Abstract
Sulfide:quinone oxidoreductase (SQR) catalyzes sulfide oxidation during sulfide-dependent chemo- and phototrophic growth in bacteria. The green sulfur bacterium Chlorobaculum tepidum (formerly Chlorobium tepidum) can grow on sulfide as the sole electron donor and sulfur source. C. tepidum contains genes encoding three SQR homologs: CT0117, CT0876, and CT1087. This study examined which, if any, of the SQR homologs possess sulfide-dependent ubiquinone reduction activity and are required for growth on sulfide. In contrast to CT0117 and CT0876, transcripts of CT1087 were detected only when cells actively oxidized sulfide. Mutation of CT0117 or CT1087 in C. tepidum decreased SQR activity in membrane fractions, and the CT1087 mutant could not grow with ≥6 mM sulfide. Mutation of both CT0117 and CT1087 in C. tepidum completely abolished SQR activity, and the double mutant failed to grow with ≥4 mM sulfide. A C-terminal His6-tagged CT1087 protein was membrane localized, as was SQR activity. Epitope-tagged CT1087 was detected only when sulfide was actively consumed by cells. Recombinantly produced CT1087 and CT0117 proteins had SQR activity, while CT0876 did not. In summary, we conclude that, under the conditions tested, both CT0117 and CT1087 function as SQR proteins in C. tepidum. CT0876 may support the growth of C. tepidum at low sulfide concentrations, but no evidence was found for SQR activity associated with this protein.
Many bacteria can utilize sulfide at micro- to millimolar concentrations as an electron donor. Sulfide oxidation can be catalyzed by the enzyme sulfide:quinone oxidoreductase (SQR) (20, 52, 56) or flavocytochrome c (FCC; also known as flavocytochrome c sulfide dehydrogenase) (9, 43). Many phototrophic bacteria contain genes that encode both enzymes, and the most recent models of sulfur oxidation in both the green sulfur and purple sulfur bacteria indicate that these enzymes are alternate routes that result in the production of either polysulfide (green sulfur) or protein-encapsulated elemental sulfur globules (purple sulfur) in the periplasm (10, 16, 17). SQR donates electrons from sulfide to the electron transport chain at the level of quinone, upstream of the cytochrome b/c1 complex (menaquinol:cytochrome c oxidoreductase), while FCC donates electrons at the level of cytochrome c (41). Theoretically, the energy yield should be greater for organisms utilizing SQR than for those utilizing FCC, because proton motive force is generated when electrons are passed through the b/c1 complex en route to the reaction center (41). Oxidation of sulfur/polysulfides produced by sulfide oxidation in purple sulfur bacteria requires the action of gene products encoded by the dissimilatory sulfite oxidoreductase (Dsr) gene cluster (10-12). In green sulfur bacteria, the Dsr system has been proposed to be involved in elemental sulfur oxidation, but this has not been experimentally demonstrated as yet (16, 17).
For bacteria, evidence suggests that SQR is more important than FCC for chemo- and phototrophic sulfide oxidation. First, sequence analyses indicate that homologs of FCC seem to be confined to autotrophs that can utilize thiosulfate in addition to sulfide as an energy source (17, 20, 56). In these organisms, in vitro FCC activity is found in the SoxF protein (42), whose gene is part of a large cluster encoding the entire sulfur-oxidizing (Sox) enzyme system (14, 15). SoxF enhances thiosulfate and sulfide oxidation activity in vivo (3) but inhibits in vitro sulfide oxidation via the reconstituted Sox complex (44). In contrast, homologs of SQR are widely distributed in bacteria, and similar proteins have been identified in the archaea (17, 20, 56) and eukaryotes (21, 33, 48). Second, mutation of the fccAB genes in the phototrophic purple sulfur bacterium Allochromatium vinosum did not inhibit its ability to oxidize sulfide and grow photolithoautotrophically, though the specific growth rates and biomass yields were not reported (43). A. vinosum also contains SQR and the Dsr and Sox systems. Third, in the chemolithotrophic sulfur oxidizer Acidithiobacillus ferrooxidans NASF-1, sqr transcripts were threefold more abundant in sulfide-grown than in iron-grown cells (60). Finally, sulfide oxidation activities directly linked to energy production or detoxification have been demonstrated with the purified SQR proteins from the proteobacterium Rhodobacter capsulatus (46, 47, 51) and the cyanobacteria Oscillatoria limnetica and Aphanothece halophytica (1, 4, 49). R. capsulatus contains neither the Dsr nor the Sox sulfur oxidation system, and while genomic information is not available for O. limnetica and A. halophytica, other cyanobacterial genome sequences (28, 35, 39, 40) do not contain the Dsr or Sox systems.
Here we report the functional analysis of multiple SQR homologs encoded by CT0117, CT0876, and CT1087 from the green sulfur phototrophic bacterium Chlorobaculum tepidum (27) (formerly Chlorobium tepidum [59]). With the exception of Chlorobium ferrooxidans (25), all green sulfur bacteria can utilize sulfide as an electron donor to support growth, and all green sulfur bacterial genome sequences encode at least one SQR homolog, including C. ferroxidans (16, 17). Biochemically, Chlorobium limicola forma thiosulfatophilum membranes have been shown to catalyze electron transfer from sulfide to plastoquinone in the dark (50). On the basis of sequence comparisons, CT0117 has been proposed to be the bona fide SQR in C. tepidum, while CT0876 and CT1087 have been labeled SQR-like-proteins (SQRLPs) (16), and it is unclear whether these gene products contribute to SQR activity in C. tepidum.
Recently, we reported that a C. tepidum mutant strain (C5, ΔCT0867-CT0876::TnOGm) in which the 5′ half of the SQR homolog, carrying the CT0876 gene, was replaced with a transposon insertion oxidized sulfide normally and grew well with sulfide as the sole electron donor (8). In the experiments reported here, we sought to define the roles of the three C. tepidum SQR homologs more precisely. The results indicate that either CT0117 or CT1087 is required for sulfide-dependent growth at >2 mM sulfide, while CT1087 is required for growth above 4 mM sulfide. Both proteins displayed SQR activity in C. tepidum and when produced recombinantly with a standard activity assay, clearly indicating that CT1087 is not a SQRLP but a bona fide SQR. Our results did not rule out a requirement for CT0876 for sulfide-dependent growth at concentrations less than or equal to 2 mM, but no evidence was found that CT0876 contributes to SQR activity in C. tepidum or that the recombinant protein possesses SQR activity, suggesting that CT0876 may indeed be a SQRLP.
MATERIALS AND METHODS
Bacterial growth conditions and media.
Bacterial strains, plasmids, and antibiotics are listed in Table 1. The Escherichia coli cloning strain TOP10 (Invitrogen, Carlsbad, CA) was grown in Luria-Bertani (LB) medium at 37°C (2). Strain BL21λDE3/pLysS (Novagen, San Diego, CA), used for the recombinant expression of SQR homologs, was grown anaerobically at 37°C in LB medium supplemented with 0.4% (wt/vol) glucose, 20 mM morpholinepropanesulfonic acid (MOPS), and 25 mM NaNO3 (pH 7.8). For anaerobic culture, stopper-sealed 25-ml serum vials were filled with 10 ml of medium and were pressurized under a 95% N2-5% CO2 atmosphere. For larger volumes, 150-ml bottles filled with 100 ml medium were used. For anaerobic growth on plates, LB plates inoculated with E. coli cells were incubated at 37°C in sealed anaerobic jars. Growth of E. coli in liquid medium was routinely monitored by measuring the optical density at 600 nm (OD600).
TABLE 1.
