Abstract
The intracellular level of cyclic 3′,5′-AMP (cAMP), a signaling molecule that mediates a variety of cellular processes, is finely modulated by the regulation of its synthesis, excretion, and degradation. In this study, cAMP phosphodiesterase (CpdA), an enzyme that catalyzes the conversion of cAMP to AMP, was characterized in a pathogenic bacterium, Vibrio vulnificus. The cpdA gene exists in an operon composed of mutT, yqiB, cpdA, and yqiA, the transcription of which was initiated at position −22 upstream of mutT. A cpdA-null mutant of V. vulnificus contained significantly higher levels of cAMP than the wild type but showed no detectable cAMP when a multicopy plasmid of the cpdA gene was provided in trans, suggesting that CpdA is responsible for cAMP degradation. Cellular contents of the CpdA protein decreased dramatically in both cya and crp mutants. In addition, levels of expression of the cpdA::luxAB transcription fusion decreased in cya and crp mutants. The level of expression of cpdA::luxAB in the cya mutant increased in a concentration-dependent manner upon the exogenous addition of cAMP. The cAMP-cAMP receptor protein (CRP) complex bound directly to the upstream region of mutT, which includes a putative CRP-binding sequence centered at position −95.5 relative to the transcription start site. Site-directed mutagenesis or the deletion of this sequence in the cpdA::luxAB transcription fusion resulted in the loss of regulation by cAMP and CRP. Thus, this study demonstrates that CpdA plays a crucial role in determining the intracellular cAMP level and shows for the first time that the expression of cpdA is activated by the cAMP-CRP complex via direct binding to the regulatory region.
The pathogenic bacterium Vibrio vulnificus is a normal inhabitant of marine estuary environments and infects humans via ingestion of seafood or contact with seawater. Thus, the life cycle of this pathogen involves periods of stress when environmental parameters are fluctuated (25). V. vulnificus must survive such conditions and be able to proliferate with high metabolic activity when the proper host conditions are encountered to cause fatal septicemia or gastroenteritis (32). This bacterium is therefore expected to be able to sense the fluctuations in the surrounding environment and to respond to conditions in its physicochemically distinct niches.
Cyclic 3′,5′-AMP (cAMP) is a cellular signaling metabolite that is involved in relieving the carbon catabolite repression of many genes and operons that encode diverse catabolic enzymes (10). It also mediates multiple global regulatory networks by controlling the expression of major transcription factors in a variety of microorganisms (4, 11). For example, bacterial global regulators such as sigma factor S (RpoS) or ferric uptake regulator (Fur) have been shown to be regulated by cAMP complexed with another global regulator, cAMP receptor protein (CRP), which is itself regulated by cAMP (9, 13, 20). Recently, the cAMP-CRP complex has been reported to be involved in the regulation of numerous virulence factors in pathogenic bacteria. The production of the potent virulence factors of pathogenic Vibrio species, cholera toxin (ctxAB) and toxin-coregulated pilus (tcpPH) in Vibrio cholerae (31) and metalloprotease (vvpE) and cytolytic hemolysin (vvhBA) in V. vulnificus (2, 8, 14), has been shown to be regulated by the cAMP-CRP complex. Thus, it has been suggested that cAMP is one of the key molecules for the timely expression of virulence factors in pathogenic bacteria (10).
Microorganisms are required to modulate cAMP levels to mediate various cellular processes in response to physiological demands (19). The final intracellular concentration of cAMP is determined by a fine-tuned regulatory system that includes its synthesis by adenylate cyclase, its extracellular excretion, and its cleavage into 5′-AMP by 3′,5′-cAMP phosphodiesterase (5, 28). Although we have extensive knowledge about the synthesis and excretion of cAMP (5), the enzymatic hydrolysis of cAMP and the genes encoding these enzymes have been studied in only a limited number of bacterial species. The genes encoding the cytoplasmic 3′,5′-cAMP phosphodiesterase (CpdA) have been isolated and characterized for only two bacterial species so far, Escherichia coli (12) and Haemophilus influenza (23). Studies of some bacterial species, including E. coli (12), Salmonella enterica serovar Typhimurium (4), Bradyrhizobium japonicum (7), and Myxococcus xanthus (18), indicate that the CpdA proteins are involved in decreasing intracellular cAMP levels. The regulation of cpdA expression, however, has not yet been studied.
We have isolated the cpdA gene from the genomic library of V. vulnificus (GenBank accession number AY221025) (20). When the putative amino acid sequence of V. vulnificus CpdA was aligned with those deduced from the corresponding genes of E. coli (GenBank accession number BAA03986.1) and H. influenzae (GenBank accession number NP_438561.1), it showed significant identities of 51 and 45%, respectively (see Fig. S1 in the supplemental material). In the present study, we investigated the role of this gene product in determining intracellular cAMP levels and examined the regulation of cpdA gene expression in V. vulnificus.
MATERIALS AND METHODS
Strains, plasmids, and culture cultivation.
