Abstract
Certain bacteria use cell-to-cell chemical communication to coordinate community-wide phenotypic expression, including swarming motility, antibiotic biosynthesis, and biofilm production. Here we present a marine gram-positive bacterium that secretes secondary metabolites capable of quenching quorum sensing-controlled behaviors in several gram-negative reporter strains. Isolate C42, a Halobacillus salinus strain obtained from a sea grass sample, inhibits bioluminescence production by Vibrio harveyi in cocultivation experiments. With the use of bioassay-guided fractionation, two phenethylamide metabolites were identified as the active agents. The compounds additionally inhibit quorum sensing-regulated violacein biosynthesis by Chromobacterium violaceum CV026 and green fluorescent protein production by Escherichia coli JB525. Bacterial growth was unaffected at concentrations below 200 μg/ml. Evidence is presented that these nontoxic metabolites may act as antagonists of bacterial quorum sensing by competing with N-acyl homoserine lactones for receptor binding.
Taxonomically diverse marine bacteria have proven to be a rich resource for the discovery of structurally unique and bioactive secondary metabolites (5). Given the intense microbial competition for resources such as space and nutrients, it is probable that many excreted metabolites help mediate microbe-microbe interactions. Various antibiotics have been implicated as chemical defenses for marine bacteria, thus suggesting a role for the biosynthesis of toxic metabolites. For example, a pelagic Alteromonas species produces the antibiotic 2-n-pentyl-4-quinolinol, capable of influencing bacterial community structure on particles (25), and production of the antibiotic andrimid by a marine Vibrio species prevents colonization of surfaces by the particle specialist Vibrio cholerae (26).
Though not yet widely studied, the secretion of nontoxic molecules could also play important roles in antagonistic marine microbial interactions. Quorum sensing pathways of competing bacteria are potential targets for such nontoxic chemical defenses. Bacterial communication is facilitated by the production and subsequent recognition of small signaling molecules (autoinducers) and can regulate important phenotypes, including bioluminescence, biofilm formation, swarming motility, antibiotic biosynthesis, and virulence factor production (3, 7, 15). Gram-negative bacteria commonly use N-acyl homoserine lactones (AHL) as signaling molecules, which bind their cognate receptor proteins to activate gene expression (10). These autoinducers share a conserved l-homoserine lactone moiety, and the length and sites of oxidation on the acyl chain dictate the species specificity (37). In contrast, gram-positive bacteria generally accomplish quorum sensing using posttranslationally modified peptides as autoinducers. For example, Staphylococcus aureus uses cyclic oligopeptides to regulate virulence factor production (11).
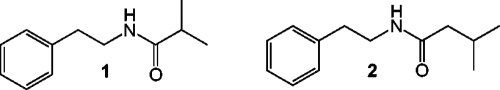
Here we report the production of nontoxic secondary metabolites by a marine gram-positive bacterium that interfere with quorum sensing-regulated phenotypes in several gram-negative species. Using a cocultivation experiment, a marine Halobacillus salinus isolate was discovered to inhibit bioluminescence, a quorum sensing-controlled phenotype, by Vibrio harveyi. Bioassay-guided fractionation led to the identification of two secondary metabolites responsible for the observed activity (Fig. 1). These compounds further quenched quorum sensing-controlled activities in two additional reporter strains. To our knowledge, this is the first example of secondary metabolites secreted by bacilli that inhibit quorum sensing-regulated behaviors in gram-negative species.
FIG. 1.
Structures of cell signaling antagonists produced by marine Halobacillus salinus isolate C42. Compound 1, N-(2′-phenylethyl)-isobutyramide; compound 2, 3-methyl-N-(2′-phenylethyl)-butyramide.
MATERIALS AND METHODS
Media.
Isolation and propagation media consisted of the following: (i) Luria-Bertani broth (LB) (4), (ii) LB containing 4 g sodium chloride (LB4), (iii) nutrient broth (NB) containing 3 g beef extract and 5 g peptone per liter deionized H2O, and (iv) marine broth (MB) containing 1 g yeast extract and 5 g peptone per liter synthetic seawater (Instant Ocean; 36 g per liter). For agar media, 15 g agar per liter of H2O was used. For soft agar media, 8 g agar per liter of H2O was used.