Bacterial strains, plasmids, and antibiotic markers
| Strain or plasmid | Characteristic(s) | Antibiotica | Source or reference |
|---|---|---|---|
| Strains | |||
| C. tepidum | |||
| WT2321 | Wild type | None | 59 |
| CT0117::TnOGm | TnOGm insertion in CT0117 | Gm | This study |
| CT0876::TnOGm | TnOGm insertion in CT0876 | Gm | This study |
| CT1087::TnOGm | TnOGm insertion in CT1087 | Gm | This study |
| CT0117::TnOGm, CT1087::TnEm | TnOGm and TnEm insertions in CT0117 and CT1087, respectively | Gm, Em | This study |
| CT1087-His6::aacC1 | Encodes CT1087 protein with C-terminal His6 extension, followed by the insertion of an aacC1 gene downstream of the CT1087 coding sequence | Gm | This study |
| E. coli strains | |||
| TOP10 | General cloning strain; F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araΔ139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | None | Invitrogen |
| BL21λDE3/pLysS | Recombinant protein expression strain; ompT hsdSB(rB− mB−) gal dcm | Cm | Novagen |
| Plasmids | |||
| pCR2.1 | Cloning vector | Km | Invitrogen |
| pET16b | T7 expression vector, N-terminal His10 tag | Car | Novagen |
| pET28b | T7 expression vector, N- and C-terminal His6 tags | Km | Novagen |
| pET28b-CT0117 | CT0117 cloned into EcoRI-HindII sites | Km, Cm | This study |
| pET16b-CT0876 | CT0876 cloned into NdeI-BamHI sites | Car, Cm | This study |
| pET16b-CT1087 | CT1087 cloned into NdeI-BamHI sites | Car, Cm | This study |
Gm, gentamicin (4 μg ml−1 for liquid medium and 8 μg ml−1 for plates); Em, erythromycin (for C. tepidum, 5 μg ml−1 for liquid medium and 10 μg ml−1 for plates; for E. coli, 20 μg ml−1, used to construct a plasmid to create CT0117::TnOGm and CT1087::TnEm); Cm, chloramphenicol (20 μg ml−1); Km, kanamycin (50 μg ml−1); Car, carbenicillin (50 μg ml−1).
C. tepidum was grown in liquid Pf-7-BTP, a mineral medium with no supplements, or on CP solid medium as previously described (8, 24, 59). Stationary-phase cultures (∼30 h of growth; ∼120 μg of protein ml−1 and ∼40 μg of bacteriochlorphyll c ml−1) of the wild-type and mutant strains were pelleted by centrifugation, washed with sulfur-free Pf-7-BTP medium, and incubated overnight in sulfur-free medium before being inoculated to a density of 0.5 μg of bacteriochlorophyll c ml−1, which corresponds to ∼4 μg of protein ml−1, in a medium with specific electron donors for physiological and growth experiments (8). Since the antibiotic resistance conferred by the transposon TnOGm is temperature sensitive (6), all growth results described here are from cultures grown in the absence of antibiotic selection in well-buffered medium (8). Cultures of mutant strains were routinely screened by PCR and reverse transcriptase PCR (RT-PCR) with gene-specific primers (see Table S1 in the supplemental material) to ensure that no reversion of mutagenized genes had occurred. The growth of C. tepidum was monitored by measuring total cellular protein with the Bradford microassay (38).
Nucleic acid purification, PCR, and sequencing.
Primers for all PCRs and RT-PCR amplification and sequencing reactions are listed in Table S1 in the supplemental material. DNA was purified using the genomic DNA extraction kit from either Fermentas (Glen Burnie, MD) or Qiagen (Valencia, CA). PCR was conducted with the FailSafe PCR system (Epicentre, Madison, WI) or SuperMix high-fidelity PCR mix (Invitrogen) under standard conditions. Plasmid DNA was isolated with the Qiaprep miniprep kit (Qiagen). Reagents and T4 ligase for DNA ligation were from the LigaFast rapid DNA ligation system (Promega, Madison, WI). RNA was purified with the NuceloSpin RNA purification kit from Macherey-Nagel (Bethlehem, PA), and trace DNA contamination was removed by treating samples with the Turbo DNA-free kit (Ambion, Austin, TX). RT-PCR was performed with Epicentre's high-fidelity RT-PCR kit according to the manufacturer's instructions. When needed, PCR products or plasmids were sequenced by standard procedures at the College of Agriculture and Natural Resources DNA Sequencing Facility at the University of Delaware, Newark.
IVTM of SQR-encoding genes in C. tepidum.
In vitro transposition mutagenesis (IVTM) of C. tepidum was performed as previously described (8). PCR products carrying CT0117, CT0876, and CT1087 were amplified from C. tepidum wild-type genomic DNA with target-specific primers (see Table S1 in the supplemental material) and inserted into the pCR2.1 vector (Invitrogen), which was used to transform E. coli TOP 10 electrocompetent cells (Invitrogen). Positive transformants were selected with 50 μg kanamycin ml−1 on LB plates. Purified plasmids were used for transposition reactions with the EZ::TN transposase (Epicentre) and transposon TnOGm, generated by PCR from pTnModOGm (13) using primers GmMEori-F and GmMEori-R (see Table S1 in the supplemental material) (8). TnOGm-mutagenized plasmids were recovered in E. coli by selection with 50 μg kanamycin ml−1 and 20 μg gentamicin ml−1 on LB plates. Plasmids carrying the mutagenized SQR-encoding genes were identified by colony PCR. Purified plasmid DNA was used to transform C. tepidum as described previously, with selection for C. tepidum strains carrying TnOGm at 8 μg gentamicin ml−1 on CP plates (8). Genomic DNA was purified from the densely grown cultures, and the precise positions of transposon insertions were determined by sequencing of the CT0117, CT0876, or CT1087 PCR products with primer TnOGm-Seq-L (see Table S1 in the supplemental material). Three C. tepidum strains—CT0117::TnOGm, CT0876::TnOGm, and CT1087::TnOGm—were constructed by this method.
The C. tepidum double-mutant strain CT0117::TnOGm, CT1087::TnEm was constructed similarly. A transposon (TnEm) carrying the erythromycin resistance marker from plasmid pRL21 (5) was PCR amplified with primers Em-ME-F and Em-ME-R (see Table S1 in the supplemental material). Transposase and TnEm were used to construct an E. coli TOP10 strain carrying mutagenized CT1087 in pCR2.1 by selection with 20 μg erythromycin ml−1. The purified plasmid was then used to transform C. tepidum strain CT0117::TnOGm. Positive transformants containing both mutations were selected with 8 μg gentamicin ml−1 and 10 μg erythromycin ml−1 on plates and were subsequently grown with 4 μg gentamicin ml−1 and 5 μg erythromycin ml−1 in liquid Pf-7-BTP medium.
Epitope tagging of CT1087 in C. tepidum.
A C. tepidum strain expressing CT1087-His6 from the native genomic location of CT1087 (strain CT1087-His6::aacC1) was constructed according to the method of Morgan-Kiss and Cronan (36). Two PCR products, each carrying a unique restriction site, were generated by using C. tepidum wild-type genomic DNA as the template with primers CT1087-His-F and CT1087-His-R (see Table S1 in the supplemental material). The first PCR product contains sequences downstream of the 3′ end of the CT1087 gene, and the second amplification product carries homology to sequences upstream of the 5′ end of the CT1087 gene. A stretch of nucleotides, (CAT)6, was incorporated into primer CT1087-His-R (see Table S1 in the supplemental material), resulting in the addition of a C-terminal His6 tag to the protein product. The PCR products were treated with restriction endonucleases EcoRI and Acc65I and then ligated to the gentamicin resistance marker gene aacC1 in the EcoRI- and Acc65I-treated vector pCM351, and the ligation was used to transform E. coli TOP10. Positive transformants were selected with 20 μg gentamicin ml−1 and 50 μg kanamycin ml−1 in LB plates. Plasmids containing the correct product were purified and transformed into C. tepidum, followed by gentamicin selection as described above for constructing the gentamicin-resistant IVTM mutants. The accuracy of the nucleotide sequence at the CT1087 locus in C. tepidum CT1087-His6::aacC1 was verified by sequencing the PCR product amplified with primers CT1087-His-F and pCM351-Gm-R (see Table S1 in the supplemental material).
Quantitative RT-PCR (Q-RT-PCR) of SQR and FccB homologs.
Cultures of wild-type C. tepidum and strain CT0117::TnOGm, CT1087::TnEm were grown with 0.7 mM sulfide and 9.2 mM thiosulfate, and total RNA was purified 15 h after inoculation. Reactions were performed on a Mastercycler ep realplex (Eppendorf) system. cDNAs were generated as described above with gene-specific reverse primers and were added to a reaction mixture containing both forward and reverse primers and realMasterMix with SYBR green (2.5×; Eppendorf). A control reaction lacking cDNA was performed for each primer set, and all reactions were performed in triplicate with RNA samples from independent cultures. Amplified products were confirmed by melting-curve analysis and gel electrophoresis. The expression levels of each gene were analyzed by the ΔΔCT method (32) using sigA (CT1551) as the reference gene. The threshold cycles (CT) for sigA were found to be similar throughout the growth of C. tepidum cultures and when the wild type and double mutant were compared, indicating that sigA was expressed at a constant level.