The strains and plasmids used in this study are listed in Table 1. E. coli strains used for plasmid DNA preparation and conjugational transfer were grown at 37°C in Luria-Bertani (LB) medium supplemented with appropriate antibiotics. V. vulnificus strains were grown in LB medium supplemented with an additional 1.5% (wt/vol) NaCl (LBS) at 30°C unless stated otherwise. All medium components were purchased from Difco, and chemicals and antibiotics were obtained from Sigma.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| V. vulnificus | ||
| ATCC 29307 | Clinical isolate; virulent | 14 |
| AR | ATCC 29307 but spontaneous rifampin resistance | 26 |
| HY101 | AR but ΔcpdA | 20 |
| KP301 | AR but Δcya | 20 |
| KC74 | ATCC 29307 but crp::nptI | 14 |
| E. coli | ||
| DH5α | (φ80 lacZΔM15) recA1 endA1 gyrA96 relA1 thi-1 hsdR17(rK− mK−) supE44 deoR Δ(lacZYA-argF)U169 | Laboratory collection |
| SM10λpir | thi-1 thr leu tonA lacY supE recA::Rp4-2-Tc::Muλpir; OriT of RP4; Kmr | 30 |
| JM109 | endA1 recA1 gyrA96 thi-1 hsdR17(rK− mK−) relA1 supE44 Δ(lac-proAB) [F′ traD3 6proAB lacIqZΔM15] | Promega |
| Plasmids | ||
| pUC19 | Cloning vector; lacZ Apr | 34 |
| pINE45 | pUC19 with 1.72-kb Sau3AI fragment of V. vulnificus DNA containing the cpdA gene | This study |
| pINE45-1 | pUC19 with 4.3-kb Sau3AI fragment of V. vulnificus DNA containing the cpdA gene | This study |
| pLAFR5 | IncP Tcr; derivative of pLAFR3 containing double cos cassettes | 16 |
| pHS51 | pLAFR5 containing EcoRI and HindIII fragment of pINE45 | This study |
| pQE30 | Expression vector; Apr | Qiagen |
| pQE30-cpdA | pQE30 with V. vulnificus cpdA | This study |
| pHK0011 | Transcriptional fusion plasmid with promoterless luxAB; Tcr | 8 |
| pSMK-cpdA-1 | pHK0011 with cpdA upstream region (positions −1521 to +41 relative to cpdA IC) | This study |
| pSMK-cpdA2 | pHK0011 with cpdA upstream region (positions −1178 to −1051 relative to cpdA IC) | This study |
| pSMK-cpdA3 | pHK0011 with cpdA upstream region (positions −1163 to −1051 relative to cpdA IC) | This study |
| pGEM-11zf(+) | General cloning vector; Apr | Promega |
| pGEM-11zf(+)-cpdA2mt | pGEM-11zf(+) with the mutagenized sequence for the putative CRP-binding site of cpdA regulatory region | This study |
| pSMK-cpdA2mt | pSMK-cpdA2 but mutagenized CRP-binding site | This study |
Determination of cAMP concentrations.
Wild-type and Δcya and ΔcpdA mutant V. vulnificus strains were grown in LBS. The ΔcpdA mutant carrying either a broad-host-range vector (pLAFR5) (16) or cpdA-containing pLAFR5 (pHS51) was grown in LBS supplemented with 3 μg/ml tetracycline. Exponential-phase (the optical density at 595 nm [OD595] ranged from 0.6 to ∼0.7) and stationary-phase (the OD595 ranged from 3.3 to ∼4.0) cells were harvested and lysed, and the amount of cAMP in the lysates was estimated using the cAMP Biotrak enzyme immunoassay system according to the manufacturer's instructions (Amersham). To determine concentrations of secreted cAMP, the same procedure was applied to cell-free spent medium, which had been filtered through a 0.22-μm-pore-size membrane.
Western blot analysis of CpdA.
A pair of oligonucleotides, cpdAexp-F (5′-CCCGGATCCTTGCAACATACATCCAGTGATACG-3′ [underlined sequence indicates a BamHI restriction site]) and cpdAexp-R (5′-GGGCTGCAGTTAGGACGGCTCATATCAGTAACC-3′ [underlined sequence indicates a PstI restriction site]), were used to amplify an 840-bp DNA fragment containing the full sequence of the cpdA open reading frame (ORF) from the genomic DNA of V. vulnificus. Recombinant CpdA (rCpdA) was overexpressed in E. coli JM109 cells carrying pQE30-cpdA with His6-tagged CpdA and purified using a Ni-nitrilotriacetic acid affinity column as directed by the manufacturer (Qiagen). Purified rCpdA was used to generate polyclonal antibodies by three immunizations of Sprague-Dawley rats (200 μg of CpdA protein per immunization) at 3-week intervals. Cell lysates of wild-type and ΔcpdA, crp, and Δcya mutant V. vulnificus were prepared by sonication in TNT buffer (10 mM Tris-HCl, 150 mM NaCl, and 0.05% [vol/vol] Tween 20 [pH 8.0]) (29). One hundred micrograms of each bacterial lysate was fractionated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred onto a Hybond P membrane (Amersham). The membrane was incubated with polyclonal antibodies against rCpdA (1:5,000 dilution) and then incubated with alkaline phosphatase-conjugated rabbit anti-rat immunoglobulin G (1:5,000; Sigma). Immunoreactive protein bands were visualized using a nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) system (Promega). To investigate the effect of added cAMP on CpdA levels, cAMP was added to the Δcya mutant at a final concentration of 1.0 mM for 1.5 h before extracts were prepared.
Northern blot analysis.
Total RNA was extracted from wild-type V. vulnificus ATCC 29307 cells using Trizol reagent (Gibco BRL) according to the manufacturer's instructions and quantified by spectrophotometric readings at 260 nm. Thirty micrograms of RNA was fractionated by 1% formaldehyde agarose gel electrophoresis in a running buffer (0.1 M MOPS [morpholinepropanesulfonic acid], 40 mM sodium acetate, and 5 mM EDTA), blotted onto a Hybond-N membrane (Amersham) by capillary transfer in 20× SSPE (3 M NaCl, 0.2 M NaH2PO4, 0.02 M EDTA), and immobilized using a UV cross-linker (CL-1000; UVP). Blots were incubated for 2 h at 42°C in a prehybridization solution (5× SSPE, 50% formamide, 5× Denhardt's solution, 0.5% SDS, 200 μg/ml salmon sperm DNA, and 10% dextran sulfate). Hybridization at 42°C was continued overnight in the presence of a labeled cpdA probe. For the preparation of the probe, cpdA gene-containing plasmid pINE45 was digested with EcoRI and HindIII, and the 1.7-kb inserted DNA fragment was isolated using the GeneClean kit (Bio101) and labeled with [α-32P]ATP using a Random Primer kit (Takara). The membrane was washed twice with 2× SSPE-0.1% SDS at room temperature for 15 min and twice with 0.1× SSPE-0.5% SDS at 60°C for 30 min and then exposed to X-ray film (29).
Primer extension analysis.
A primer, yqiE-R (5′-TTCGATCACATTGCTCCACC-3′), was designed to be complementary to positions −976 to −956 with respect to the initiation codon (IC) of cpdA. The primer was labeled at the 5′ terminus with [γ-32P]ATP using T4 polynucleotide kinase (Takara), annealed to 150 μg RNA in hybridization buffer (10 mM Tris-HCl, 1 mM EDTA, 1.25 M KCl [pH 8.0]), and then incubated at 65°C for 3 min. RNA was converted to cDNA with SuperScript II reverse transcriptase (Invitrogen). The resultant cDNA products were precipitated and resolved on a sequencing gel beside sequencing ladders generated with the same primer used for primer extension. The sequence of pINE45-1 was determined using the dideoxy chain termination method using AccuPower DNA sequencing kit (Bioneer) as previously described (27). Sequencing gels were dried and then visualized with a phosphorimager (Personal Molecular Imager FX; Bio-Rad).
