Abstract
A truncated derivative of the phage endolysin LysK containing only the CHAP (cysteine- and histidine-dependent amidohydrolase/peptidase) domain exhibited lytic activity against live clinical staphylococcal isolates, including methicillin-resistant Staphylococcus aureus. This is the first known report of a truncated phage lysin which retains high lytic activity against live staphylococcal cells.
Staphylococcus aureus is associated with a variety of clinical manifestations, ranging from skin infections to more acute conditions such as necrotizing pneumonia and septicemia (20). Since methicillin-resistant S. aureus (MRSA) is now the most commonly reported antibiotic-resistant bacterium in clinical settings (5), the development of alternative antimicrobials is warranted.
Bacteriophage-encoded endolysins are a group of enzymes that act by digesting the peptidoglycan of bacterial cell walls. The potential of these molecules for controlling bacterial infections and preventing the pathogenic colonization of mucosal membranes has been demonstrated previously (2, 6, 10, 11, 14, 15, 21, 23, 29). In general, phage endolysins have a modular organization with an N-terminal catalytic domain and a C-terminal cell-binding domain (9, 12, 18). To our knowledge, only a few phage endolysins, such as LysK, phi11, MV-L, and LysH5, have been reported to lyse live staphylococcal cultures (4, 25-27). LysK has a modular structure similar to the structure of these endolysins, with two catalytic domains, a CHAP (cysteine- and histidine-dependent amidohydrolase/peptidase) domain and a central amidase-2 domain (N-acetylmuramoyl-l-alanine amidase), as well as a C-terminal SH3b cell-binding domain (4, 22, 25-27).
In this study, we examined the involvement of each of the three domains of LysK during exolysis by performing a deletion analysis and identified truncated LysK proteins containing only the CHAP domain, which still showed lytic activity against live clinical staphylococcal isolates, including MRSA. In previous studies other workers have obtained similar results upon deletion of the cell wall-binding domains (1, 8, 16, 17, 19). Construction of a single-domain protein for therapeutic purposes is desirable since, as well as facilitating protein production, this may decrease the likelihood of a significant immunogenic response. Unlike antibiotics, intact endolysins are large proteins which are capable of stimulating a humoral immune response, especially when they are used intravenously (7, 24, 30).
Generation of deletion derivatives of LysK.
LysK protein deletion mutants were constructed based on the domain organization of this protein. The primer pairs used for domain deletion analysis were FLysK plus Rami (CHAP and amidase-2), Fami plus RSH3b (amidase-2 and SH3b), Fami plus Rami (amidase-2), FSH3b plus RSH3b (SH3b), and FLysK plus R313 to R156 (CHAP) (Table 1). Amplified products were cloned into the pQE60 (Qiagen) expression system and transformed into Escherichia coli XL1-Blue for expression. Cells were induced as previously described (3). However, induction was performed at 26°C for 14 h to avoid inclusion bodies.
TABLE 1.
Oligonucleotides used for construction of plasmids
| Oligonucleotide | Sequencea |
|---|---|
| Full LysK protein | |
| FLysK | CATGCCATGGCTAAGACTCAAGCAG |
| RLysKb | GCAGATCTTTTGAATACTCCCCAGG |
| Initial domain deletions | |
| R313 | GGAAGATCTCTAACCCCATTCTTTAAATTTC |
| Fami | CATGCCATGGCGGTATTTACATCCGGTAG |
| Rami | GGAAGATCTCTAACCTATCCAAATGTGACC |
| FSH3b | CATGCCATGGAATTTGTACCAACTGC |
| RSH3b | GGAAGATCTCTATTTGAATACTCCCCAGGC |
| C-terminal CHAP deletionsc | |
| R203b | GGAAGATCTTTTATCCATTGTATAGTTAA |
| R165 | GGAAGATCTCTATGCTTTTACAGGTATTTC |
| R164 | GGAAGATCTCTATTTTACAGGTATTTCAAT |
| R163 | GGAAGATCTCTATACAGGTATTTCAATGAA |
| R162 | GGAAGATCTCTAAGGTATTTCAATGAAGTG |
| R161 | GGAAGATCTCTATATTTCAATGAAGTGAGT |
| R160 | GGAAGATCTCTATTCAATGAAGTGAGTTAAT |
| R159 | GGAAGATCTCTAAATGAAGTGAGTTAATCC |
| R156 | GGAAGATCTCTAAGTTAATCCGTAATAATTATC |
NcoI, BglII, and HindIII sites are underlined, and stop and start codons are indicated by bold type.