Recombinant expression of C. tepidum SQRs in E. coli.
C. tepidum genes encoding SQR homologs were produced recombinantly by expression from pET-based plasmids in E. coli BL21λDE3/pLysS (Novagen). PCR products containing genes with unique restriction sites were amplified from C. tepidum wild-type genomic DNA, digested with restriction endonucleases, and ligated to the T7 expression vector pET16b or pET28b (Novagen). Clones were recovered in E. coli TOP10 cells, and the insert was completely sequenced prior to the introduction of the clones into E. coli BL21λDE3/pLysS. All recombinant SQR clones carried His tags, which were provided by the pET vectors. For induction of the recombinant proteins, E. coli cultures were inoculated to an OD600 of 0.01 and grown under anaerobic conditions at 37°C for 4 h (OD600, ∼0.3); then 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added, and 1 h later, sulfide was added to a final concentration of 5 mM. Cells were harvested 20 h after sulfide addition.
Subcellular fractionation.
E. coli cells expressing recombinant SQR proteins and C. tepidum strain CT1087-His6::aacC1 were pelleted, resuspended in anaerobic 100 mM Tris-HCl (pH 7.0), and lysed by sonication using a model 450 Sonifier equipped with a microtip probe (Branson Ultrasonics, Danbury, CT), housed in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI). The power setting was 3, with a duty cycle of 30%, and cells were sonicated for two periods of 2 min each, with cooling on ice between each sonication. Unbroken cells (and inclusion bodies in E. coli strains) were removed by low-speed (∼17,900 × g) centrifugation. Membrane and cytosolic fractions were separated by ultracentrifugation at 126,000 × g for 60 min. In C. tepidum, the membrane pellet also contains the chlorosome, the unique light-harvesting complex (19, 26). When needed, C. tepidum chlorosome-depleted membranes were prepared by sucrose gradient density ultracentrifugation (7, 57). The membrane fraction was further treated with the surfactant Nonidet P-40 and centrifuged at 126,000 × g for 60 min (7, 53). The Nonidet P-40 pellet is enriched in outer membrane and peripherally bound inner membrane proteins, whereas the Nonidet P-40-soluble fraction is enriched in integral membrane proteins (7).
SDS-PAGE and immunoblotting.
Equal amounts of protein from purified subcellular fractions or proteins extracted from intact cells by boiling in 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer were separated by SDS-PAGE on acrylamide gels (15% resolving, 4% stacking, 6 mM urea) according to the method of Laemmli (31). When needed, proteins were visualized by staining with BioSafe Coomassie dye (Bio-Rad, Hercules, CA). For immunoblotting, proteins separated on the acrylamide gel were transferred to polyvinylidene difluoride membranes (Bio-Rad) with 25 mM Tris, 192 mM glycine, 20% (vol/vol) methanol, and 0.5% (wt/vol) SDS at 100 V for 2 h at 4°C with continuous buffer stirring. Polyvinylidene difluoride blots were incubated in the primary antibody (mouse anti-penta-His [α-penta-His]; Qiagen) and secondary antibody (alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G; Bio-Rad) in Tris-buffered saline and 5% (wt/vol) nonfat milk (10× Tris-buffered saline is 0.2 M Tris-HCl [pH 7.5] and 1.5 M NaCl). Blots were incubated with ECF chemifluorescence substrate (GE Healthcare Life Sciences, Piscataway, NJ) for 5 min in the dark in order to detect bound alkaline phosphatase-conjugated immunoglobulin G on a Typhoon 8600 scanner (GE Healthcare Life Sciences) with 532-nm excitation and 526-nm emission wavelengths. Band intensities from SDS-PAGE or immunoblots were quantified with Scion Image (Scion, Frederick, MD) according to the manufacturer's instructions.
SQR activity assay.
Sulfide-dependent decyl-ubiquinone (dUQ) reduction activity was measured under anaerobic conditions in N2-flushed quartz cuvettes with septum screw caps (Starna Cells, Atascadero, CA) as previously described (49-51) with minor modifications. The typical assay mixture was prepared in the anaerobic chamber and consisted of 10 mM Tris-HCl (pH 7.4), 20 μg membrane protein, and 100 μM dUQ (Sigma, St. Louis, MO). Assays were performed at 47°C (for C. tepidum) or 37°C and 47°C (for E. coli) in a Peltier temperature-controlled cuvette holder attached to a DU7400 spectrophotometer (Beckman Coulter, Fullerton, CA). SQR activity was determined by calculating the difference between A294 and A284, which represent the maximum absorbances of oxidized and reduced dUQ, respectively. The absorbance of the assay mixture was measured immediately after dUQ addition, and the reaction was initiated by adding sulfide to a final concentration of 1 mM or 0.5 mM from a sterile, neutralized, anoxic stock solution of Na2S·9H2O. All C. tepidum SQR activities were corrected for nonenzymatic dUQ reduction, measured with an appropriate boiled membrane fraction. Recombinant SQR activities were corrected with membrane fractions prepared from empty-vector control strains. An extinction coefficient for dUQ of 15 (mmol cm)−1, measured by Morton (37), was used to convert absorbance to molar concentrations.
Amino acid sequence analysis and comparison.
SQR and FCC sequences were identified by BLASTP (http://www.ncbi.nih.gov/BLAST) with C. tepidum SQR homolog sequences as queries. A neighbor-joining (45) phylogram of selected sequences was constructed with tools in MEGA 4 (55). SQR and FCC sequences were classified as previously described (20, 48, 56).
RESULTS
RT-PCR analysis of C. tepidum sqr homologs.
To investigate if C. tepidum might regulate the SQR homologs encoded by CT0117, CT0876, and CT1087, RT-PCR was performed on total RNAs isolated at different times during growth in medium containing both 0.7 mM sulfide and 9.2 mM thiosulfate (Fig. 1a). As previously described in detail (7, 8), C. tepidum oxidizes sulfide during early growth (≤15 h) and produces extracellularly deposited elemental sulfur. After sulfide is depleted, elemental sulfur is consumed (15 to 24 h), followed by the oxidation of thiosulfate (24 to 48 h). Sulfate is produced as the end product of sulfur metabolism. While the transcripts of both CT0117 and CT0876 were found at all times, the transcript of CT1087 was observed only at early time points during sulfide oxidation and elemental sulfur production (Fig. 1a).
FIG. 1.
RT-PCR analysis of gene transcripts with total RNA harvested from wild-type C. tepidum either at the indicated time when the organism was grown in medium containing 0.7 mM sulfide and 9.2 mM thiosulfate (a) or at 15 h in medium with sulfide or thiosulfate as the sole electron donor at the indicated concentration (b). The gene encoding the major housekeeping sigma factor (CT1551, or sigA) was used as a control gene that was not expected to be regulated under these conditions. Negative-control reactions omitted reverse transcriptase (RT −).
RT-PCR was also performed on total RNA harvested at ∼15 h from wild-type C. tepidum grown in medium supplemented with 4, 6, or 8 mM sulfide or 10, 20, 30, or 50 mM thiosulfate as the sole electron donor (Fig. 1b). Transcripts of CT0117 and CT0876 were observed at all concentrations of sulfide and thiosulfate (Fig. 1b). The CT1087 transcript was readily detected in sulfide-grown cultures but was much lower in abundance in cultures supplemented with thiosulfate alone (Fig. 1b). The mRNA of sigA (CT1551), encoding the major housekeeping sigma factor of RNA polymerase (22, 23), which is not expected to be regulated by sulfide, was observed under all growth conditions (Fig. 1a and b).
Genetics and physiology of C. tepidum mutant strains lacking SQR homologs.
