect of GlKTth overexpression (Table 6). This demonstrated that GlKTth is able to interact with PtsG to release Mlc as a malT repressor, but it is not able to interact with MalT to affect its activity.
DISCUSSION
We describe here a complex regulatory network connected to glucose and glucokinase that affects the mal gene expression in E. coli. In the wild type under laboratory growth conditions these regulatory inputs are hardly recognizable. They may become important under the real-life conditions where varying carbon sources and stress conditions are normal. The effect of uptake and metabolism of glucose on the utilization of alternative carbon sources (mostly carbohydrates) is a well-known phenomenon. Catabolite repression and inducer exclusion in E. coli are connected to the uptake of glucose via the glucose-specific PTS, and the state of phosphorylation of the EIIAGlc has been identified as a major player in this global signal transduction pathway controlling the activity of the cAMP/CAP complex to stimulate the expression of catabolite sensitive genes, as well as the activity of the cognate transporter (40). Certainly, the E. coli maltose system is prominently affected by both catabolite repression (10) and inducer exclusion (24). However, the phenomena connected to glucose described in this publication are not related to catabolite repression even though the EIIBCGlc (PtsG), the specific PTS for glucose, and Mlc, the regulator for ptsG (as well as for malT), are integral parts in this regulation. Here, it is not the PtsG-mediated transport of glucose but cytoplasmic glucose released by the metabolism of maltose and maltodextrins (and by glucose containing disaccharides, such as trehalose and lactose) that has regulatory functions. In addition, it is glucokinase itself that exerts regulatory effects on malT expression, as well as on MalT activity.
Regulation of MalQ activity by glucose.
In a strain wild type for the mal genes, as well as glycogen-synthesizing and -degrading enzymes, the mal genes exhibit an “uninduced” level of expression when grown on a nonmaltodextrin carbon source (16, 18). This situation reflects endogenous induction by glycogen-derived maltotriose, the identified inducer of MalT, the transcriptional regulator of all mal genes (42). We now understand that the concentration of maltotriose is balanced on the one hand by its synthesis from glycogen and on the other hand by its MalQ-mediated removal (formation of glucose plus larger maltodextrins; Fig. 1). It is the removal of maltotriose by MalQ that is controlled by glucose. Thus, excess cytoplasmic glucose will lead to a partial MalQ− phenotype and therefore to an increase of the inducer maltotriose. In contrast, removal of glucose by glucokinase will activate MalQ and lead to the removal of the inducer maltotriose (Fig. 6). These effects have only become apparent by applying nonphysiological test conditions. Thus, mal gene expression was assayed by following the activity of a malK-lacZ fusion. The loss of MalK activity renders MalT more responsive to inducer (24) for easier measurement of mal gene expression. Similarly, the plasmid-encoded glk overexpression was used for more dramatic effects.
One could argue that with the loss of glucose phosphorylating activity in a Glk mutant (and therefore reduced MalQ activity) but wild type for all mal genes one should observe a reduced ability to grow on maltose. This is not the case. Only by the introduction of a second mutation removing PtsG, a strong reduction of growth on maltose is observed (13). Indeed, the inability to grow on maltose in strains lacking glucokinase and PtsG (which apparently is also able to phosphorylate cytoplasmic glucose) has been observed previously (6). We propose that it is the strong inhibition of MalQ by internally accumulated glucose (which can no longer be removed by phosphorylation) that is responsible for the growth defect on maltose.
Glucokinase affects MalT activity.