Complementation of the cpdA gene.
An intact cpdA gene was isolated as EcoRI and HindIII from pINE45, a pUC19-based plasmid with a 1.72-kb Sau3AI fragment of V. vulnificus ATCC 29307 genomic DNA including the coding sequence of cpdA. The cpdA-containing DNA fragment was cloned into EcoRI- and HindIII-digested pLAFR5 to produce pHS51. The resultant plasmid in E. coli SM10 λpir was transferred into the ΔcpdA mutant (HY101) (20) by conjugation, and exconjugants were selected on TCBS (thiosulfate-citrate-bile salts-sucrose) agar containing tetracycline.
Construction of the cpdA::luxAB transcriptional reporter fusions.
A set of cpdA::luxAB fusions was made by subcloning DNA fragments encompassing upstream regions of the cpdA gene into pHK0011, which contains promoterless luxAB genes (8). To construct a transcriptional fusion, pSMK-cpdA-1, a DNA fragment containing the whole upstream region of the cpdA gene up to the 3′ end of the tolC gene, was obtained by PCR using primers SMK-F1K (5′-GCGGTAATAAGGTACCACGG-3′ [underlining indicates a KpnI site]) and SMK-RX-1 (5′-GCTCTAGAATACTGTTCTCGCTGAGCG 3′ [underlining indicates an XbaI site]), digested with KpnI and XbaI, and ligated into KpnI/XbaI-digested pHK0011. The resulting plasmid includes the region from positions −1,521 to +41 relative to the IC of V. vulnificus cpdA. The PCR product amplified with primers SMK-F2K (5′-GGGGTACCGTCCAGTTGTGCAATAAATG-3′ [underlining indicates a KpnI restriction site]) and SMK-RX (5′-AGAACCTCTAGATCTTCTGG-3′ [underlining indicates an XbaI restriction site]) was cloned into pHK0011 to produce pSMK-cpdA2, which includes only the intergenic space between tolC and mutT (positions −1178 to −1051 relative to the cpdA IC). pSMK-cpdA3 was constructed by inserting the DNA encompassing positions −1163 to −1051 relative to the cpdA IC that was amplified using SMK-F3K (5′-GGGGTACCAAATGAATTGTTTAAACCTAAA-3′ [underlining indicates a KpnI restriction site]) and SMK-RX.
The cpdA::luxAB reporters were then mobilized into the wild type and the ΔcpdA, crp, and Δcya V. vulnificus mutants via conjugal transfer. Cultures of bacterial cells grown overnight (16 to 18 h) that contained the reporters were inoculated into fresh LBS medium with tetracycline (3 μg/ml) and grown to stationary phase. At various time points, a portion of the samples was diluted 100-fold with LBS medium. Expression from various lengths of the cpdA upstream region was measured by monitoring light production in the presence of 0.006% (vol/vol) n-decyl aldehyde using a luminometer (TD-20/20; Turners Designs). Light production was expressed in arbitrary relative light units (RLU), and the specific bioluminescence was calculated by normalizing RLU with cell mass (OD595) as described previously (26). cAMP at concentrations up to 1.0 mM was added exogenously to the Δcya mutant culture, and light emission was monitored.
Site-directed mutagenesis of the cpdA promoter.
Based on the CRP-binding consensus sequence (AAATGTGATCTAGATCACATTT) (6), a putative CRP-binding site, 5′-AGTTGTGCAATAAATGAATTGT-3′, was found in the upstream region of the promoter for the mutT-yqiB-cpdA-yqiA operon. This site was mutagenized using the GeneEditor in vitro site-directed mutagenesis kit (Promega). Briefly, a DNA fragment containing the cpdA promoter region in pSMK-cpdA2 (see above) was cloned into pGEM-11zf(+) to produce pGEM-11zf(+)-cpdA2. Next, cpdA-siteF (5′-GGGGTACCGTCCCGTAAAACAAAAAATGAATTGTTTAAACC-3′ (underlining indicates the r mutagenesis site, and italics indicate a KpnI site]) was used to substitute six bases in the putative CRP-binding site. The resultant plasmid was named pGEM-11zf(+)-cpdA2mt. The insert DNA of pGEM-11zf(+)-cpdA2mt was isolated after digestion with KpnI and XbaI and inserted into pHK0011 to produce pSMK-cpdA2mt.
Electrophoretic mobility shift assay.
The V. vulnificus rCRP protein was overexpressed in E. coli BL21 carrying pHK0201 (15), a pRSETA (Invitrogen)-based expression plasmid, and purified by Ni-nitrilotriacetic acid affinity chromatography according to the manufacturer's instructions (Qiagen). The 373-bp upstream region of the mutT gene was PCR amplified using SMK-F1K and SMK-R2X (5′-GCTCTAGACTCAAAGGACATTGCTC-3′) and labeled with [γ-32P]ATP using T4 polynucleotide kinase. The labeled DNA fragment (225 nM) was incubated with various concentrations of purified rCRP protein (50 to ∼200 nM) for 30 min at 37°C in a 20-μl reaction mixture containing 1× binding buffer (26) with 500 μM cAMP (Sigma). Following the addition of 3 μl of loading buffer, the samples were separated on a 6% nondenaturing polyacrylamide gel. For competition analyses, the identical but unlabeled sequence was included as a competitor. Competitor DNA from 22.5 to 675 nM was added to reaction mixtures containing 225 nM of labeled DNA prior to the addition of 200 nM CRP. A 378-bp DNA fragment encompassing the promoter of the gap gene, encoding glyceraldehyde-3-phosphate dehydrogenase, was amplified from V. vulnificus genomic DNA using primers gap-F (5′-CATTAATCTAGATATCGTCG-3′) and gap-R (5′-AATCAGTGGATCCAAAGCGC-3′) and included as nonspecific DNA in the binding assay.
RESULTS
Genetic organization of the cpdA gene in V. vulnificus.