Primer that allows translation of a six-His tag present in the pQE60 vector.
The forward primer used was FLysK.
CHAP domain exhibits lytic activity against heat-killed staphylococcal cells.
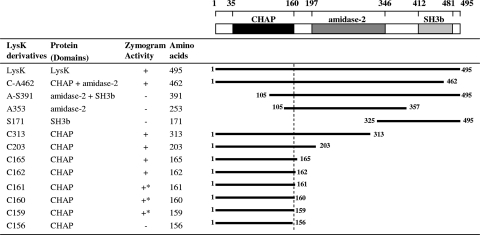
Preparation of protein samples, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and zymographic analysis were performed as previously described (13, 26). Zones of lysis on the zymogram gel were used as an indication of putative catalytic activity. The results suggested that in the absence of other domains, the LysK CHAP domain retains lytic activity, while the amidase-2 and SH3b domains appear to have no significant lytic activity when they are expressed alone or in combination (Fig. 1).
FIG. 1.
Schematic diagram of LysK and its truncated derivatives. LysK (495 residues) contains a CHAP domain (residues 35 to 160), an amidase-2 domain (residues 197 to 346), and an SH3b domain (residues 412 to 481). Activity was determined using zymogram analysis with heat-killed MRSA strain DPC5645. The dotted line indicates the end of the CHAP domain. The numbers are the positions of the first and last amino acids. +, zone of clearing; −, no zone of clearing; +*, very faint zone of clearing.
To determine the smallest fully functional catalytic region of LysK, a sequential deletion analysis of the CHAP domain was performed (Fig. 1, plasmids C203 to C156). Zymogram analysis indicated that while there was some residual activity with a shorter variant with only 159 amino acids (C159), full endopeptidase activity required amino acids 1 to 162 (C162), suggesting that the N-terminal CHAP domain is sufficient for lysis of S. aureus, including MRSA. In addition, the truncated derivatives showed a spectrum of inhibition similar to that of LysK, lysing all S. aureus strains tested, including MRSA, heterogeneous vancomycin-intermediate S. aureus, and other antibiotic-resistant variants (data not shown), but not lysing members of genera other than Staphylococcus among the strains tested.
CHAP domain exhibits lytic activity against live cells.
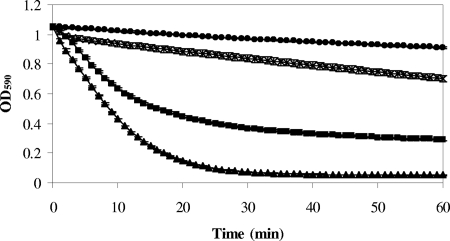
The native LysK protein and two derivatives of interest, C203 (CHAP and six residues of amidase-2) and C165 (CHAP) (Fig. 1), were selected to determine the lytic activity against live staphylococcal cells. LysK and C165 were purified by ion-exchange and size exclusion chromatography, while C203 was purified by nickel affinity chromatography (unpublished data). The specific activity of each protein was estimated as previously described (2, 23), with some modifications. Briefly, MRSA strain DPC5645 (Moorepark Food Research Centre Culture Collection) was grown to an optical density at 590 nm (OD590) of 0.3, centrifuged, and then resuspended to a final OD590 of 0.8 in 50 mM sodium acetate buffer (pH 6.5). Serial dilutions of 100 μl purified lysin were mixed with 100 μl of the bacterial suspension and incubated at 37°C. The amount of lysin that reduced the OD590 by 50% in 15 min was defined as 1 U of activity. The protein concentration was measured using a Bradford protein assay kit (Bio-Rad). Based on the data obtained, the specific activities of LysK, C165, and C203 were calculated to be 34 U nmol−1 (621 U mg−1), 68 U nmol−1 (3,690 U mg−1), and 2.4 U nmol−1 (100 U mg−1), respectively. Increases and decreases in activity were determined as previously described (1). Compared to the activity of LysK, the activity of C165 was approximately twofold higher, demonstrating that a LysK derivative containing only the CHAP domain is more active against live staphylococcal cells than the native enzyme. By contrast, the activity of C203 was approximately 14-fold lower, which may have been due to a change in protein folding. It should also be emphasized that while C165 did not contain a His tag, the native protein and C203 did contain such a tag, which potentially could have altered its specific activity. Nonetheless, the results show that C165 had high activity in this case. To compare the catalytic activities, DPC5645 cells were treated with the same quantity (0.5 nmol) of LysK, C203, and C165 (Fig. 2).
