The small molecule cyclic (5′→3′)-di-GMP (c-di-GMP), created through the condensation of two molecules of GTP, is a novel secondary messenger in the Bacteria. In recent years, the fundamental principles of c-di-GMP metabolism and the identities of downstream receptors have been unraveled (Fig. 1) (11, 17).
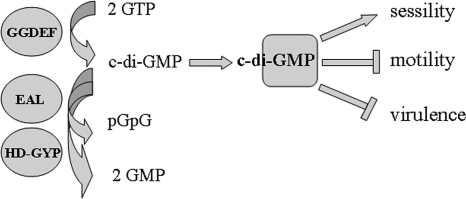
FIG. 1.
Principal components of the c-di-GMP signaling pathway. C-di-GMP is synthesized by GGDEF domain proteins, which are di-guanylate cyclases. Degradation of c-di-GMP occurs through c-di-GMP-specific PDEs. These enzymatic activities are present in the unrelated EAL and HD-GYP domains and degrade c-di-GMP to pGpG and GMP, respectively. Subsequently, c-di-GMP binds to downstream proteins or RNA receptors (17, 20). c-di-GMP thereby stimulates sessility and inhibits motility and virulence properties required for acute infection.
c-di-GMP is the central component of an evolutionarily conserved regulatory system that controls the physiological transition between sessility and motility in many bacteria (17). This life style switch occurs predominantly during the formation of structured multicellular communities (biofilms), a major form of microbial life in various environments. Consequently, not only is the c-di-GMP pathway frequently found in environmental bacteria, but the c-di-GMP network is often also highly complex, with numerous c-di-GMP-synthesizing and -degrading enzymes and c-di-GMP receptors (7, 20). Although biofilm formation plays a role in chronic infections and colonization, the current paradigm predicts that virulence properties are inhibited by c-di-GMP signaling (4, 21), as c-di-GMP affects virulence indirectly through inhibition of motility and directly through inhibition of the expression of virulence factors, such as cholera toxin in Vibrio cholerae. However, as exceptions to this paradigm have been noted, the contribution of c-di-GMP to virulence seems complex.
In this issue of the Journal of Bacteriology, Lai et al. provide for the first time evidence for a functional c-di-GMP signaling pathway in an obligate intracellular pathogen, Anaplasma phagocytophilum (14). The results of this work indicate that regulated c-di-GMP production contributes to the virulence of A. phagocytophilum.
A HOMOLOGUE OF THE DI-GUANYLATE CYCLASE PleD IN A. PHAGOCYTOPHILUM
A. phagocytophilum is the cause of human granulocytic anaplasmosis, an acute flulike illness, potentially fatal in the elderly or immunocompromised individuals. A. phagocytophilum persists in wild-animal (vertebrate) reservoirs. Infection of humans occurs accidentally through ticks, an arthropod vector.
A. phagocytophilum is an intracellular pathogen which primarily infects neutrophils, professional phagocytic cells of the innate immune response. A. phagocytophilum survives and replicates in membrane-bound vacuoles where it prevents the fusion of the phagosome with the deadly lysosome.
The development of intracellular parasitism and host dependence is paralleled by substantial genome reduction whereby regulatory pathways are preferentially lost (2). Surprisingly, however, despite its small genome size of 1.5 Mb, A. phagocytophilum has retained a functional homologue of the di-guanylate cyclase PleD (the A. phagocytophilum homologue is here named PleDAp) (14).
A c-DI-GMP SIGNALING PATHWAY PRESENT IN THE ORDER RICKETTSIALES
The di-guanylate cyclase PleD was first identified in the free-living aquatic bacterium Caulobacter crescentus (8), which belongs, like A. phagocytophilum, to the alphaproteobacteria. In C. crescentus, PleD is required for the substantial morphological changes observed during development from a free-swimming swarmer cell into a sessile stalk cell.
Usually, the inherent di-guanylate cyclase activity of a GGDEF domain is low and requires allosteric activation through N-terminal sensor or signaling domains (19). A significant fraction of GGDEF domain proteins possess an N-terminal receiver domain, indicating their integration into the phosphorelay system of two-component systems as response regulators with c-di-GMP readout upon activation. In C. crescentus, phosphorylation of the conserved aspartate in the first of two receiver domains stimulates the di-guanylate cyclase activity of PleD (16). Similarily, Lai et al. (14) demonstrate that phosphorylated PleDAp is able to produce c-di-GMP, although they report the di-guanylate cyclase activity as being low.