V. vulnificus genomic library plasmids containing the cpdA gene, pINE45 and pINE45-1, were obtained from a screening experiment to isolate the factors for which gene products caused the change in rpoS gene expression (20). Sequencing of insert DNA in these plasmids revealed the flanking regions of the cpdA gene. The upstream region of the cpdA gene contains two ORFs homologous to mutT and yqiB of E. coli that are transcribed in the same direction as cpdA (Fig. 1A). Further upstream, an ORF homologous to the tolC gene of E. coli was found, which is transcribed in the opposite direction from mutT-yqiB-cpdA. An ORF downstream of cpdA is homologous to E. coli yqiA. Downstream and in the opposite direction of the mutT-yqiB-cpdA-yqiA gene cluster, an ORF homologous to the topoisomerase gene is present.
FIG. 1.
Genetic organization and transcription of the mutT-yqiB-cpdA-yqiA operon in V. vulnificus. (A) Based upon the genetic organization of the cpdA gene, two different cpdA::luxAB transcriptional fusions were constructed: one (pSMK-cpdA2) includes the upstream region of mutT, the first gene of the tentative operon, and the other (pSMK-cpdA-1) includes the entire upstream region of cpdA. (B) For Northern blot analysis of cpdA mRNA, total RNA extracted from stationary-phase V. vulnificus cells was hybridized with a 32P-labeled cpdA probe and visualized by autoradiography. The left lane (lane 1) is ethidium bromide-stained total RNA from wild-type V. vulnificus in a formamide agarose gel, and the right lane (lane 2) is a Northern hybridization using the cpdA probe. Arrows and numbers on the left side indicate the molecular sizes of 23S and 16S RNAs in kilobases (kb). (C) Wild-type V. vulnificus carrying each fusion or the vector only (pKH0011) was grown in LBS medium supplemented with 3 μg/ml tetracycline, aliquots were sampled, and their cell masses (OD595) and their bioluminescence (RLU) were determined. Luciferase activities are expressed as normalized values that were obtained by dividing the RLU by the OD595 of each sample. The averages of three independent experiments are shown, along with their standard deviations.
Identification of the cpdA promoter.
To define the promoter of the cpdA gene, two experiments were performed. Northern blotting using a cpdA gene probe showed the presence of a single band approximately 2.5 kb long (Fig. 1B). Because the cpdA gene seems to be organized as an operon composed of mutT, yqiB, cpdA, and yqiA, this suggested that the cpdA gene was transcribed with its flanking genes from a promoter upstream of the first gene, mutT. To test this hypothesis, two different cpdA::luxAB transcriptional reporter fusions were constructed: pSMK-cpdA-1 includes the whole upstream region of the cpdA gene to the 3′ end of the tolC gene, and the other, pSMK-cpdA2, includes only the intergenic space between tolC and mutT (Fig. 1A). Both fusions were highly expressed compared to the luciferase activity from the vector plasmid without an insert DNA and showed exactly the same expression pattern and level of luciferase activity (Fig. 1C), indicating that the cpdA gene is a member of the mutT-yqiB-cpdA-yqiA operon, and its transcript is produced as a polycistronic mRNA.
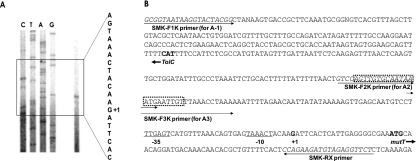
The transcription start site for the mutT-yqiB-cpdA-yqiA operon was determined by primer extension (Fig. 2A) to be at position −22 relative to the IC of mutT (Fig. 2B). The putative promoter showed the presence of relatively well-conserved −10 and −35 regions (TAAACT and TTGAGT, respectively). In addition, a sequence homologous to the CRP-binding consensus sequence (5′-AAATGTGATCTAGATCACATTT-3′) (6) was discernible at position −95.5 relative to the transcription start site.
FIG. 2.
Determination of the transcriptional start site of the cpdA gene. (A) Primer extension using V. vulnificus RNA and oligonucleotide primer yqiE-R (annealing to positions +130 to +150 relative to the IC of mutT). Lanes C, T, A, and G represent sequencing ladders of pINE45-1. The +1 indicates the site of transcriptional initiation. (B) Sequence of the upstream region of mutT, the first gene of the operon composed of mutT, yqiB, cpdA, and yqiA. The initiation codon for mutT is in boldface type, and the promoter and the putative −10 and −35 regions are underlined. The putative binding site for the cAMP-CRP complex is designated with a box. The primers used for the construction of the cpdA::luxAB transcription fusions are indicated with horizontal arrows.
Role of CpdA in modulating cellular cAMP levels.
To investigate the function of CpdA, the level of cellular cAMP of a V. vulnificus ΔcpdA mutant (20) was compared to that of the wild-type strain and a Δcya mutant deficient in the cAMP-synthesizing enzyme adenylate cyclase. The intracellular concentrations of cAMP in wild-type cells grown in LBS medium at exponential and stationary phases were 26 and 6.3 pmol/mg protein, respectively (Table 2). The Δcya mutant had nondetectable levels of cAMP in both growth phases. In contrast, the ΔcpdA mutant showed highly elevated levels of cellular cAMP, estimated at 99 to ∼137 and 37 to ∼45 pmol/mg protein from exponential- and stationary-phase cells, respectively. When the intact cpdA gene was introduced into the ΔcpdA mutant on the multicopy plasmid pHS51, its intracellular cAMP level decreased to below the detection limit.
TABLE 2.
Intracellular cAMP contents in various V. vulnificus strains grown in LBS medium
| Growth phase | cAMP concn (pmol/mg protein) (SD)a
|
||||
|---|---|---|---|---|---|
| Wild type | Δcya | ΔcpdA | ΔcpdA (pLAFR5) | ΔcpdA (pHS51)b | |
| Exponentialc | 26.2 (3.6) | ND | 98.8 (7.1) | 137.3 (16.7) | ND |
| Stationaryd | 6.3 (0.8) | ND | 36.5 (0.2) | 44.7 (0.7) | ND |
Intracellular cAMP concentration (moles of cAMP in the unit mass of bacterial cells determined by the protein amount in lysate) was expressed as an average value from four independent experiments, with the standard deviation in parentheses. ND, the cAMP concentration was not detectable because it was below the detection limit of the assay used in this study.