The situation becomes more complicated by the observation that even a strain that lacks glycogen and can therefore no longer produce endogenous maltotriose still showed considerable mal gene expression (strain RD38, Table 3). Here, too, the overexpression of glucokinase reduced mal gene expression ∼20-fold. For this to happen, either MalQ (MAD108) or MalP (RD105, GW15) or both (MAD106) had to be present (Table 3). In a mutant lacking both MalQ and MalP the overexpression of glucokinase only reduced mal gene expression by ca. 50% (strain GW11, Table 3). Note that a similar phenomenon was observed in a background producing glycogen (Table 3). The decisive difference there was the behavior of the MalQ− MalP+ strain (strain MAD107). Whereas in a glgA background overexpression of glucokinase reduced mal gene expression nearly 20-fold (MAD108, Table 3), in the corresponding MalQ− MalP+ GlgA+ strain the overexpression only led to ca. 30% reduction (strain MAD107, Table 2). We interpret the difference in the two strains by the massive production of maltotriose from glycogen in strain MAD107 which apparently conteracts the effect of glucokinase overproduction. Therefore, the effect of glucokinase on mal gene expression must be caused at a level that is competed by the inducer maltotriose. The obvious target for glucokinase action must therefore be MalT, the central regulator of mal gene expression (Fig. 7). In the past, MalT has been shown to interact with several proteins (3). The interaction of these enzymes with MalT results in a strong inhibition of its activity as transcriptional regulator and in each case the inhibition is counteracted by maltotriose. In addition, The D65E mutations in malT that render MalT largely independent of maltotriose and resistant to the inhibition by Aes (50) also became completely resistant to the inhibition by overexpressed Glk (Table 4). Thus, we conclude that the effect of Glk overproduction falls into the same category as the other known MalT protein inhibitors. When the C-terminally truncated form of Glk (Δ305-321) was used in a strain lacking GlgA, as well as lacking Mlc, the repression was minimal (Table 6, CL19), in contrast to a strain lacking GlgA but producing Mlc (Table 6, MAD106). This demonstrates that it is the C-terminal helix of Glk that is interacting with MalT, whereas this helix is not needed for the interaction with PtsG (Fig. 3 and 5). The additional requirement of either MalQ or MalP in the effect of Glk on MalT is at present not understood. Possibly, a tripartite complex has to be formed for the inhibition to occur. It will take a major effort to biochemically elucidate this interaction with MalT. The interaction of Glk with MalT must be weak, and high concentrations of Glk must be necessary to observe the effect. pCSF2, the plasmid harboring glk under its natural promoter, is much less effective than pBK1 where glk is under the IPTG-inducible promoter. The corresponding relative amounts of Glk can be estimated in Fig. 4. Preliminary attempts to coelute His-tagged MalT or MalT fragments during molecular sieve chromatography with Glk (in the presence of MalP and MalQ) have failed, presumably due to the low affinity between the two proteins.
Coming back to the effect of Glk overproduction on mal gene expression in a strain that is producing glycogen (Table 2), we realized that there must be additional effects concerning MalT expression and activity. We had discussed the effect of glucose on the enzymatic activity of MalQ. Removal of glucose by overproduced Glk would activate MalQ, which in turn would remove maltotriose as an inducer. After we discovered the effect of Glk overproduction in a strain lacking glycogen (Table 3), it became obvious that the effects seen in Table 2 are not only due to MalQ activation but also due to the effects of Glk on MalT activity and on the interaction with PtsG releasing Mlc as a malT repressor. The data of Table 2 and 3 obtained with the glk mutation clearly differentiate the two phenomena; whereas in the glgA+ strain the loss of glucokinase activity has a significantly stimulating effect (MAD104 versus TB04), the glk mutation in the glgA background had hardly any effect (MAD106 versus RD38, Table 3).
Glucokinase binding to PtsG affects malT expression via the release of Mlc.
The third level of mal gene regulation became visible by the effect of Glk overproduction in a strain that lacks glycogen, as well as MalP and MalQ (GW11, Table 3). We found that mal gene expression was reduced ca. 50% by Glk overproduction. We reasoned that Glk, due to its similar structure to Mlc, could be bound by PtsG and release Mlc that might be partially bound by PtsG (Fig. 8). Since Mlc is the transcriptional repressor of malT, increased Mlc concentration would lead to a reduction in malT transcription and therefore to a reduction in mal gene expression. To demonstrate the validity of this scheme, we used GW12, an mlc derivative of strain GW11 (Table 3). Indeed, Glk overproduction in GW12 had hardly any effect on mal gene expression. The effect of Glk to release Mlc from PtsG was surprising. In previous work we had shown that the state of PtsG phosphorylation (EIIBGlc) is the major signaling for the binding and inactivation of Mlc. Transport of glucose would lead to EIIBGlc-P dephosphorylation, the state that would be recognized by Mlc, and lead to its inactivation as a specific repressor. In the absence of glucose transport EIIBGlc should be fully phosphorylated and should be unable to bind Mlc. Obviously, this picture is not quite correct. Even in the absence of glucose transport, Mlc must be able to be bound by PtsG at least to some extent since it can be competed for by binding by Glk. This conclusion is corroborated by the observation that overproduction of PtsG led to an increase in mal gene expression in an Mlc+ strain but not in an Mlc− strain.