A. phagocytophilum has recently been assigned to the genus Anaplasma in the order Rickettsiales (6). An obligate intracellular lifestyle is characteristic for species in the order Rickettsiales. Intriguingly, homologues of PleD can be found in all sequenced species in this order, which includes six different genera (Anaplasma, Ehrlichia, Neorickettsia, and Wolbachia in the family Anaplasmataceae and Rickettsia and Orienta in the family Rickettsiaceae) (Fig. 2). However, not all species are human or animal pathogens; rather, some, such as Wolbachia spp., live as parasites in arthropods or nematodes (Fig. 2) (5). Therefore, the c-di-GMP signaling pathway might play a role not only in virulence but also in parasitism and symbiosis.
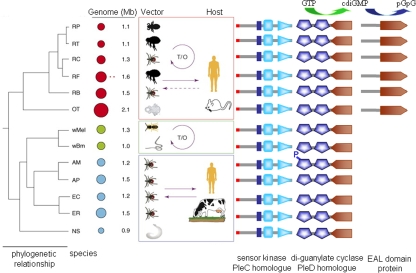
FIG. 2.
Occurrence of proteins involved in the c-di-GMP signaling pathway in species of the order Rickettsiales. PleC-like sensor kinases, as represented by the protein APH_0944 of A. phagocytophilum and the di-guanylate cyclase PleDAp (represented by APH_0551), are present in all species. A putative PDE (exemplified as protein RP246 from Rickettsia prowazekii) is only present in species of the genus Rickettsia. Abbreviations: RP, R. prowazekii; RT, Rickettsia typhi; RC, Rickettsia conorii; RF, Rickettsia felis; RB, Rickettsia bellii; OT, Orientia tsutsugamushi; wMel, Wolbachia from Drosophila melanogaster; wBM, Wolbachia from Brugia malayi; AM, Anaplasma marginale; AP, Anaplasma phagocytophilum; EC, Ehrlichia chaffeensis; ER, Ehrlichia ruminantium; NS, Neorickettsia sennetsu; T/O, vertical (transovarial) transmission from mother to offspring. In the “Genome” column, circles represent genomes and plasmids and are proportioned to show their relative sizes. (Modified from Trends in Genetics [5] with permission of the publisher.)
PleDAP IS PART OF THE PleCAP-PleDAP TWO-COMPONENT SYSTEM
In C. crescentus, the di-guanylate cyclase PleD is part of a two-component system network activated by phosphotransfer from the sensor kinase PleC in vivo (15). A. phagocytophilum encodes on its chromosome three sensor kinases and three response regulators. Lai et al. identified one of the sensor kinases as a PleC-like sensor kinase (PleCAp) with significant similarity to PleC in the histidine kinase domain but not the sensor domain (14). PleCAp specifically phosphorylated PleDAp but not the two other response regulators in vitro. Similarly, the two sensor kinases did not donate the phosphoryl group to PleDAp. Although PleC and PleD are not located adjacent to each other on the chromosome, the PleC-PleD two-component system seems to be conserved in Rickettsiales species (Fig. 2), which all harbor PleCAp homologues, with some variations in the signaling domain. In addition, PleC-PleD constitutes a two-component system in Ehrlichia chaffeensis (13).
LACK OF A c-DI-GMP PDE HOMOLOGUE IN A. PHAGOCYTOPHILUM
Secondary messenger signals respond dynamically to changes in environmental and physiological conditions by signal integration to the enzymes that synthesize and degrade the messenger (10). However, c-di-GMP degradation seems to be absent in A. phagocytophilum, as a c-di-GMP phosphodiesterase (PDE), an EAL domain protein or an HD-GYP domain protein, cannot be identified (14). This finding opens the intriguing possibility that an alternative, not-yet-identified PDE exists in A. phagocytophilum. Alternatively, as Lai et al. speculate, the di-guanylate cyclase activity of PleDAp might be sufficiently low that the lack of PDE has no adverse effect (14). c-di-GMP levels might then be altered passively as cells divide, or c-di-GMP might be pumped out of the cell.
If the PDE activity is indeed lacking, the c-di-GMP signaling pathway is rudimentary in A. phagocytophilum. Possibly, the entire degradation of a c-di-GMP signaling pathway is observed in Staphylococcus epidermidis (9). Although S. epidermidis possesses the GGDEF domain protein GdpS with intact GGD/EEF and GTP binding motifs, GdpS does not possess di-guanylate cyclase activity, possibly because nonhomologous substitutions in invariable consensus residues have occurred outside the GTP binding pocket. Indeed, GdpS plays a role in biofilm formation, but the GGDEF domain is not required for the expression of the phenotype.