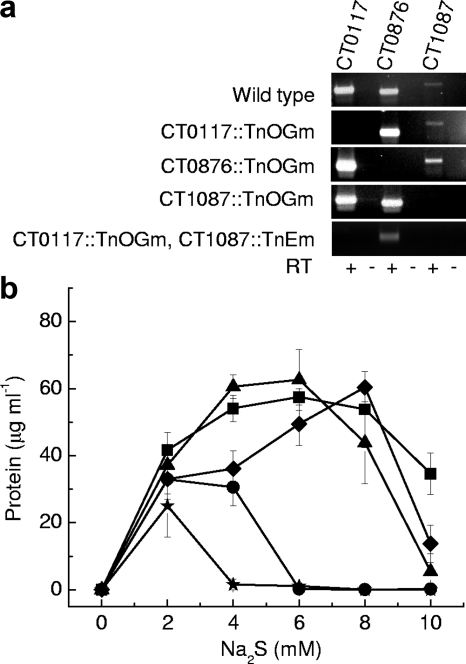
To determine their physiological roles in C. tepidum, the SQR-encoding homologs CT0117, CT0876, and CT1087 were individually inactivated by IVTM with the transposon TnOGm. A double-mutant strain carrying TnOGm and TnEm insertions in CT0117 and CT1087, respectively, was also constructed. Transcripts of each gene were assayed in the wild-type and mutant strains by RT-PCR using total RNA isolated at ∼15 h from cultures grown in medium containing both sulfide and thiosulfate (Fig. 2a). As expected, transcripts for each SQR homolog were found in the wild type, while the correct mRNA was lacking in each mutant strain, without any obvious effects on the mRNAs of the other putative SQR-encoding genes (Fig. 2a).
FIG. 2.
Genetics and physiology of wild-type and mutant C. tepidum strains. (a) RT-PCR analysis of gene transcripts using total RNA prepared at ∼15 h from cultures grown with 0.7 mM sulfide and 9.2 mM thiosulfate. Negative-control reactions omitted reverse transcriptase (RT −). (b) Protein yield measured at 48 h postinoculation in cultures grown with sulfide at varying concentrations as the sole electron donor. Data are averages and standard deviations (error bars) for three or more independent replicates. Symbols: ♦, wild type; ▪, CT0117::TnOGm; ▴, CT0876::TnOGm; •, CT1087::TnOGm; ★, CT0117::TnOGm, CT1087::TnEm.
All C. tepidum strains were analyzed for their abilities to grow with sulfide as the sole electron donor at concentrations ranging from 2 to 10 mM by determining the growth yield at 48 h after inoculation. Wild-type C. tepidum had a maximum growth yield with 6 to 8 mM sulfide, but growth was strongly inhibited with 10 mM sulfide (Fig. 2b). In the range of 2 to 8 mM sulfide, the wild type displayed a linear increase in the growth yield of 9 g protein (mol sulfide)−1 (linear regression [R2] = 0.96). This yield for C. tepidum is somewhat lower than the yield of 14 g protein (mol sulfide)−1 calculated from sulfide-dependent cell production data for the green sulfur bacterium Prosthecochloris aestuarii (54) and the yield of 13 g protein (mol sulfide)−1 calculated from C. tepidum growth on sulfide (38). This decrease likely reflects differences between the simple batch tube culture system employed here and the elaborate bioreactor systems utilized in the other studies.
The growth yields of mutant strains CT0117::TnOGm and CT0876::TnOGm were similar to that of the wild type, except that the former strains had a ∼40% yield increase relative to the wild type with 4 mM sulfide (Fig. 2b). These mutant strains also displayed maximal growth yield with 6 to 8 mM sulfide (Fig. 2b). In contrast, strain CT1087::TnOGm failed to grow at ≥6 mM sulfide and displayed a 25% growth yield reduction with 4 mM sulfide (Fig. 2b). The double-mutant strain CT0117::TnOGm, CT1087::TnEm failed to grow with ≥4 mM sulfide, and its growth yield was 35% lower than that of the wild type with 2 mM sulfide (Fig. 2b).
Between 2 and 8 mM sulfide, the wild type and mutants had equivalent doubling times of about 2 h, in agreement with previously published studies (8, 24, 59). These data indicate that in this range, the sulfide concentration affects the growth yield, but not the growth rate, of C. tepidum. In addition, wild-type C. tepidum and all mutant strains displayed similar growth yields of 99 to 110 μg of protein ml−1 after 48 h of growth with 10 mM thiosulfate, indicating that the growth yield defects observed for the mutant strains are limited to growth on sulfide alone.
SQR activity in wild-type and mutant C. tepidum strains.
Prior studies demonstrated that the membrane fraction of C. limicola forma thiosulfatophilum catalyzed sulfide oxidation with ubiquinone as the electron acceptor (50), so a similar assay was used to determine if mutations in the putative SQR-encoding genes affected this ability in C. tepidum.
Under anaerobic conditions, freshly prepared C. tepidum membrane fractions readily catalyzed sulfide oxidation with dUQ as an electron acceptor, but this activity was not found in the cytosolic protein fraction. SQR activity could be eliminated by boiling or exposing the membrane fraction to air and was also decreased >30% if membrane fractions were frozen prior to the assay. SQR activity measured with 20 μg of freshly prepared C. tepidum wild-type membranes and 100 μM dUQ saturated at a concentration of 1 mM sulfide.
With 1 mM sulfide, wild-type C. tepidum membranes displayed an SQR activity of 87 ± 7 μmol of dUQ reduced (mg of protein)−1 min−1 (Table 2). Mutant strains CT0117::TnOGm and CT1087::TnOGm displayed 64% ± 5% and 39% ± 7% reductions in SQR activity from that of the wild type (Table 2). With 0.5 mM sulfide, the SQR activities of strains CT0117::TnOGm and CT1087::TnOGm were reduced by 88% ± 4% and 28% ± 13%, respectively, from that of the wild type (Table 2). Strain CT0876::TnOGm displayed no significant reduction in SQR activity from that of the wild type at either sulfide concentration (Table 2).
TABLE 2.
SQR activities of wild-type and mutant C. tepidum strains
| Strain | Sp act (μmol of dUQ reduced [mg of protein]−1 min−1) with:
|
|
|---|---|---|
| 1.0 mM Na2S | 0.5 mM Na2S | |
| Wild type | 87.0 ± 6.9 | 63.0 ± 4.5 |
| CT0117::TnOGm | 31.5 ± 4.5a | 7.5 ± 2.6a |
| CT0876::TnOGm | 82.5 ± 2.6 | 60.0 ± 6.9 |
| CT1087::TnOGm | 52.5 ± 5.2a | 45.0 ± 9.0a |
| CT0117::TnOGm, CT1087::TnEm | NDb | ND |
P < 0.05 (n ≥ 3) by the t test for comparison to the wild-type strain assayed under the same conditions.
ND, not detected.
SQR activity could not be detected in the double-mutant strain CT0117::TnOGm, CT1087::TnEm under any conditions tested (Table 2). This is consistent with the observation that strain CT0876::TnOGm displayed no change in SQR activity from that of the wild type. Furthermore, if the residual activities observed for strains CT1087::TnOGm and CT0117::TnOGm are added, the combined activities are identical to that observed for the wild type at either sulfide concentration (Table 2). Therefore, one would expect to see no SQR activity in double-mutant strain CT0117::TnOGm, CT1087::TnEm, which expresses only CT0876.
Growth of strain CT0117::TnOGm, CT1087::TnEm at low sulfide concentrations.
The mechanism by which the double-mutant strain (CT0117::TnOGm, CT1087::TnEm), which lacks detectable SQR activity, grows at low sulfide concentrations was investigated. To determine if the FCC sulfide dehydrogenase of C. tepidum can support growth at low sulfide concentrations, RT-PCR was carried out for both genes encoding homologs of the FccB subunit: CT1015 (fccB1) and CT2081 (fccB2). Transcripts of both CT1015 and CT2081 were detected at all stages of growth on sulfide (Fig. 3a). However, we failed to detect any FCC sulfide dehydrogenase activity in C. tepidum soluble or membrane fractions prepared from cultures that were actively oxidizing sulfide. The assays utilized horse heart cytochrome c as an electron acceptor, which was successful for measuring this activity in extracts of A. vinosum (43). However, this experiment may simply indicate that horse heart cytochrome c is not a suitable acceptor for C. tepidum FCC sulfide dehydrogenase.