Previously, we had concluded that the effect of glucokinase was primarily to abolish internal glucose assumed to be involved in the formation of an alternative endogenous inducer, different from maltotriose (13). The observation that glucokinase activity is not needed for the interaction with PtsG renders this possibility no longer valid. The 16-amino-acid C-terminally truncated Glk protein encoded by glk15::Tn10 (Cam) which lacks glucokinase activity is as effective for the release of Mlc from PtsG as is the wild-type Glk, whereas it is no longer able to interact with MalT.
Inducer-dependent and inducer-independent endogenous induction.
When glycogen is present, there is continuous synthesis and degradation to small maltodextrins, including maltotriose, the inducer of the maltose system (42). Therefore, glycogen-dependent endogenous induction occurs predominantly at the level of inducer concentration. In the absence of glycogen there is still considerable mal gene expression, but the causative relation to a distinct inducer is lacking. As we have demonstrated, glucokinase itself is controlling mal gene expression either by controlling MalT expression or activity. Therefore, the most likely explanation is that in vivo MalT, even in the absence of maltotriose, exhibits basal transcriptional activity. The latter is subject to various protein inhibitors. The postulation for an alternative inducer is no longer necessary.
These conclusions in regard to the regulatory role of glucokinase in mal gene expression were obtained under conditions of severe imbalance by the use of mutations, as well as protein overproduction, that are hardly considered physiological. One may argue that they are the product of artificial situations never occurring in vivo. However, the level of glucokinase is not constant in different carbon sources, and it is under the control of the Cra global regulator known to affect genes encoding glycolytic and gluconeogenic enzymes (27, 47). This opens a link of central metabolism to the modulation of mal gene expression.
There is still another level of mal gene expression that thus far has escaped a reasonable explanation. It is the sensitivity of glycogen-independent endogenous mal gene expression to the osmolarity of the medium (7, 15). As with Glk/MalP/MalQ effects on MalT activity, the osmolarity-controlled regulation is competed for by maltotriose (7). The obvious explanation that glucokinase might be either strongly induced or activated by high osmolarity could be excluded. Possibly, thus-far-unrecognized proteins induced by high medium osmolarity might interact with MalT to affect its activity in the same way as Glk/MalP/MalQ shown here.
Acknowledgments
We thank Michael Krug for providing the structural presentation of the domain interaction in Glk. We are grateful to Gabriele Witz for technical assistance. We thank Iris Andernach for cloning and purifying glucokinase from T. thermophilus. The Δglk strain JW2385 was obtained from the National BioResource Project (NIG, Japan: E. coli).
Financial support was provided by the Deutsche Forschungsgemeinschaft.
Footnotes
Published ahead of print on 21 November 2008.
REFERENCES
- 1.Böhm, A., and W. Boos. 2004. Gene regulation in prokaryotes by subcellular relocalization of transcription factors. Curr. Opin. Microbiol. 7151-156. [DOI] [PubMed] [Google Scholar]
- 2.Böhm, A., and W. Boos. 2004. Transport-dependent gene regulation by sequestration of transcriptional regulators, p. 47-66. In E. Boles and R. Krämer (ed.), Molecular mechanisms controlling transmembrane transport. Springer, Heidelberg, Germany.