Interestingly, however, species of the genus Rickettsia harbor an EAL-like putative PDE (Fig. 1). The N-terminal part of this EAL domain protein is unique to the genus, suggesting that Rickettsia species degrade c-di-GMP when a specific signal is received. The life cycle of Rickettsia species differs somewhat from the life cycle of Anaplasma and Ehrlichia spp. Rickettsia species infect endothelial cells, where they escape from the phagosome and live in the cytoplasm. Through the polymerization of actin, some species move in the cytoplasm and spread to adjacent cells. In the arthropod vector, Rickettsia bacteria can be vertically inherited from mother to offspring, which is not the case for Anaplasma and Ehrlichia (Fig. 2) (2, 5). Whether one of these differences in life style requires a c-di-GMP PDE remains to be shown.
REGULATED c-DI-GMP PRODUCTION PLAYS A ROLE IN A. PHAGOCYTOPHILUM VIRULENCE
When a promyelocytic cell line was infected with synchronized A. phagocytophilum, Lai et al. observed a temporal pattern of expression of PleCAp and PleDAp whereby PleCAp and PleDAp increased in parallel (14). Expression of the PleCAp-PleDAp two-component system was not observed up to 24 h after the beginning of the infection, when aggregates (morulae) of loosely packed bacteria were present. The onset of PleCAp and PleDAp expression coincided with the observation of morulae consisting of densely packed bacteria at 36 h and peaked at 60 h, when morulae with clumped bacteria were present.
The impact of these observations is twofold. First, it suggests that c-di-GMP is required for the formation of intracellular communities of bacterial cells in a manner similar to that observed for bacteria on the outside of host cells or in the environment. Intracellular biofilm communities have also been observed to be formed by Escherichia coli causing urinary tract infections (1). c-di-GMP signaling could also be required for the morphological transition of A. phagocytophilum from intracellular large reticulate cells to small dense-cored cells with the ability to enter host cells, which parallels morulae formation in the host cell. c-di-GMP-regulated morphological transition inside the host cell might occur more frequently, as concerted upregulation of c-di-GMP metabolism was also observed when Legionella pneumophilae switched from replicative, nonmotile cells to highly motile transmissive cells during infection of amoebae (3).
Second, the above observations suggest that c-di-GMP production is required for virulence of A. phagocytophilum. The detection of PleCAp and PleDAp expression in a patient suffering from human granulocytic anaplasmosis greatly strengthens this assumption. Intriguingly, therefore, the findings of Lai et al. challenge the current paradigm of the stimulation of acute infections by low c-di-GMP levels (4, 14, 21).
Genetic tools to investigate the functions of genes do not exist in A. phagocytophilum. To study the role of c-di-GMP in virulence more directly, bacteria were pretreated with a hydrophobic c-di-GMP analog. The application of this molecule did not prevent the initial infection, but the replication of A. phagocytophilum in the promyelocytic cell line was affected. In bacterial cells, the c-di-GMP analog might interact with the allosteric inhibitory side of PleDAp (11) or a 47-kDa protein identified by Lai et al. as a c-di-GMP binding protein (14). As possible mechanisms, c-di-GMP receptors which subsequently signal constitutively are targeted or signaling is blocked by the c-di-GMP analog. Whatever mechanism turns out to be true, the work by Lai et al. indicates that regulation of the c-di-GMP concentration is necessary for a successful infection process, and therefore, c-di-GMP has a dual role in infections (14).
Conclusions and perspectives.
The establishment of an acute infection is a complex process which involves various interactions with the host components and immune cells. The paradigm that low c-di-GMP concentrations stimulate virulence properties stems from observations that high c-di-GMP concentrations inhibit the production of virulence factors and acute disease phenotypes (12, 21). Indeed, my group has observed inhibition of the invasion phenotype of Salmonella enterica serovar Typhimurium by high c-di-GMP levels (A. Lamprokostopoulou, C. Monteiro, and U. Römling, submitted for publication).
Studying a pathogen with only one c-di-GMP-synthesizing enzyme, Lai et al. gained convincing evidence for a role of c-di-GMP in virulence (14). Consequently, the work by Lai et al. offers a clue for the puzzling outcome of c-di-GMP phenotype screens which indicate a stimulating and inhibiting effect of c-di-GMP in virulence (18). If phenotypes, such as the formation of intracellular communities, which might require c-di-GMP to develop are an integral part of the disease phenotype, then c-di-GMP contributes to virulence.
Usually the c-di-GMP signaling network is complex in bacteria. However, like A. phagocytophilum, other bacterial species harbor only one GGDEF/EAL domain protein pair. Among these bacteria are not only pathogenic bacteria, such as Bartonella and Borrelia spp., but also environmental bacteria, such as Mycobacterium smegmatis (13a), Corynebacterium glutamicum, and Lactobacillus acidophilus. Those bacterial species provide intriguing model systems to study the fundamental principles of c-di-GMP signaling at the lowest possible complexity.