FIG. 3.
RT-PCR and Q-RT-PCR analyses of transcripts encoding CT0876 and FccB homologs. (a) RT-PCR to detect transcripts of CT1015 (fccB1) and CT2081 (fccB2) in total RNA harvested from wild-type C. tepidum at the indicated times during growth in a medium containing 0.7 mM sulfide and 9.2 mM thiosulfate. (b) Q-RT-PCR analysis of the abundances of CT0876, CT1015, and CT2081 transcripts relative to that of CT1551 (sigA) after 15 h of growth in medium with both thiosulfate and sulfide in the wild type (open bars) and strain CT0117::TnOGm, CT1087::TnEm (filled bars). An expression ratio of 1 indicates that an equal number of copies of the target gene and CT1551 were present in the sample. The P value above the CT1015 data indicates a slight, but statistically significant, increase in transcript abundance in strain CT0117::TnOGm, CT1087::TnEm relative to the wild type. Data are means ± standard errors for three independent experiments.
If either a cryptic SQR activity in CT0876 or FCC sulfide dehydrogenase is responsible for the growth of the double mutant, one might expect compensatory positive regulation of the relevant gene in strain CT0117::TnOGm, CT1087::TnEm. This was tested by Q-RT-PCR analysis of the CT0876- and FccB homolog-encoding genes CT1015 and CT2081 in the wild type and the double-mutant strain. The results indicated that the abundance of the CT0876 transcript relative to that of a control gene (sigA [CT1551]) was not statistically different between the wild-type and double-mutant strains (Fig. 3b). The abundance of CT1015 transcripts, encoding FccB1, was statistically significantly increased by ∼10% in the double mutant, while CT2081 transcripts, encoding FccB2, were not significantly different. Transcripts of all three genes were present at levels similar to those of sigA. Thus, either CT0876 or FCC sulfide dehydrogenase may support growth at low sulfide concentrations, but these activities clearly cannot support growth at higher sulfide concentrations in the absence of the other SQR homologs.
Epitope tagging and localization of CT1087.
To test if a specific C. tepidum SQR homolog is membrane associated, as would be predicted from the SQR activity measurements, we constructed a strain harboring a C-terminal His6 epitope by modifying CT1087 in its native location in the C. tepidum genome to produce strain CT1087-His6::aacC1. The epitope-tagged version of CT1087, CT1087-His6, should therefore reflect any regulation of CT1087 gene expression. The growth yield of strain CT1087-His6::aacC1 with 6 mM sulfide and 10 mM thiosulfate was similar to that of the wild type, suggesting that CT1087-His6 does not cause any physiological defects.
C. tepidum strain CT1087-His6::aacC1 was grown on 6 mM sulfide, and cells were harvested at 10 to 15 h after inoculation. For localization, immunoblotting targeting the His6 epitope was performed on subcellular fractions equally loaded with 30 μg of cytoplasmic, chlorosome, and Nonidet-P40-soluble and -insoluble membrane proteins. The Nonidet-P40-soluble fraction enriches integral membrane proteins, whereas outer membrane proteins and peripherally associated inner membrane proteins tend to partition to the Nonidet-P40-insoluble fraction (7). CT1087-His6 was found primarily in the Nonidet-P40-insoluble membrane fraction (Fig. 4a), suggesting that CT1087-His6 is membrane localized and is likely a peripherally associated inner membrane protein. The observed mass of CT1087-His6 is ∼44 kDa, which closely matches the size of 43.9 kDa predicted from the C. tepidum genome sequence.
FIG. 4.
Localization and abundance of CT1087-His6 in C. tepidum, determined by immunoblotting with an α-His5 antibody. All lanes were equally loaded with 30 μg of protein. (a) Cultures grown in the presence of 6 mM sulfide were harvested at ∼15 h postinoculation. Lanes: cytosolic, ∼126,000 × g supernatant; chlorosome, chlorosome-containing sucrose gradient fraction; Nonidet P-40 soluble, proteins solubilized from the sucrose gradient membrane pellet by NP-40; Nonidet P-40 insoluble, NP-40-insoluble proteins in the sucrose gradient membrane pellet. (b) Membrane proteins were harvested from cultures grown with 0.7 mM sulfide and 9.2 mM thiosulfate at the indicated times. (c) Membrane proteins were harvested at ∼15 h from cultures grown in medium with sulfide or thiosulfate as the sole electron donor at the indicated concentrations.
To corroborate the apparent regulation of CT1087 mRNA levels by sulfide, CT1087-His6 was monitored in membrane fractions throughout the growth of C. tepidum on both sulfide and thiosulfate by immunoblot analysis (Fig. 4b). CT1087-His6 was abundant during early growth (≤15 h) in C. tepidum (Fig. 4b), coinciding with active sulfide oxidation and elemental sulfur production. While CT1087-His6 was readily detectable in sulfide-grown cultures, it was present at a much lower abundance in thiosulfate-grown cultures (Fig. 4c).
Analysis of recombinant SQRs.
To further analyze the SQR activities of C. tepidum SQR homologs, CT0876 and CT1087 were recombinantly expressed from pET16b in E. coli, while CT0117 was expressed from pET28b due to the presence of an NdeI site in the native coding sequence. The recombinant proteins all contained His tags and so were detectable by immunoblotting with an α-His5 antibody. In these experiments, all E. coli strains were cultured under anaerobic conditions in LB medium with glucose plus nitrate, and expression was initiated by the addition of 0.4 mM IPTG and 5 mM sulfide. Subcellular fractionation and immunoblotting indicated that most of the recombinant proteins were found as inclusion bodies, with a small portion of each protein found in the membrane fraction (data not shown). The observed masses of the recombinant proteins in immunoblots were similar to the sizes predicted from the sequence of each expression construct.
Because some recombinant CT0117-His, CT0876-His, and CT1087-His protein was found in the membrane fractions of E. coli, these were assayed for SQR activity at both 37°C and 47°C. SQR activity was detected only in CT0117-His- and CT1087-His-containing membrane fractions. At 37°C, the activities of CT0117-His and CT1087-His were <4.5 and 31.5 ± 8.4 μmol of dUQ reduced (mg of protein)−1 min−1, respectively. At 47°C, the activities for these strains were 5.6 ± 3.0 and 79.5 ± 9.4 μmol of dUQ reduced (mg of protein)−1 min−1, respectively. SQR activity was never detected in membranes containing recombinant CT0876-His or in the empty-vector controls.
Phylogenic analysis of C. tepidum SQR homologs.
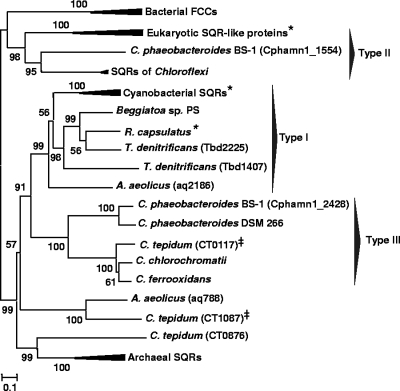
Because our data indicated that C. tepidum possesses two active SQRs and one apparently inactive homolog, we sought to use phylogenic (Fig. 5) comparisons with previously studied SQR, SQR-like, and FCC sequences to provide context for our observations. Our analysis successfully reconstructed the three types (I to III) of SQR and SQR-like homologs in a neighbor-joining phylogeny (Fig. 5) that had been defined previously (20, 48, 56). Type I SQR consists of sequences from the cyanobacteria O. limnetica, Anabaena variabilis, and A. halophytica, together with one of the two SQR orthologs from Aquifex aeolicus (AQ2186), R. capsulatus, and a Beggiatoa sp., and both SQR homologs of Thiobacillus denitrificans (Tbd1407 and Tbd2225) (Fig. 5). Type II sequences include those from the Chloroflexi and the eukaryotic SQR-like homologs and one of the two SQR homologs (Cphamn1_1554) from Chlorobium phaeobacteroides BS-1 (Fig. 5). Type II SQRs are more similar to FCCs and other disulfide oxidoreductases than the other SQR types. Type III SQRs include CT0117 and the SQRs of most other green sulfur bacteria, including the second SQR homolog from C. phaeobacteroides BS-1 (Cphamn1_2428), C. phaeobacteroides DSM 266, Chlorobium chlorochromatii, and C. ferrooxidans, even though the latter strain cannot grow on sulfide (25). CT1087 clustered with the AQ0788 protein of A. aeolicus, and these proteins together form a cluster that branches as deeply as the division between type I and type III SQRs. Similarly, CT0876, the apparently inactive SQR homolog, appears to be affiliated with archaeal proteins annotated as SQR in a deeply branching cluster (Fig. 5). To the best of our knowledge, SQR activity has never been demonstrated in any archaeon, which may suggest that this cluster represents a branch of the SQR family with altered catalytic properties.