- 3.Boos, W., and A. Böhm. 2000. Learning new tricks from an old dog: MaIT of the Escherichia coli maltose system is part of a complex regulatory network. Trends Genet. 16404-409. [DOI] [PubMed] [Google Scholar]
- 4.Boos, W., and H. A. Shuman. 1998. The maltose/maltodextrin system of Escherichia coli; transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 62204-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremer, E., T. J. Silhavy, and J. M. Weinstock. 1985. Transposable lplac Mu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Escherichia coli. J. Bacteriol. 1621092-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buhr, A., K. Flukiger, and B. Erni. 1994. The glucose transporter of Escherichia coli: overexpression, purification, and characterization of functional domains. J. Biol. Chem. 26923437-23443. [PubMed] [Google Scholar]
- 7.Bukau, B., M. Ehrmann, and W. Boos. 1986. Osmoregulation of the maltose regulon in Escherichia coli. J. Bacteriol. 166884-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104541-555. [DOI] [PubMed] [Google Scholar]
- 9.Chapon, C. 1982. Expression of malT, the regulator gene of the maltose region in Escherichia coli, is limited both at transcription and translation. EMBO J. 1369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapon, C. 1982. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J. Bacteriol. 150722-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clausen, T., A. Schlegel, R. Peist, E. Schneider, C. Steegborn, Y.-S. Chang, A. Haase, G. P. Bourenkov, H. D. Bartunik, and W. Boos. 2000. X-ray structure of MalY from Escherichia coli: a pyridoxal 5′-phosphate-dependent enzyme acting as a modulator in mal gene expression. EMBO J. 19831-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decker, K., R. Peist, J. Reidl, M. Kossmann, B. Brand, and W. Boos. 1993. Maltose and maltotriose can be formed endogenously in Escherichia coli from glucose and glucose-1-phosphate independently of enzymes of the maltose system. J. Bacteriol. 1755655-5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decker, K., J. Plumbridge, and W. Boos. 1998. Negative transcriptional regulation of a positive regulator: the expression of malT, encoding the transcriptional activator of the maltose regulon of Escherichia coli, is negatively controlled by Mlc. Mol. Microbiol. 27381-390. [DOI] [PubMed] [Google Scholar]
- 15.Dippel, R., T. Bergmiller, A. Böhm, and W. Boos. 2005. The maltodextrin system of Escherichia coli: glycogen-derived endogenous induction and osmoregulation. J. Bacteriol. 1878332-8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dippel, R., and W. Boos. 2005. The maltodextrin system of Escherichia coli: metabolism and transport. J. Bacteriol. 1878322-8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrmann, M., and W. Boos. 1987. Identification of endogenous inducers of the mal system in Escherichia coli. J. Bacteriol. 1693539-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eppler, T., and W. Boos. 1999. Glycerol-3-phosphate-mediated repression of malT in Escherichia coli does not require metabolism, depends on enzyme IIAGlc and is mediated by cAMP levels. Mol. Microbiol. 331221-1231. [DOI] [PubMed] [Google Scholar]
- 19.Eppler, T., P. Postma, A. Schütz, U. Völker, and W. Boos. 2002. Glycerol-3-phosphate-induced catabolite repression in Escherichia coli. J. Bacteriol. 1843044-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freundlieb, S., and W. Boos. 1986. a-amylase of Escherichia coli, mapping and cloning of the structural gene, malS, and identification of its product as a periplasmic protein. J. Biol. Chem. 2612946-2953. [PubMed] [Google Scholar]
- 21.Johansson, J., B. Dagberg, E. Richet, and B. E. Uhlin. 1998. H-NS and StpA proteins stimulate expression of the maltose regulon in Escherichia coli. J. Bacteriol. 1806117-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joly, N., A. Böhm, W. Boos, and E. Richet. 2004. MalK, the ATP-binding cassette component of the Escherichia coli maltodextrin transporter, inhibits the transcriptional activator MalT by antagonizing inducer binding. J. Biol. Chem. 27933123-33130. [DOI] [PubMed] [Google Scholar]