FIG. 2.
Comparison of lytic activities of purified LysK, C203, and C165 determined by using live DPC5645 cells in vitro. The amount of purified C203 (×), LysK (▪), or C165 (▴) used was 0.5 nmol. Control experiments (•) were performed under the same conditions with no enzyme added. The error bars indicate standard errors of the means.
LysK (26), phi11 (4), MV-L (27), and LysH5 (25) are the only staphylococcal endolysins that have been reported to kill untreated staphylococcal cells. However, deletion of additional domains of phi11 changed it to a barely active lysin (4, 28), and while the LysK derivatives lyse cells of all members of the genus Staphylococcus, MV-L and LysH5 have only been shown to lyse S. aureus, as well as Staphylococcus simulans and Staphylococcus epidermidis, respectively. We determined that the CHAP domain alone (C162, 33%) can exhibit activity against live staphylococci. This phenomenon was also observed with other staphylococcal phage endolysins with a similar structure, such as PlyTW (24) and Ply187 (23), but lysis was observed with only heat-killed staphylococcal cells. The LysK CHAP domain provides a valuable functional unit for domain-swapping studies. It would be interesting to investigate if a chimeric protein with the LysK CHAP domain and a different substrate-binding domain would have an altered spectrum of inhibition, since we demonstrated that the CHAP domain alone has the same spectrum of inhibition in all of the strains tested. Environments such as hospitals and nursing homes in which there are high numbers of MRSA infections could benefit considerably from exploitation of the CHAP domain of LysK.
Acknowledgments
We thank Susan Mills and David Donovan for their scientific advice and Kieran Kilcawley for his technical assistance.
This research was funded by Teagasc and Science Foundation, Ireland. M. Horgan was the recipient of a Teagasc Walsh Fellowship.
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Cheng, Q., and V. A. Fischetti. 2007. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl. Microbiol. Biotechnol. 74:1284-1291. [DOI] [PubMed] [Google Scholar]
- 2.Cheng, Q., D. Nelson, S. Zhu, and V. A. Fischetti. 2005. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob. Agents Chemother. 49:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donovan, D. M., J. Foster-Frey, S. Dong, G. M. Rousseau, S. Moineau, and D. G. Pritchard. 2006. The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl. Environ. Microbiol. 72:5108-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donovan, D. M., M. Lardeo, and J. Foster-Frey. 2006. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol. Lett. 265:133-139. [DOI] [PubMed] [Google Scholar]
- 5.EARSS Management Team, Members of the Advisory Board, and National Representatives of EARSS. 2007. EARSS Annual Report 2006. EARSS, Bilthoven, The Netherlands.