FIG. 5.
Neighbor-joining phylogram of SQR and FCC sequences constructed with MEGA 4 using pairwise gap elimination. The scale bar represents 0.1 substitution per site, and values at the nodes indicate the bootstrap support observed in 1,000 trials. Gene identifiers for each homolog are shown when more than one SQR or FCC homolog was identified for the same strain. SQR types were classified as described previously (20, 48, 56). Stars indicate that SQR activity was detected with purified protein (Schizosaccharomyces pombe, O. limnetica, A. halophytica, and R. capsulatus); double daggers indicate that SQR activity was observed in this study. Strains (with GenBank accession numbers in parentheses) are as follows: bacterial FCCs, Allochromatium vinosum (AAB86576) and Chlorobaculum tepidum TLS (FccB1, NP_661907; FccB2, NP_662956); eukaryotic SQR-like proteins, Schizosaccharomyces pombe 972 h− (NP_596067), Mus musculus (NP_067482), and Homo sapiens (NP_067022); SQRs of Chloroflexi, Chloroflexus aurantiacus J-10-fl (YP_001634207) and Chloroflexus aggregans DSM 9485 (ZP_01515968); cyanobacterial SQRs, Oscillatoria limnetica “Solar Lake” (AAF72962), Anabaena variabilis ATCC 29413 (YP_323880), and Aphanothece halophytica (AAF72963); archaeal SQRs, Thermoplasma acidophilum DSM 1728 (NP_394588), Sulfolobus solfataricus P2 (NP_343636), and Picrophilus torridus DSM 9790 (YP_023317); other bacterial SQRs, Aquifex aeolicus VF5 (AQ788, NP_213539; AQ2186, NP_214500), Thiobacillus denitrificans ATCC 25259 (Tbd1407, YP_315165; Tbd2225, YP_315983), Rhodobacter capsulatus (CAA66112), Beggiatoa sp. strain PS (ZP_02002714), Chlorobium phaeobacteroides BS1 (Cphamn1_2428, YP_001960804; Cphamn1_1554, YP_001959958), Chlorobaculum tepidum TLS (CT0117, NP_661023; CT0876, NP_661769; CT1087, NP_661978), Chlorobium chlorochromatii CaD3 (YP_379190), Chlorobium ferrooxidans DSM 13031 (ZP_01385816), and Chlorobium phaeobacteroides DSM 266 (YP_912981).
DISCUSSION
This paper reports the first analysis of multiple SQR homologs found in a single microbe, the green sulfur phototrophic bacterium Chlorobaculum tepidum. The data reported above led us to conclude that the C. tepidum genes CT0117 and CT1087 encode functional SQRs and that either can support sulfide-dependent growth at sulfide concentrations of ≥2 mM. While the results support the annotation of CT0117 and CT1087 as SQRs, they are not absolutely definitive, due to the lack of a complementation system in C. tepidum. While C. tepidum will apparently accept and maintain conjugally transferred plasmids (58), there has been no report of plasmid-based complementation to date, and efforts to develop this in our laboratory have been unsuccessful. The recent report of heterologous gene expression from the chromosomal integration of a carotenoid biosynthetic gene (34) will hopefully allow us to address this issue in the near future by integration of the wild-type SQR genes at an alternative chromosomal location in the mutant strains.
The data were not able to resolve whether or not CT0876 encodes an active SQR. While CT0876 is expressed in the double-mutant strain and this strain grows at 2 mM sulfide, no SQR activity was found in this strain or in recombinantly produced CT0876 protein. If the CT0876 protein does possess SQR activity, it must not be able to use dUQ as an electron acceptor in vitro. We are in the process of purifying native quinones from C. tepidum in order to test whether any of these may serve as an electron acceptor for CT0876. Transcripts of genes encoding both homologs of the FccB subunit of FCC sulfide dehydrogenase were also found in the mutant strain, suggesting that this activity may support growth at low sulfide levels. Alternatively, the growth of the double-mutant strain with 2 mM sulfide might be due to a low level of thiosulfate consistently observed when sulfide is added to Pf-7-BTP medium, as has been previously documented (8). Experiments in which strain CT0117::TnOGm, CT1087::TnEm was grown at the levels of thiosulfate (∼0.75 mM) observed in medium with 2 mM sulfide as the sole electron donor indicate that this strain achieves a density (20 to 30 μg of protein ml−1) similar to that reported in Fig. 2b.
This study has expanded the range of sulfide concentrations known to be utilized for growth by C. tepidum. C. tepidum was initially reported to have an upper limit for sulfide tolerance of 4 mM (59), while our data indicate a maximal yield at 8 mM sulfide for the wild type. The observations that CT1087 appears to be expressed only during active sulfide oxidation and that it is required for growth at high levels of sulfide suggest that this protein may be adapted to function at high concentrations of sulfide. This suggestion is supported by the observation that a higher SQR-specific activity was observed in strain CT0117:TnOGm than in CT1087:TnOGm when both were assayed at a saturating sulfide concentration. Both CT0117 and CT1087 could be recombinantly expressed in an active, His-tagged form in E. coli, which will facilitate protein purification to examine the kinetic differences between these proteins in greater detail. The preliminary data reported here indicate that the SQR activities of both CT0117 and CT1087 are adapted to high temperatures, as would be expected for an enzyme from a moderate thermophile such as C. tepidum.
CT1087-His6 can be produced in C. tepidum by epitope tagging, the first demonstration of this technique in this organism. Unfortunately, attempts to produce a His6-tagged version of CT0117 in C. tepidum have failed. Our results indicate that CT1087-His6 is membrane associated, and Nonidet-P40 treatment suggests that it is a peripherally associated inner membrane protein. Previous analysis indicates that FmoA, another peripherally associated inner membrane protein, was, like CT1087-His6, observed in both the total-membrane and Nonidet P-40-insoluble fractions, while the integral membrane protein PscD, a component of the photosynthetic reaction center, was not found in the Nonidet P-40-insoluble fraction (7). A CT1087::phoA fusion protein should help to confirm the precise location of CT1087 in C. tepidum. This technique was used to suggest that the R. capsulatus SQR is associated with the periplasmic face of the cytoplasmic membrane (46).
While this study has significantly improved our understanding of sulfide oxidation in C. tepidum, intriguing questions remain. Quinone specificity, noted above as a possible reason for the failure to observe activity in CT0876, may provide a rationale for multiple SQRs in C. tepidum, which possesses both menaquinone and chlorobiumquinone (18, 30, 37). Chlorobiumquinone is found in membranes and the chlorosome, where it is thought to help mediate the quenching of excited states of bacteriochlorophyll and prevent the photodegradation of chlorosomes (29). Menaquinone in C. tepidum is found associated with the photosynthetic reaction center (30) and in the cytoplasmic membrane. A larger question is the mechanism of growth inhibition by sulfide in C. tepidum, which is poorly understood. Since the strains lacking SQRs displayed differing levels of resistance to sulfide, they may help to provide insights into sulfide tolerance specifically in C. tepidum and in the green sulfur bacteria generally, especially when these studies are coupled to proteomic and genome-wide expression analyses.
Supplementary Material
Acknowledgments
This project was supported by a National Science Foundation CAREER award (MCB-0447649, to T.E.H.) and utilized common instrumentation facilities provided in part by the National Institutes of Health, P20-RR116472-04 from the IDeA Networks of Biomedical Research Excellence program of the National Center for Research Resources.
Bruce Kingham is acknowledged for providing DNA sequencing services. We also thank Nicole Donofrio and Kun Huang for their expertise in Q-RT-PCR analysis and David Kirchman for assistance in editing the manuscript.