A broad-host-range vector, pLAFR5-based plasmid DNA including the V. vulnificus cpdA gene.
V. vulnificus cells freshly grown in LBS medium at an OD595 ranging from 0.6 to ∼0.7.
V. vulnificus cells grown in LBS medium at an OD595 ranging from 3 to ∼4.
Similarly, the level of extracellular cAMP in cell-free spent medium from wild-type and ΔcpdA mutant cultures was also determined. Spent medium sampled from the ΔcpdA mutant culture showed a 1.7-fold-higher concentration of cAMP than that of the wild type (S.-M. Kim and K.-H. Lee, unpublished data). This implied that the higher intracellular cAMP level in the ΔcpdA mutant was not from a reduced excretion of cAMP but possibly from an absence of cAMP phosphodiesterase activity. Therefore, this suggests that CpdA controls the level of intracellular cAMP through degradation.
Contents of CpdA in wild-type, Δcya mutant, and crp mutant V. vulnificus strains.
Since V. vulnificus CpdA appears to degrade cellular cAMP via a cAMP phosphodiesterase activity, as is seen in other bacteria (12, 23), the effect of the substrate cAMP on the level of the CpdA protein was examined. Western blotting using polyclonal anti-CpdA antibodies produced an immunoreactive band of ∼30.5 kDa in all V. vulnificus strains except the ΔcpdA mutant (Fig. 3A). Interestingly, the Δcya mutant, which does not contain detectable levels of cAMP (Table 2), showed significantly decreased levels of CpdA compared to those of the wild type. Densitometric quantification of immunoreactive bands indicated about a threefold decrease in levels in the Δcya mutant (Fig. 3B). This suggested that cAMP may play a role in inducing the production of an enzyme that uses cAMP as a substrate. cAMP induction is known to be associated with CRP (19), so the CpdA content was also measured in a V. vulnificus crp mutant. The crp mutant showed a CpdA level similar to that of the Δcya mutant. In addition, the crp Δcya double mutant showed essentially the same pattern as that of each single mutant (data not shown), which suggested that cpdA expression is activated by the cAMP-CRP complex.
FIG. 3.
Cellular contents of the cAMP phosphodiesterase (CpdA) in wild-type (wt), crp mutant, and Δcya mutant strains of V. vulnificus. (A) Lysates of V. vulnificus strains grown to stationary phase (OD595 of 1.5) were used to estimate CpdA levels by Western blotting. One hundred micrograms of each bacterial lysate was fractionated by SDS-polyacrylamide gel electrophoresis. The blotted membrane was incubated with polyclonal antibodies raised against the recombinant CpdA and then incubated with alkaline phosphatase-conjugated rabbit anti-rat immunoglobulin G. Upon incubation with the nitroblue tetrazolium-BCIP system, the CpdA protein appeared as an immunoreactive band as indicated by an arrow. (B) The intensities of bands corresponding to CpdA were estimated by densitometry, and the densitometric readings are presented in the plot as values relative to those of CpdA of the wild-type strain.
Effect of cAMP and CRP on cpdA gene expression.
Because the upstream region of the mutT-yqiB-cpdA-yqiA operon includes a putative CRP-binding site (Fig. 2B) and the Δcya and crp mutants contained less CpdA protein than did the wild type (Fig. 3), the effect of cAMP on cpdA gene expression was further confirmed using a transcriptional reporter fusion. The cpdA::luxAB transcriptional fusion pSMK-cpdA2 showed reduced levels of expression in the Δcya or crp mutant, which were about three- to fourfold lower than the levels of expression in the wild type (Fig. 4). The degree of the decrease in the level of transcription fusion expression in the Δcya or crp mutant was similar to the extent of the decrease in the level of the CpdA protein in the same mutants as shown in Fig. 3.
FIG. 4.
Effects of crp or cya mutations on expression of cpdA::luxAB transcription fusion. Wild-type (wt), crp, and Δcya V. vulnificus strains carrying pSMK-cpdA2 were grown to stationary phase in LBS medium supplemented with 3 μg/ml tetracycline, and aliquots were sampled and measured for cell mass (OD595) and bioluminescence (RLU). Luciferase activities are expressed as normalized values by dividing the RLU by the OD595 of each sample. The activities of two independent experiments were averaged and are shown with their standard deviations. An asterisk indicates P values less than 0.005.
To investigate whether the lowered level of expression of pSMK-cpdA2 in the Δcya mutant was caused by the absence of cAMP, various concentrations of cAMP were added, and the level of expression of pSMK-cpdA2 was measured after 1.5 h (Fig. 5A). The level of expression increased in a dose-dependent manner, with maximal expression observed in the Δcya mutant incubated with ≥0.5 mM cAMP. In a subsequent experiment, cAMP was exogenously added to the Δcya mutant carrying pSMK-cpdA2. In the presence of 1.0 mM cAMP in the medium, the mutant expressed the fusion at increasingly higher rates than the control in a time-dependent manner, peaking at 2 h after the addition of cAMP (Fig. 5B). Similarly, 1.0 mM cAMP was exogenously added to either the wild type or the Δcya mutant, and their cellular levels of CpdA protein were compared. A Western blot using polyclonal antibodies against rCpdA showed that the wild type was not influenced by the exogenous addition of cAMP, but the Δcya mutant showed higher levels in the presence of added cAMP (Fig. 5C). All of the above-described results suggest that the cellular amount of CpdA is regulated at the transcriptional level via cAMP-CRP complex-mediated activation.
FIG. 5.
Expression of cpdA in the Δcya mutant V. vulnificus strain in the presence of exogenous cAMP. (A) Various concentrations of cAMP ranging from 0 to 1.5 mM were added to the Δcya strain carrying pSMK-cpdA2 freshly grown in LBS medium supplemented with 3 μg/ml tetracycline. After a 1.5-h incubation, specific bioluminescence was estimated as described in the legend of Fig. 4. (B) The Δcya strain carrying pSMK-cpdA2 was grown in LBS medium supplemented with 3 μg/ml tetracycline, and half of the culture was treated with 1.0 mM cAMP at the time point indicated by a vertical arrow. Growth and luminescence of the mutant in the presence or absence of exogenously added cAMP were compared. Luciferase activities are expressed as described in the legend of Fig. 4. (C) Wild-type (wt) and Δcya cells in exponential phase were treated with 1.0 mM cAMP for 1.5 h and used for Western blot analysis to measure the contents of CpdA, as described in the legend of Fig. 3. Lane 1, wild type without cAMP; lane 2, wild type with cAMP; lane 3, Δcya strain without cAMP; lane 4, Δcya strain with cAMP.