Source of quorum sensing autoinducers and antagonists.
N-Hexanoyl-l-homoserine lactone (HHL) and N-decanoyl-dl-homoserine lactone (DHL) were synthesized as described by Chhabra et al. (6). DHL was used as a positive control for quorum sensing inhibition in the disc diffusion assays at 200 μg per disc (31). 4-Bromo-5-(bromomethylene)-2-(5H)-furanone was synthesized as reported previously (28) and used as a positive control in broth dilution assays (1). The 3-oxo-hexanoyl-homoserine lactone (OHHL) was purchased from Sigma-Aldrich.
Isolation and identification of bacterial isolate C42.
Bacterial strain C42 was isolated from a sea grass sample collected in 2002 from Point Judith Salt Pond, South Kingstown, RI. The sea grass was sectioned with a sterile scalpel, and a portion was submerged in 1 ml of autoclaved sterile seawater, vortexed for several seconds, and heat shocked at 70°C for 6 min. One hundred microliters of the resulting water was spread onto an MB agar plate and incubated at ambient temperature for several days. Isolate C42 was picked as a single colony and restreaked a minimum of twice to ensure a pure strain. C42 is cultivatable in MB at 25°C. The isolate was stored in 25% glycerol at −80°C.
Phylogenetic identification of C42 was accomplished using 16S rRNA gene sequence comparison. Total DNA was extracted using the DNeasy tissue kit (Qiagen, Valencia, CA) per the manufacturer's protocol. Amplification of the bacterial 16S rRNA gene was performed with B27F forward primer (5′-AGA GTT TGA TCC TGG CTC AG-3′) and B1392R reverse primer (5′-ACG GGC GGT GTG TRC-3′) and an annealing temperature of 60°C. Sequencing was conducted on an Applied Biosystems 3130xl genetic analyzer at the Rhode Island Genomics and Sequencing Center (Kingston, RI).
Quorum sensing reporter strains and assays.
V. harveyi BB120 (2), a wild-type bioluminescent strain, was cultivated at 30°C in MB. Bioluminescence was observed using a Typhoon 9410 variable mode imager (GE Healthcare Bio-Sciences) in chemiluminescence mode. Chromobacterium violaceum ATCC 12472 produces the pigment violacein in response to threshold concentrations of the autoinducer HHL (33). C. violaceum was cultured at 29°C with shaking in NB. C. violaceum CV026 is a mini-Tn5 transposon mutant of C. violaceum ATCC 31532 that produces violacein only with exogenous addition of HHL (31). CV026 was cultured in LB at 29°C, and disc diffusion assays were conducted with 30 μM HHL added as a supplement to the soft agar. Escherichia coli JB525 is E. coli MT102 harboring the gfp plasmid pJBA132. This mutant produces an unstable green fluorescent protein (GFP) in response to C6-C8 AHL autoinducers (1). E. coli JB525 was cultured in LB4 at 30°C.
A bacterium-bacterium competition assay was used to assess the ability of isolate C42 to inhibit bioluminescence by V. harveyi BB120. Two microliters of overnight culture of C42 in MB was spotted onto an MB agar plate and incubated at 23°C for 48 h. The colony was covered with a sterile 12,000- to 14,000-molecular-weight-cutoff (MWCO) dialysis membrane (Spectra/Por; Spectrum Medical Industries, Inc., Houston, TX), overlaid with 5 ml of MB soft agar seeded with 50 μl of overnight V. harveyi BB120, and incubated at 30°C for 12 to 16 h. Bioluminescence was observed using a Typhoon 9410 variable mode imager in chemiluminescence mode. Zones of no light production were measured to the nearest mm. The competition assay was also conducted using sterilized 3,000- to 4,000-MWCO dialysis membranes.
Disc diffusion assays were performed with pure compounds or crude mixtures at 500 μg/disc. Fifty microliters of overnight bacterial culture was added to 5 ml of molten soft agar, vortexed, and poured atop an agar plate. Impregnated, sterile discs were laid onto the test plates and incubated overnight. Zones of inhibition (ZOIs; light or pigment production) were measured to the nearest mm.