This article is dedicated to the memory of Tom Wahlund (1948-2008), who isolated C. tepidum and facilitated the application of molecular genetics to the Chlorobiaceae. Without his efforts, this work and that of many other groups would not have been possible.
Footnotes
Published ahead of print on 21 November 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Arieli, B., Y. Shahak, D. Taglicht, G. Hauska, and E. Padan. 1994. Purification and characterization of sulfide-quinone reductase, a novel enzyme driving anoxygenic photosynthesis in Oscillatoria limnetica. J. Biol. Chem. 2695705-5711. [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Green Publishing Associates and Wiley Interscience, New York, NY.
- 3.Bardischewsky, F., A. Quentmeier, and C. G. Friedrich. 2006. The flavoprotein SoxF functions in chemotrophic thiosulfate oxidation of Paracoccus pantotrophus in vivo and in vitro. FEMS Microbiol. Lett. 258121-126. [DOI] [PubMed] [Google Scholar]
- 4.Bronstein, M., M. Schutz, G. Hauska, E. Padan, and Y. Shahak. 2000. Cyanobacterial sulfide-quinone reductase: cloning and heterologous expression. J. Bacteriol. 1823336-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, Y., and C. P. Wolk. Use of a conditionally lethal gene in anabaena sp. strain Pcc 7120 to select for double recombinants and to entrap insertion sequences. J. Bacteriol. 1723138-3145. [DOI] [PMC free article] [PubMed]
- 6.Chan, L.-K., R. Morgan-Kiss, and T. E. Hanson. 2008. Genetic and proteomic studies of sulfur oxidation in Chlorobium tepidum (syn. Chlorobaculum tepidum), p. 357-373. In R. Hell, C. Dahl, D. Knaff, and T. Leustek (ed.), Sulfur metabolism in phototrophic organisms. Springer, New York, NY.
- 7.Chan, L.-K., R. Morgan-Kiss, and T. E. Hanson. 2008. Sulfur oxidation in Chlorobium tepidum (syn. Chlorobaculum tepidum): genetic and proteomic analyses, p. 117-126. In C. Dahl and C. G. Friedrich (ed.), Microbial sulfur metabolism. Springer, Berlin, Germany.
- 8.Chan, L.-K., T. S. Weber, R. M. Morgan-Kiss, and T. E. Hanson. 2008. A genomic region required for phototrophic thiosulfate oxidation in the green sulfur bacterium Chlorobium tepidum (syn. Chlorobaculum tepidum). Microbiology 154818-829. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Z. W., M. Koh, G. Van Driessche, J. J. Van Beeumen, R. G. Bartsch, T. E. Meyer, M. A. Cusanovich, and F. S. Mathews. 1994. The structure of flavocytochrome c sulfide dehydrogenase from a purple phototrophic bacterium. Science 266430-432. [DOI] [PubMed] [Google Scholar]
- 10.Dahl, C. 2008. Inorganic sulfur compounds as electron donors in purple sulfur bacteria, p. 289-317. In R. Hell, C. Dahl, D. Knaff, and T. Leustek (ed.), Sulfur metabolism in phototrophic organisms. Springer, Dordrecht, The Netherlands.
- 11.Dahl, C., S. Engels, A. S. Pott-Sperling, A. Schulte, J. Sander, Y. Lubbe, O. Deuster, and D. C. Brune. 2005. Novel genes of the dsr gene cluster and evidence for close interaction of Dsr proteins during sulfur oxidation in the phototrophic sulfur bacterium Allochromatium vinosum. J. Bacteriol. 1871392-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahl, C., G. Rákhely, A. S. Pott-Sperling, B. Fodor, M. Takács, A. Tóth, M. Kraeling, K. Győrfi, Á. Kovács, J. Tusz, and K. L. Kovács. 1999. Genes involved in hydrogen and sulfur metabolism in phototrophic sulfur bacteria. FEMS Microbiol. Lett. 180317-324. [DOI] [PubMed] [Google Scholar]
- 13.Dennis, J. J., and G. J. Zylstra. 1998. Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 642710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich, C. G., F. Bardischewsky, D. Rother, A. Quentmeier, and J. Fischer. 2005. Prokaryotic sulfur oxidation. Curr. Opin. Microbiol. 8253-259. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich, C. G., D. Rother, F. Bardischewsky, A. Quentmeier, and J. Fischer. 2001. Oxidation of reduced inorganic sulfur compounds by bacteria: emergence of a common mechanism? Appl. Environ. Microbiol. 672873-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frigaard, N., and D. Bryant. 2008. Genomic insights into the sulfur metabolism of phototrophic green sulfur bacteria, p. 337-355. In R. Hell, C. Dahl, D. Knaff, and T. Leustek (ed.), Sulfur metabolism in phototrophic organisms. Springer, New York, NY.
- 17.Frigaard, N.-U., and D. A. Bryant. 2008. Genomic and evolutionary perspectives on sulfur metabolism in green sulfur bacteria, p. 60-76. In C. Dahl and C. G. Friedrich (ed.), Microbial sulfur metabolism. Springer, New York, NY.
- 18.Frigaard, N.-U., S. Takaichi, M. Hirota, K. Shimada, and K. Matsuura. 1997. Quinones in chlorosomes of green sulfur bacteria and their role in the redox-dependent fluorescence studied in chlorosome-like bacteriochlorophyll c aggregates. Arch. Microbiol. 167343-349. [Google Scholar]
- 19.Frigaard, N. U., H. Li, K. J. Milks, and D. A. Bryant. 2004. Nine mutants of Chlorobium tepidum each unable to synthesize a different chlorosome protein still assemble functional chlorosomes. J. Bacteriol. 186646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griesbeck, C., G. Hauska, and M. Schutz. 2000. Biological sulfide-oxidation: sulfide-quinone reductase (SQR), the primary reaction, p. 129-203. In S. G. Pandalai (ed.), Recent research developments in microbiology. Research Signpost, Trivandrum, India.
- 21.Grieshaber, M. K., and S. Volkel. 1998. Animal adaptations for tolerance and exploitation of poisonous sulfide. Annu. Rev. Physiol. 6033-53. [DOI] [PubMed] [Google Scholar]
- 22.Gruber, T. M., and D. A. Bryant. 1998. Characterization of the group 1 and group 2 sigma factors of the green sulfur bacterium Chlorobium tepidum and the green non-sulfur bacterium Chloroflexus aurantiacus. Arch. Microbiol. 170285-296. [DOI] [PubMed] [Google Scholar]
- 23.Hanson, T. E., and F. R. Tabita. 2003. Insights into the stress response and sulfur metabolism revealed by proteome analysis of a Chlorobium tepidum mutant lacking the Rubisco-like protein. Photosynth. Res. 78231-248. [DOI] [PubMed] [Google Scholar]
- 24.Hanson, T. E., and F. R. Tabita. 2001. A ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO)-like protein from Chlorobium tepidum that is involved with sulfur metabolism and the response to oxidative stress. Proc. Natl. Acad. Sci. USA 984397-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heising, S., L. Richter, W. Ludwig, and B. Schink. 1999. Chlorobium ferrooxidans sp. nov., a phototrophic green sulfur bacterium that oxidizes ferrous iron in coculture with a “Geospirillum” sp. strain. Arch. Microbiol. 172116-124. [DOI] [PubMed] [Google Scholar]
- 26.Hohmann-Marriott, M. F., and R. E. Blankenship. 2007. Hypothesis on chlorosome biogenesis in green photosynthetic bacteria. FEBS Lett. 581800-803. [DOI] [PubMed] [Google Scholar]
- 27.Imhoff, J. F. 2003. Phylogenetic taxonomy of the family Chlorobiaceae on the basis of 16S rRNA and FMO (Fenna-Matthews-Olson protein) gene sequences. Int. J. Syst. Evol. Microbiol. 53941-951. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko, T., and S. Tabata. 1997. Complete genome structure of the unicellular cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 381171-1176. [DOI] [PubMed] [Google Scholar]
- 29.Kim, H., H. Li, J. A. Maresca, D. A. Bryant, and S. Savikhin. 2007. Triplet exciton formation as a novel photoprotection mechanism in chlorosomes of Chlorobium tepidum. Biophys. J. 93192-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kjaer, B., N. U. Frigaard, F. Yang, B. Zybailov, M. Miller, J. H. Golbeck, and H. V. Scheller. 1998. Menaquinone-7 in the reaction center complex of the green sulfur bacterium Chlorobium vibrioforme functions as the electron acceptor A1. Biochemistry 373237-3242. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 32.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 33.Luo, Q., L. R. Krumholz, F. Z. Najar, A. D. Peacock, B. A. Roe, D. C. White, and M. S. Elshahed. 2005. Diversity of the microeukaryotic community in sulfide-rich Zodletone Spring (Oklahoma). Appl. Environ. Microbiol. 716175-6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maresca, J. A., S. P. Romberger, and D. A. Bryant. 2008. Isorenieratene biosynthesis in green sulfur bacteria requires the cooperative actions of two carotenoid cyclases. J. Bacteriol. 1906384-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meeks, J. C., J. Elhai, T. Thiel, M. Potts, F. Larimer, J. Lamerdin, P. Predki, and R. Atlas. 2001. An overview of the genome of Nostoc punctiforme, a multicellular, symbiotic cyanobacterium. Photosynth. Res. 7085-106. [DOI] [PubMed] [Google Scholar]
- 36.Morgan-Kiss, R. M., and J. E. Cronan. 2004. The Escherichia coli fadK (ydiD) gene encodes an anerobically regulated short chain acyl-CoA synthetase. J. Biol. Chem. 27937324-37333. [DOI] [PubMed] [Google Scholar]
- 37.Morton, R. A. 1965. Biochemistry of quinones. Academic Press, London, United Kingdom.