Direct interaction of the cAMP-CRP complex with the regulatory region of cpdA.
The above-described results clearly indicate that both cAMP and CRP positively affect cpdA expression. To determine whether the cAMP-CRP complex acts by binding to the regulatory region of the mutT-yqiB-cpdA-yqiA operon, an electrophoretic mobility shift assay was performed using the V. vulnificus CRP protein and a 373-bp DNA encompassing the region used for the construction of pSMK-cpdA2. As shown in Fig. 6, the addition of CRP and cAMP resulted in a slower mobility of the DNA fragment in a dose-dependent manner. The binding of cAMP-CRP to the DNA was specific, because excess unlabeled DNA abolished the formation of the slower-moving band, although retarded mobility was retained in the presence of an unrelated sequence, namely, the V. vulnificus gap promoter.
FIG. 6.
Binding of the cAMP-CRP complex to the upstream region of the mutT-yqiB-cpdA-yqiA operon. A gel shift assay was performed to determine the direct interaction between cAMP-CRP and the upstream region of the mutT gene. A 32P-labeled 373-bp DNA probe of the upstream region (225 nM) was incubated with increasing amounts of CRP up to 200 nM in the presence of cAMP (1.0 mM). The reaction mixtures were then subjected to 6% native polyacrylamide gel electrophoresis. For competition analysis, the same, but unlabeled, 373-bp DNA was included. As noncompetitive and nonspecific DNA, an unlabeled 378-bp DNA fragment containing the promoter of the gap gene (Pgap) was added in excess. Lane 1, probe only; lane 2, probe with 50 nM CRP; lane 3, probe with 75 nM CRP; lane 4, probe with 100 nM CRP; lane 5, probe with 150 nM CRP; lane 6, probe with 200 nM CRP; lane 7, probe with 200 nM CRP and 22.5 nM unlabeled upstream DNA; lane 8, probe with 200 nM CRP and 225 nM unlabeled upstream DNA; lane 9, probe with 200 nM CRP and 675 nM unlabeled upstream DNA; lane 10, probe with 200 nM CRP and 200 nM unlabeled Pgap DNA. The arrow on the left side indicates the unbound DNA probe, whereas the arrow on the right side indicates the DNA bound with CRP.
Site-directed mutagenesis of the CRP-binding site at the regulatory region of cpdA.
The sequence of the upstream region of the mutT-yqiB-cpdA-yqiA operon was analyzed for the cAMP-CRP-binding sequence. A region with considerable homology to the E. coli CRP-binding site (AAATGTGATCTAGATCACATTT; underlining indicates highly conserved residues) was found in the upstream region centered at position −95.5 relative to the transcription start site (Fig. 3B). To determine whether the putative binding site plays a role in cpdA transcription, the site was modified by either site-directed mutagenesis or the deletion of the first 11 out of 22 nucleotides of the putative CRP-binding site (Fig. 7A and B). The altered DNAs were used to construct the luxAB transcriptional fusions. pSMK-cpdA2mt is the same as pSMK-cpdA2 but includes the mutated CRP-binding site. pSMK-cpdA3 is missing 15 nucleotides at the 5′ end of the pSMK-cpdA2 insert.
FIG. 7.
Effect of mutating the putative CRP binding site on cpdA expression. (A) A putative CRP-binding site, based upon the conserved nucleotide sequences for CRP binding in E. coli (6), was found in the upstream region of mutT centered at position −95.5 upstream of the transcription start site (designated with a +1) and indicated as an open box in the pSMK-cpdA2 fusion. The same transcription fusion with an altered putative CRP-binding site, pSMK-cpdA2mt, was constructed by site-directed mutagenesis to change the nucleotides as shown in B (hatched box). The upstream region of mutT in pSMK-cpdA3 has a deletion of the first 11 of the 22 nucleotides that comprise the putative CRP-binding site. Wild-type, crp, and Δcya strains carrying pSMK-cpdA2mt (C) or pSMK-cpdA3 (D) were grown in LBS medium supplemented with 3 μg/ml tetracycline, and aliquots were sampled to estimate specific luciferase activities. Luciferase activities are expressed as described in the legend of Fig. 4. (E) Assay of binding of the cAMP-CRP complex to the DNA fragments carrying the original sequence (wt probe) (the same DNA used in Fig. 6) or the mutagenized sequence (mt probe) (as indicated in B) was performed as described in the legend of Fig. 6. Lane 1, wild-type probe without CRP; lane 2, wild-type probe with 200 nM CRP; lane 3, mutagenized probe without CRP; lanes 4, 5, and 6, mutagenized probe with 20, 100, and 200 nM CRP, respectively. The arrow on the left side indicates the unbound DNA probe, whereas the arrow on the right side indicates the DNA bound with CRP.
These mutant fusions showed similar basal levels of expression in the wild-type, crp, and Δcya strains (Fig. 7C and D) compared to the level of pSMK-cpdA2 expression in the crp and Δcya strains. In addition, to verify if cAMP-CRP binding to this putative site occurs, the DNA fragment used in Fig. 6 and the same DNA but with the mutagenized putative CRP-binding site were used for electrophoretic mobility shift assays. No binding of cAMP-CRP to the mutagenized probe was observed (Fig. 7E, lanes 3 to 6), while the wild-type probe was efficiently bound by the cAMP-CRP complex (Fig. 6 and 7E, lanes 1 to 2). These results suggest that cpdA expression is activated by cAMP-CRP acting on the region between positions −106 and −85 relative to its transcription start site.
DISCUSSION
The activity of 3′,5′-cAMP phosphodiesterase has been found to play a role in optimizing cAMP concentrations in some bacteria, to induce the starvation response, to regulate catabolite-sensitive operons, or to protect against a high influx of cAMP (1, 4). Using the cpdA gene isolated from V. vulnificus, we investigated the role of CpdA with respect to modulating cAMP concentrations, which may subsequently result in an adjustment of bacterial responses to diverse stimuli and the control of virulence factor expression within host environments.