Acknowledgments
Work in the laboratory of the author is funded by Vetenskapsrådet, the European Commission, under contract number MEST-CT-2004-8475 and by the Carl Trygger Foundation.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301105-107. [DOI] [PubMed] [Google Scholar]
- 2.Batut, J., S. G. Andersson, and D. O'Callaghan. 2004. The evolution of chronic infection strategies in the alpha-proteobacteria. Nat. Rev. Microbiol. 2933-945. [DOI] [PubMed] [Google Scholar]
- 3.Bruggemann, H., A. Hagman, M. Jules, O. Sismeiro, M. A. Dillies, C. Gouyette, F. Kunst, M. Steinert, K. Heuner, J. Y. Coppee, and C. Buchrieser. 2006. Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila. Cell. Microbiol. 81228-1240. [DOI] [PubMed] [Google Scholar]
- 4.Cotter, P. A., and S. Stibitz. 2007. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr. Opin. Microbiol. 1017-23. [DOI] [PubMed] [Google Scholar]
- 5.Darby, A. C., N. H. Cho, H. H. Fuxelius, J. Westberg, and S. G. Andersson. 2007. Intracellular pathogens go extreme: genome evolution in the Rickettsiales. Trends Genet. 23511-520. [DOI] [PubMed] [Google Scholar]
- 6.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol Microbiol. 512145-2165. [DOI] [PubMed] [Google Scholar]
- 7.Galperin, M. Y. 2005. A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht, G. B., and A. Newton. 1995. Identification of a novel response regulator required for the swarmer-to-stalked-cell transition in Caulobacter crescentus. J. Bacteriol. 1776223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holland, L. M., S. T. O'Donnell, D. A. Ryjenkov, L. Gomelsky, S. R. Slater, P. D. Fey, M. Gomelsky, and J. P. O'Gara. 2008. A staphylococcal GGDEF domain protein regulates biofilm formation independently of cyclic dimeric GMP. J. Bacteriol. 1905178-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houslay, M. D., and G. Milligan. 1997. Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem. Sci. 22217-224. [DOI] [PubMed] [Google Scholar]
- 11.Jenal, U., and J. Malone. 2006. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 40385-407. [DOI] [PubMed] [Google Scholar]
- 12.Kulasekara, H., V. Lee, A. Brencic, N. Liberati, J. Urbach, S. Miyata, D. G. Lee, A. N. Neely, M. Hyodo, Y. Hayakawa, F. M. Ausubel, and S. Lory. 2006. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc. Natl. Acad. Sci. USA 1032839-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumagai, Y., Z. Cheng, M. Lin, and Y. Rikihisa. 2006. Biochemical activities of three pairs of Ehrlichia chaffeensis two-component regulatory system proteins involved in inhibition of lysosomal fusion. Infect. Immun. 745014-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Kumar, M., and D. Chatterji. 2008. Cyclic di-GMP: a second messenger required for long-term survival, but not biofilm formation, in Mycobacterium smegmatis. Microbiology 1542942-2955. [DOI] [PubMed] [Google Scholar]
- 14.Lai, T.-H., Y. Kumagai, M. Hyodo, Y. Hayakawa, and Y. Rikihisa. 31 October 2008. The Anaplasma phagocytophilum PleC histidine kinase and PleD diguanylate cyclase two-component system and role of cyclic di-GMP in host cell infection. J. Bacteriol. 191693-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul, R., T. Jaeger, S. Abel, I. Wiederkehr, M. Folcher, E. G. Biondi, M. T. Laub, and U. Jenal. 2008. Allosteric regulation of histidine kinases by their cognate response regulator determines cell fate. Cell 133452-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul, R., S. Weiser, N. C. Amiot, C. Chan, T. Schirmer, B. Giese, and U. Jenal. 2004. Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 18715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Römling, U., and D. Amikam. 2006. Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol. 9218-228. [DOI] [PubMed] [Google Scholar]
- 18.Ryan, R. P., Y. Fouhy, J. F. Lucey, B. L. Jiang, Y. Q. He, J. X. Feng, J. L. Tang, and J. M. Dow. 2007. Cyclic di-GMP signalling in the virulence and environmental adaptation of Xanthomonas campestris. Mol. Microbiol. 63429-442. [DOI] [PubMed] [Google Scholar]
- 19.Ryjenkov, D. A., M. Tarutina, O. V. Moskvin, and M. Gomelsky. 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 1871792-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudarsan, N., E. R. Lee, Z. Weinberg, R. H. Moy, J. N. Kim, K. H. Link, and R. R. Breaker. 2008. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science 321411-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamayo, R., J. T. Pratt, and A. Camilli. 2007. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu. Rev. Microbiol. 61131-148. [DOI] [PMC free article] [PubMed] [Google Scholar]