- 6.Entenza, J. M., J. M. Loeffler, D. Grandgirard, V. A. Fischetti, and P. Moreillon. 2005. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob. Agents Chemother. 49:4789-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischetti, V. A. 2005. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol. 13:491-496. [DOI] [PubMed] [Google Scholar]
- 8.Gaeng, S., S. Scherer, H. Neve, and M. J. Loessner. 2000. Gene cloning and expression and secretion of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 66:2951-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia, P., J. L. Garcia, E. Garcia, J. M. Sanchez-Puelles, and R. Lopez. 1990. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene 86:81-88. [DOI] [PubMed] [Google Scholar]
- 10.Grandgirard, D., J. M. Loeffler, V. A. Fischetti, and S. L. Leib. 2008. Phage lytic enzyme cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J. Infect. Dis. 197:1519-1522. [DOI] [PubMed] [Google Scholar]
- 11.Jado, I., R. Lopez, E. Garcia, A. Fenoll, J. Casal, and P. Garcia. 2003. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 52:967-973. [DOI] [PubMed] [Google Scholar]
- 12.Khosla, C., and P. B. Harbury. 2001. Modular enzymes. Nature 409:247-252. [DOI] [PubMed] [Google Scholar]
- 13.Leclerc, D., and A. Asselin. 1989. Detection of bacterial cell wall hydrolases after denaturing polyacrylamide gel electrophoresis. Can. J. Microbiol. 35:749-753. [DOI] [PubMed] [Google Scholar]
- 14.Loeffler, J. M., S. Djurkovic, and V. A. Fischetti. 2003. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 71:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeffler, J. M., D. Nelson, and V. A. Fischetti. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170-2172. [DOI] [PubMed] [Google Scholar]
- 16.Loessner, M. J., S. Gaeng, and S. Scherer. 1999. Evidence for a holin-like protein gene fully embedded out of frame in the endolysin gene of Staphylococcus aureus bacteriophage 187. J. Bacteriol. 181:4452-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loessner, M. J., S. Gaeng, G. Wendlinger, S. K. Maier, and S. Scherer. 1998. The two-component lysis system of Staphylococcus aureus bacteriophage Twort: a large TTG-start holin and an associated amidase endolysin. FEMS Microbiol. Lett. 162:265-274. [DOI] [PubMed] [Google Scholar]
- 18.Loessner, M. J., K. Kramer, F. Ebel, and S. Scherer. 2002. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 44:335-349. [DOI] [PubMed] [Google Scholar]
- 19.Low, L. Y., C. Yang, M. Perego, A. Osterman, and R. C. Liddington. 2005. Structure and lytic activity of a Bacillus anthracis prophage endolysin. J. Biol. Chem. 280:35433-35439. [DOI] [PubMed] [Google Scholar]
- 20.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 21.McCullers, J. A., A. Karlstrom, A. R. Iverson, J. M. Loeffler, and V. A. Fischetti. 2007. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog. 3:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarre, W. W., H. Ton-That, K. F. Faull, and O. Schneewind. 1999. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage phi11. Identification of a d-alanyl-glycine endopeptidase activity. J. Biol. Chem. 274:15847-15856. [DOI] [PubMed] [Google Scholar]
- 23.Nelson, D., L. Loomis, and V. A. Fischetti. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 98:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson, J. B., T. K. Nilsson, J. H. Jansson, K. Boman, S. Soderberg, and U. Naslund. 2002. The effect of streptokinase neutralizing antibodies on fibrinolytic activity and reperfusion following streptokinase treatment in acute myocardial infarction. J. Intern. Med. 252:405-411. [DOI] [PubMed] [Google Scholar]
- 25.Obeso, J. M., B. Martinez, A. Rodriguez, and P. Garcia. 2008. Lytic activity of the recombinant staphylococcal bacteriophage PhiH5 endolysin active against Staphylococcus aureus in milk. Int. J. Food Microbiol. 128:212-218. [DOI] [PubMed] [Google Scholar]
- 26.O'Flaherty, S., A. Coffey, W. Meaney, G. F. Fitzgerald, and R. P. Ross. 2005. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 187:7161-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashel, M., J. Uchiyama, T. Ujihara, Y. Uehara, S. Kuramoto, S. Sugihara, K. Yagyu, A. Muraoka, M. Sugai, K. Hiramatsu, K. Honke, and S. Matsuzaki. 2007. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J. Infect. Dis. 196:1237-1247. [DOI] [PubMed] [Google Scholar]
- 28.Sass, P., and G. Bierbaum. 2007. Lytic activity of recombinant bacteriophage φ11 and φ12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 73:347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]
- 30.Squire, I. B., W. Lawley, S. Fletcher, E. Holme, W. S. Hillis, C. Hewitt, and K. L. Woods. 1999. Humoral and cellular immune responses up to 7.5 years after administration of streptokinase for acute myocardial infarction. Eur. Heart J. 20:1245-1252. [DOI] [PubMed] [Google Scholar]