Bacterial cells grown in LB-based media without the sugars transported by phosphotransferase systems such as glucose did not exhibit maximal cAMP levels when they entered stationary phase (20, 22). cAMP levels in V. vulnificus have been estimated to be approximately 20 to ∼50 pmol cAMP/mg of protein in the exponential phase, decreasing to about 5 pmol cAMP/mg of protein in stationary phase (20) (Table 2). When V. vulnificus was deficient in cAMP phosphodiesterase (CpdA), it showed highly elevated levels of cAMP compared to those of an isogenic wild-type strain during both exponential and stationary phases (Table 2). The increased level of cAMP in the ΔcpdA mutant has also been confirmed by measuring gene expression, which is tightly regulated by cAMP and thus might serve as an index of the intracellular level of cAMP. For example, the rpoS gene, encoding V. vulnificus sigma factor S, is known to be repressed by cAMP (20). We found approximately twofold-lower levels of expression of the rpoS gene in the ΔcpdA mutant than in the wild type (20). When the ΔcpdA mutant was supplied with the intact cpdA gene, its cAMP level was too low to be detected, similar to the Δcya mutant that lacks adenylate cyclase activity (Table 2). Therefore, CpdA appears to be an important factor in controlling the intracellular concentration of cAMP in V. vulnificus.
The expression of many enzymes is induced by the presence of their substrate molecules (33). This regulatory pattern for catalytic enzymes prompted us to study the effect of cAMP on the regulation of cpdA expression. cpdA expression at the transcriptional level was activated by the cAMP-CRP complex (Fig. 3 and 4). The regulatory region for cpdA includes a sequence homologous to the E. coli CRP consensus sequence (Fig. 2B), and we found that this sequence was bound by the cAMP-CRP complex (Fig. 6 and 7). This site is from positions −106 to −85 (centered at position −95.5) with respect to its transcription start site, which is considered an activation site for class III CRP-dependent promoters such as the araBAD promoter (33). Class III promoters have been reported to require a secondary regulator protein (e.g., the AraC protein for the araBAD promoter) for maximal induction. Regulation at class III promoters was also reported to involve the formation of a DNA loop (21).
Exponential-phase cells growing in LBS medium contained higher cAMP contents (Table 2), possibly due to a lowered expression or reduced activity of CpdA during this growth phase. Thus, it is required to search and identify another regulator for cpdA transcription and its possible interaction with the cAMP-CRP complex for optimal cpdA expression in V. vulnificus, as shown in the araBAD promoter (33). It is also possible that the presence of other factors acting at the posttranscriptional level finely adjusts the CpdA protein content or its enzymatic activity.
Both the Northern blot and primer extension experiments clearly suggest that the cpdA gene is organized as an operon with its upstream flanking genes mutT and yqiB as well as its downstream gene yqiA (Fig. 1 and 2). MutT is the nucleoside triphosphate pyrophosphohydrolase that catalyzes a conversion of deoxynucleoside triphosphate to deoxynucleoside monophosphate (3). The predicted gene products of yqiB and yqiA have homology to a hypothetical phosphohydrolase from Vibrio parahaemolyticus (GenBank accession no. ZP_01993039.1) and a hypothetical esterase from Yersinia pestis (GenBank accession no. NP_404305.1), respectively. Their functions, however, have not been determined. Thus, it is currently not known why the cpdA gene, which encodes an enzyme to catalyze the conversion of cAMP to AMP, is coregulated and coexpressed with the other three genes, although the gene products from this operon might be involved in the metabolism of nucleotides to produce nucleoside monophosphates or to remove undesired nucleotides. Further study is needed to elucidate their biological significance.
Cyclic phosphodiesterases have been shown to be involved in the stress response of M. xanthus against temperature and osmotic shocks (18). The ΔcpdA mutant V. vulnificus strain shows additional phenotypes. For example, scanning electron microscopy of wild-type and ΔcpdA mutant V. vulnificus strains revealed that the cpdA gene product is required for normal cellular morphology (see Fig. S2 in the supplemental material). The elongated morphology of the ΔcpdA mutant might result from altered expression of the genes involved in cell division and/or cell shape caused by a failure to adjust cAMP levels (4, 5). In addition, proteomic screening of V. vulnificus proteins required for mature biofilm formation found many proteins whose levels of expression are expected to be regulated by the cAMP-CRP complex (17). These results imply that deficiency in functional CpdA results in pleiotropic effects on the pathogenic bacterium V. vulnificus. According to data described previously by Merrell et al. (24), cpdA is one of the most important colonization factors in V. cholerae. Therefore, it will be interesting to investigate the roles of CpdA in the pathogenesis of V. vulnificus.
Supplementary Material
Acknowledgments
This study was supported by a grant from KOSEF (R01-2007-000-10159-0 to K.-H.L.) and partly by the Gyeonggi Regional Research Center program of Gyeonggi province (Protein Research Center for Bio-Industry) (to K.-H.L.), Republic of Korea.
We thank Songhee H. Kim for isolating plasmid pINE45 and Hyuk-Soon Ihm for isolating plasmid pINE45-1.