- 23.Joly, N., O. Danot, A. Schlegel, W. Boos, and E. Richet. 2002. The Aes protein directly controls the activity of MalT, the central transcriptional activator of the Escherichia coli maltose regulon. J. Biol. Chem. 27716606-16613. [DOI] [PubMed] [Google Scholar]
- 24.Kühnau, S., M. Reyes, A. Sievertsen, H. A. Shuman, and W. Boos. 1991. The activities of the Escherichia coli MalK protein in maltose transport, regulation, and inducer exclusion can be separated by mutations. J. Bacteriol. 1732180-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, S.-J., W. Boos, J.-P. Bouché, and J. Plumbridge. 2000. Signal transduction between a membrane bound transporter, PtsG, and a soluble transcription factor, Mlc, of Escherichia coli. EMBO J. 195353-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lunin, V. V., Y. Li, J. D. Schrag, P. Iannuzzi, M. Cygler, and A. Matte. 2004. Crystal structures of Escherichia coli ATP-dependent glucokinase and its complex with glucose. J. Bacteriol. 1866915-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer, D., C. Schneider-Fresenius, R. Horlacher, R. Peist, and W. Boos. 1997. Molecular characterization of glucokinase from Escherichia coli K-12. J. Bacteriol. 1791298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Monod, J., and A. M. Torriani. 1950. De l'amylomaltase d'Escherichia coli. Ann. Inst. Pasteur. 7865-77. [PubMed] [Google Scholar]
- 30.Nam, T.-W., S.-H. Cho, D. Shin, J.-H. Kim, J.-Y. Jeong, J.-H. Lee, J.-H. Roe, A. Peterkofsky, S.-O. Kang, S. Ryu, and Y.-J. Seok. 2001. The Escherichia coli glucose transporter enzyme IICBGlc recruits the global repressor Mlc. EMBO J. 20491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nam, T. W., H. I. Jung, Y. J. An, Y. H. Park, S. H. Lee, Y. J. Seok, and S. S. Cha. 2008. Analyses of Mlc-IIBGlc interaction and a plausible molecular mechanism of Mlc inactivation by membrane sequestration. Proc. Natl. Acad. Sci. USA 1053751-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldham, M. L., D. Khare, F. A. Quiocho, A. L. Davidson, and J. Chen. 2007. Crystal structure of a catalytic intermediate of the maltose transporter. Nature 450515-521. [DOI] [PubMed] [Google Scholar]
- 33.Pajatsch, M., A. Böck, and W. Boos. 1998. Enzymatic preparation of radiolabeled linear maltodextrins and cyclodextrins of high specific activity from [14C]-maltose using amylomaltase, cyclodextrin glucosyltransferase, and cyclodextrinase. Carbohydrate Res. 307375-378. [DOI] [PubMed] [Google Scholar]
- 34.Palmer, N. T., B. E. Ryman, and W. J. Whelan. 1976. The action pattern of amylomaltase from Escherichia coli. Eur. J. Biochem. 69105-115. [DOI] [PubMed] [Google Scholar]
- 35.Panagiotidis, C. H., W. Boos, and H. A. Shuman. 1998. The ATP-binding cassette subunit of the maltose transporter MalK antagonizes MalT, the activator of the Escherichia coli mal regulon. Mol. Microbiol. 30535-546. [DOI] [PubMed] [Google Scholar]
- 36.Peist, R., A. Koch, P. Bolek, S. Sewitz, T. Kolbus, and W. Boos. 1997. Characterization of the aes gene of Escherichia coli encoding an enzyme with esterase activity. J. Bacteriol. 1797679-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plumbridge, J. 1998. Expression of ptsG, the gene for the major glucose PTS transporter in Escherichia coli, is repressed by MIc and induced by growth on glucose. Mol. Microbiol. 291053-1063. [DOI] [PubMed] [Google Scholar]
- 38.Plumbridge, J. 1999. Expression of the phosphotransferase system both mediates and is mediated by Mlc regulation in Escherichia coli. Mol. Microbiol. 33260-273. [DOI] [PubMed] [Google Scholar]
- 39.Plumbridge, J. 2002. Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Curr. Opin. Microbiol. 5187-193. [DOI] [PubMed] [Google Scholar]
- 40.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1996. Phosphoenolpyruvate:carbohydrate phosphotransferase system, p. 1149-1174. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society of Microbiology, Washington, DC.