- 38.Mukhopadhyay, B., E. F. Johnson, and M. J. Ascano. 1999. Conditions for vigorous growth on sulfide and reactor-scale cultivation protocols for the thermophilic green sulfur bacterium Chlorobium tepidum. Appl. Environ. Microbiol. 65301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura, Y., T. Kaneko, S. Sato, M. Ikeuchi, H. Katoh, S. Sasamoto, A. Watanabe, M. Iriguchi, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2002. Complete genome structure of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. DNA Res. 9123-130. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura, Y., T. Kaneko, S. Sato, M. Mimuro, H. Miyashita, T. Tsuchiya, S. Sasamoto, A. Watanabe, K. Kawashima, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, N. Nakazaki, S. Shimpo, C. Takeuchi, M. Yamada, and S. Tabata. 2003. Complete genome structure of Gloeobacter violaceus PCC 7421, a cyanobacterium that lacks thylakoids. DNA Res. 10137-145. [DOI] [PubMed] [Google Scholar]
- 41.Oh-oka, H., and R. E. Blankenship. 2004. Green bacteria: secondary electron donor (cytochromes), p. 321-324. In W. J. Lennarz and M. D. Lane (ed.), Encyclopedia of biological chemistry, vol. 2. Elsevier, Boston, MA. [Google Scholar]
- 42.Quentmeier, A., P. Hellwig, F. Bardischewsky, R. Wichmann, and C. G. Friedrich. 2004. Sulfide dehydrogenase activity of the monomeric flavoprotein SoxF of Paracoccus pantotrophus. Biochemistry 4314696-14703. [DOI] [PubMed] [Google Scholar]
- 43.Reinartz, M., J. Tschape, T. Bruser, H. G. Truper, and C. Dahl. 1998. Sulfide oxidation in the phototrophic sulfur bacterium Chromatium vinosum. Arch. Microbiol. 17059-68. [DOI] [PubMed] [Google Scholar]
- 44.Rother, D., H. J. Henrich, A. Quentmeier, F. Bardischewsky, and C. G. Friedrich. 2001. Novel genes of the sox gene cluster, mutagenesis of the flavoprotein SoxF, and evidence for a general sulfur-oxidizing system in Paracoccus pantotrophus GB17. J. Bacteriol. 1834499-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4406-425. [DOI] [PubMed] [Google Scholar]
- 46.Schütz, M., I. Maldener, C. Griesbeck, and G. Hauska. 1999. Sulfide-quinone reductase from Rhodobacter capsulatus: requirement for growth, periplasmic localization, and extension of gene sequence analysis. J. Bacteriol. 1816516-6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schütz, M., Y. Shahak, E. Padan, and G. Hauska. 1997. Sulfide-quinone reductase from Rhodobacter capsulatus. Purification, cloning, and expression. J. Biol. Chem. 2729890-9894. [DOI] [PubMed] [Google Scholar]
- 48.Shahak, Y. 2008. Sulfide oxidation from cyanobacteria to humans: sulfide-quinone oxidoreductase (SQR), p. 319-335. In R. Hell, C. Dahl, D. Knaff, and T. Leustek (ed.), Sulfur metabolism in phototrophic organisms. Springer, Dordrecht, The Netherlands.
- 49.Shahak, Y., B. Arieli, B. Binder, and E. Padan. 1987. Sulfide-dependent photosynthetic electron flow coupled to proton translocation in thylakoids of the cyanobacterium Oscillatoria limnetica. Arch. Biochem. Biophys. 259605-615. [DOI] [PubMed] [Google Scholar]
- 50.Shahak, Y., B. Arieli, E. Padan, and G. Hauska. 1992. Sulfide quinone reductase (SQR) activity in Chlorobium. FEBS Lett. 299127-130. [DOI] [PubMed] [Google Scholar]
- 51.Shahak, Y., C. Klughammer, U. Schreiber, E. Padan, I. Herrman, and G. Hauska. 1994. Sulfide-quinone and sulfide-cytochrome reduction in Rhodobacter capsulatus. Photosynth. Res. 39175-181. [DOI] [PubMed] [Google Scholar]
- 52.Shahak, Y., M. Schütz, M. Bronstein, C. Griesbeck, G. Hauska, and E. Padan. 1999. Sulfide-dependent anoxygenic photosynthesis in prokaryotes—sulfide-quinone reductase (SQR), the initial step, p. 217-228. In G. A. Peschek, W. L. Loffelhardt, and G. Schmetterer (ed.), The phototrophic prokaryotes. Kluwer Academic/Plenum, New York, NY.
- 53.Shen, N., L. Dagasan, D. Sledjeski, and R. M. Weiner. 1989. Major outer membrane proteins unique to reproductive cells of Hyphomonas jannaschiana. J. Bacteriol. 1712226-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takashima, T., T. Nishiki, and Y. Konishi. 2000. Anaerobic oxidation of dissolved hydrogen sulfide in continuous culture of the phototrophic bacterium Prosthecochloris aestuarii. J. Biosci. Bioeng. 89247-251. [DOI] [PubMed] [Google Scholar]
- 55.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. Mega4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 241596-1599. [DOI] [PubMed] [Google Scholar]
- 56.Theissen, U., M. Hoffmeister, M. Grieshaber, and W. Martin. 2003. Single eubacterial origin of eukaryotic sulfide:quinone oxidoreductase, a mitochondrial enzyme conserved from the early evolution of eukaryotes during anoxic and sulfidic times. Mol. Biol. Evol. 201564-1574. [DOI] [PubMed] [Google Scholar]
- 57.Vassilieva, E. V., M. L. Antonkine, B. L. Zybailov, F. Yang, C. U. Jakobs, J. H. Golbeck, and D. A. Bryant. 2001. Electron transfer may occur in the chlorosome envelope: the CsmI and CsmJ proteins of chlorosomes are 2Fe-2S ferredoxins. Biochemistry 40464-473. [DOI] [PubMed] [Google Scholar]
- 58.Wahlund, T. M., and M. T. Madigan. 1995. Genetic transfer by conjugation in the thermophilic green sulfur bacterium Chlorobium tepidum. J. Bacteriol. 1772583-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wahlund, T. M., C. R. Woese, R. W. Castenholz, and M. T. Madigan. 1991. A thermophilic green sulfur bacterium from New Zealand hot springs, Chlorobium tepidum sp. nov. Arch. Microbiol. 15681-90. [Google Scholar]
- 60.Wakai, S., M. Kikumoto, T. Kanao, and K. Kamimura. 2004. Involvement of sulfide:quinone oxidoreductase in sulfur oxidation of an acidophilic iron-oxidizing bacterium, Acidithiobacillus ferrooxidans NASF-1. Biosci. Biotechnol. Biochem. 682519-2528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.