Footnotes
Published ahead of print on 21 November 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alper, M. D., and B. N. Ames. 1975. Cyclic 3′,5′-adenosine monophosphate phosphodiesterase mutants of Salmonella typhimurium. J. Bacteriol. 1221081-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang, Y. B., S. E. Lee, J. H. Rhee, and S. H. Choi. 1999. Evidence that expression of the Vibrio vulnificus hemolysin gene is dependent on cyclic AMP receptor protein. J. Bacteriol. 1817639-7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessman, M. J., D. N. Frick, and S. F. O'Handley. 1996. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 27125059-25062. [DOI] [PubMed] [Google Scholar]
- 4.Botsford, J. L. 1984. Cyclic AMP phosphodiesterase in Salmonella typhimurium: characteristics and physiological function. J. Bacteriol. 160826-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botsford, J. L., and J. G. Harman. 1992. Cyclic AMP in prokaryotes. Microbiol. Rev. 56100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busby, S., and R. H. Ebright. 1999. Transcriptional activation by catabolite activator protein (CAP). J. Mol. Biol. 293199-213. [DOI] [PubMed] [Google Scholar]
- 7.Catanese, C. A., D. W. Emerich, and W. L. Zahler. 1989. Adenylate cyclase and cyclic AMP phosphodiesterase in Bradyrhizobium japonicum bacteroids. J. Bacteriol. 1714531-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, H. K., N. Y. Park, D.-I. Kim, H. J. Chung, S. Ryu, and S. H. Choi. 2002. Promoter analysis and regulatory characteristics of vvhBA encoding cytolytic hemolysin of Vibrio vulnificus. J. Biol. Chem. 27747292-47299. [DOI] [PubMed] [Google Scholar]
- 9.de Lorenzo, V., M. Herrero, F. Giovannini, and J. B. Neilands. 1988. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur. J. Biochem. 173537-546. [DOI] [PubMed] [Google Scholar]
- 10.Gorke, B., and J. Stulke. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6613-624. [DOI] [PubMed] [Google Scholar]
- 11.Gottesman, S. 1984. Bacterial regulation: global regulatory networks. Annu. Rev. Genet. 18415-441. [DOI] [PubMed] [Google Scholar]
- 12.Imamura, R., K. Yamanaka, T. Ogura, S. Hiraga, N. Fujita, A. Ishihama, and H. Niki. 1996. Identification of the cpdA gene encoding cyclic 3′,5′-adenosine monophosphate phosphodiesterase in Escherichia coli. J. Biol. Chem. 27125423-25429. [DOI] [PubMed] [Google Scholar]
- 13.Ishizuka, H., A. Hanamura, T. Kunimura, and H. Aiba. 1993. A lowered concentration of cAMP receptor protein caused by glucose is an important determinant for catabolite repression in Escherichia coli. Mol. Microbiol. 10341-350. [DOI] [PubMed] [Google Scholar]
- 14.Jeong, H. S., K. C. Jeong, H. K. Choi, K.-J. Park, K.-H. Lee, J. H. Rhee, and S. H. Choi. 2001. Differential expression of Vibrio vulnificus elastase gene in a growth phase-dependent manner by two different types of promoters. J. Biol. Chem. 27613875-13880. [DOI] [PubMed] [Google Scholar]
- 15.Jeong, H. S., M. H. Lee, K.-H. Lee, S.-J. Park, and S. H. Choi. 2003. SmcR and cyclic AMP receptor protein coactivate Vibrio vulnificus vvpE encoding elastase through the RpoS-dependent promoter in a synergistic manner. J. Biol. Chem. 27845072-45081. [DOI] [PubMed] [Google Scholar]
- 16.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmid for DNA cloning in gram-negative bacteria. Gene 70191-197. [DOI] [PubMed] [Google Scholar]
- 17.Kim, H.-S., M.-A. Lee, S.-J. Chun, S.-J. Park, and K.-H. Lee. 2007. Role of NtrC in biofilm formation via controlling expression of the gene encoding an ADP-glycero-manno-heptose-6-epimerase in the pathogenic bacterium, Vibrio vulnificus. Mol. Microbiol. 63559-574. [DOI] [PubMed] [Google Scholar]
- 18.Kimura, Y., H. Nakatuma, N. Sato, and M. Ohtani. 2006. Contribution of the cyclic nucleotide phosphodiesterases PdeA and PdeB to adaptation of Myxococcus xanthus cells to osmotic or high-temperature stress. J. Bacteriol. 188823-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolb, A., S. Busby, H. Buc, S. Garges, and S. Adhya. 1993. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62749-795. [DOI] [PubMed] [Google Scholar]
- 20.Lee, H.-J. S.-J. Park, S. H. Choi, and K.-H. Lee. 2008. Vibrio vulnificus rpoS expression is repressed by direct binding of cAMP-CRP complex to its two promoter regions. J. Biol. Chem. 28330438-30450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobell, R. B., and R. F. Schleif. 1991. AraC-DNA looping: orientation and distance-dependent loop breaking by the cyclic AMP receptor protein. J. Mol. Biol. 21845-54. [DOI] [PubMed] [Google Scholar]
- 22.Ma, Z., H. Richard, and J. W. Foster. 2003. pH-dependent modulation of cyclic AMP levels and GadW-dependent repression of RpoS affect synthesis of the GadX regulator and Escherichia coli acid resistance. J. Bacteriol. 1856852-6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macfadyen, L. P., C. Ma, and R. J. Redfield. 1998. A 3′,5′ cyclic AMP (cAMP) phosphodiesterase modulates cAMP levels and optimizes competence in Haemophilus influenzae Rd. J. Bacteriol. 1804401-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merrell, D. S., D. L. Hava, and A. Camilli. 2002. Identification of novel factors involved in colonization and acid tolerance of Vibrio cholerae. Mol. Microbiol. 431471-1491. [DOI] [PubMed] [Google Scholar]
- 25.Oliver, J. D., F. Hite, D. McDougald, N. L. Andon, and L. M. Simpson. 1995. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl. Environ. Microbiol. 612624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, K.-J., M.-J. Kang, S. H. Kim, H.-J. Lee, J.-K. Lim, S. H. Choi, S.-J. Park, and K.-H. Lee. 2004. Isolation and characterization of rpoS in a pathogenic bacterium, Vibrio vulnificus: role of σS in survival of exponential phase cells under oxidative stress. J. Bacteriol. 1863304-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roh, J.-B., M.-A. Lee, H.-J. Lee, S.-M. Kim, Y. Cho, Y.-J. Kim, Y.-J. Seok, S.-J. Park, and K.-H. Lee. 2006. Transcriptional regulatory cascade for elastase production in Vibrio vulnificus: LuxO activates luxT expression and LuxT represses smcR expression. J. Biol. Chem. 28134775-34784. [DOI] [PubMed] [Google Scholar]
- 28.Saier, M. H., B. U. Feucht, Jr., and M. T. McCaman. 1975. Regulation of intracellular adenosine cyclic 3′:5′-monophosphate levels in Escherichia coli and Salmonella typhimurium. Evidence for energy-dependent excretion of the cyclic nucleotide. J. Biol. Chem. 2507593-7601. [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 31.Skorupski, K., and R. K. Taylor. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 94265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2177-188. [DOI] [PubMed] [Google Scholar]
- 33.Wagner, R. 2000. Transcription regulation in prokaryotes. Oxford University Press Inc., New York, NY.
- 34.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33103-119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.