- 41.Pugsley, A. P., and C. Dubreuil. 1988. Molecular characterization of malQ, the structural gene for the Escherichia coli enzyme amylomaltase. Mol. Microbiol. 2473-479. [DOI] [PubMed] [Google Scholar]
- 42.Raibaud, O., and E. Richet. 1987. Maltotriose is the inducer of the maltose regulon. J. Bacteriol. 1693059-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Randall-Hazelbauer, L., and M. Schwartz. 1973. Isolation of the bacteriophage lambda receptor from Escherichia coli. J. Bacteriol. 1161436-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reyes, M., N. A. Treptow, and H. A. Shuman. 1986. Transport of p-nitrophenyl-a-maltoside by the maltose transport system of Escherichia coli and its subsequent hydrolysis by a cytoplasmic maltosidase. J. Bacteriol. 165918-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richet, E., and O. Raibaud. 1989. MalT, the regulatory protein of the Escherichia coli maltose system, is an ATP-dependent transcriptional activator. EMBO J. 8981-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richet, E., and L. Søgaard-Andersen. 1994. CRP induces the repositioning of MalT at the Escherichia coli malKp promoter primarily through DNA bending. EMBO J. 134558-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saier, M. H., and T. M. Ramseier. 1996. The catabolite repressor/activator (Cra) protein of enteric bacteria. J. Bacteriol. 1783411-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiefner, A., K. Gerber, S. Seitz, W. Welte, K. Diederichs, and W. Boos. 2005. The crystal structure of Mlc, a global regulator of sugar metabolism in Escherichia coli. J. Biol. Chem. 28029073-29079. [DOI] [PubMed] [Google Scholar]
- 49.Schirmer, T., T. A. Keller, Y. F. Wang, and J. P. Rosenbusch. 1995. Structural basis for sugar translocation through maltoporin channels at 3.1 Å resolution. Science 267512-514. [DOI] [PubMed] [Google Scholar]
- 50.Schlegel, A., O. Danot, E. Richet, T. Ferenci, and W. Boos. 2002. The N terminus of the Escherichia coli transcription activator MalT represents the interaction domain with MalY. J. Bacteriol. 1843069-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider, E., S. Freundlieb, S. Tapio, and W. Boos. 1992. Molecular characterization of the MalT-dependent periplasmic a-amylase of Escherichia coli encoded by malS. J. Biol. Chem. 2675148-5154. [PubMed] [Google Scholar]
- 52.Schwartz, M. 1987. The maltose regulon, p. 1482-1502. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society of Microbiology, Washington D. C.
- 53.Schwartz, M., and M. Hofnung. 1967. La maltodextrin phosphorylase d'Escherichia coli. Eur. J. Biochem. 2132-145. [DOI] [PubMed] [Google Scholar]
- 54.Seitz, S., S.-J. Lee, C. Pennetier, W. Boos, and J. Plumbridge. 2003. Analysis of the interaction between the global regulator Mlc and EIIBGlc of the glucose-specific phosphotransferase system in Escherichia coli. J. Biol. Chem. 27810744-10751. [DOI] [PubMed] [Google Scholar]
- 55.Szmelcman, S., M. Schwartz, T. J. Silhavy, and W. Boos. 1976. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and l-resistant mutants with the dissociation constants of the maltose binding protein as measured by fluorescence quenching. Eur. J. Biochem. 6513-19. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka, Y., K. Kimata, and H. Aiba. 2000. A novel regulatory role of glucose transporter of Escherichia coli: membrane sequestration of a global repressor Mlc. EMBO J. 195344-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tapio, S., F. Yeh, H. A. Shuman, and W. Boos. 1991. The malZ gene of Escherichia coli, a member of the maltose regulon, encodes a maltodextrin glucosidase. J. Biol. Chem. 26619450-19458. [PubMed] [Google Scholar]
- 58.Watson, K. A., R. Schinzel, D. Palm, and L. N. Johnson. 1997. The crystal structure of Escherichia coli maltodextrin phosphorylase provides an explanation for the activity without control in this basic archetype of a phosphorylase. EMBO J. 161-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiesmeyer, H., and M. Cohn. 1960. The characterization of the pathway of maltose utilization by Escherichia coli. I. Purification and physical chemical properties of the enzyme amylomaltase. Biochim. Biophys. Acta 39417-426. [DOI] [PubMed] [Google Scholar]
- 60.Wiesmeyer, H., and M. Cohn. 1960. The characterization of the pathway of maltose utilization by Escherichia coli. II. General properties and mechanism of action of amylomaltase. Biochim. Biophys. Acta 39427-439. [DOI] [PubMed] [Google Scholar]
- 61.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 975978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zdych, E., R. Peist, J. Reidl, and W. Boos. 1995. MalY of Escherichia coli is an enzyme with the activity of a bC-S lyase (cystathionase). J. Bacteriol. 1775035-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]