Broth assays were performed as follows with pure compounds. An overnight culture of V. harveyi BB120 in MB was diluted (optical density at 600 nm [OD600] = 0.1), and 100 μl of the diluted culture was added to 5 ml of MB and separated into 995-μl subsamples. Five microliters of test compounds dissolved in dimethyl sulfoxide (DMSO) was added to the bacterial cultures. The treated cultures were distributed to the wells of an opaque microtiter plate (Nunc A/S, Denmark; 0.5 to 500 μM test compound; 0.5% DMSO final concentration) and incubated at 30°C with shaking for 4 h. The plates were read on a Packard Lumicount microtiter plate reader (Packard, United Kingdom). Relative luminescence units were normalized by the OD600 values obtained by transferring 100 μl to a clear-bottomed microtiter plate (SpectraMax Multimode Microplate Reader; Molecular Devices). Percent luminescence was calculated by defining the untreated cells (no inhibitor) as 100%.
Inhibition of fluorescence was determined using a method modified from the work of Andersen et al. (1). An overnight culture of E. coli JB525 in LB4 broth was diluted to an OD450 of 0.25 with fresh medium and treated with the test compounds and OHHL, each dissolved in DMSO (32 nM OHHL; 0.01 to 1,000 μM test compound; 0.8% DMSO final concentration). Two hundred microliters of the final culture was added to wells of an opaque microtiter plate and incubated with shaking at 30°C for 90 min. Fluorescence was determined using a Packard Fluorocount microtiter plate reader (λ = 480-nm excitation, λ = 515-nm emission). Relative fluorescence values were normalized by optical density values obtained by transferring 100 μl to a clear-bottomed microtiter plate (λ = 450 nm; SpectraMax Multimode Microplate Reader). The assay was also performed with increasing serial concentrations of OHHL (16 nM to 512 nM). Percent fluorescence was calculated by defining the cells with no inhibitor present as 100% for each autoinducer concentration.
Mathematical modeling of the data.
To characterize the pharmacodynamic relationship between compound 2 and OHHL, the data were fitted into the equations below. Equation 1 represents the concentration-effect relationship for two compounds working competitively on the same receptor, and equation 2 represents the composite effect of the two compounds working on different receptors (22). The data were fitted using nonlinear regression analysis implemented in SPSS version 16.0 statistical software (SPSS Inc., Chicago, IL).
 |
(1) |
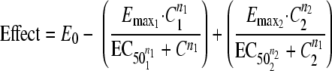 |
(2) |
E0 is the baseline effect, Emax is the maximal effect, EC50 is the concentration at half-maximal effect (50% effective concentration), n is the constant expressing the sigmoidicity of the concentration-effect relationship, and 1 and 2 denote the parameters estimated for antagonist and agonist, respectively.
Extraction and isolation of active compounds produced by isolate C42.
Two milliliters of H. salinus isolate C42 overnight culture in MB was spread evenly onto agar surfaces (16- × 30-cm aluminum trays; 250 ml MB agar per tray; 100 trays) and incubated at 23°C for 48 h. Whole cultures were extracted three times with ethyl acetate (EtOAc). The resulting extracts were concentrated in vacuo and fractionated by vacuum liquid chromatography on silica gel in 20% stepwise gradients of EtOAc in isooctane (TMP). Bioassays conducted against V. harveyi BB120 demonstrated activity in the 80% (448-mg) and 100% (440-mg) EtOAc fractions. 1H nuclear magnetic resonance (NMR) spectra of these two fractions appeared nearly identical. Reversed-phase high-pressure liquid chromatography (X-Terra Prep RP C18 column, 100 × 19 mm; 10% to 60% methanol [MeOH] in H2O at 10 ml per min; UV detection at λ = 220 and 254 nm) of the 100% EtOAc fraction yielded active metabolites 1 (96 mg) and 2 (123 mg). Mass spectral data were acquired in MeOH-H2O (1:1) containing 0.1% acetic acid (Applied Biosystems Mariner Mass Spectrometer). NMR spectra were recorded on a Bruker Biospin spectrometer (400 MHz for 1H, 100 MHz for 13C) and were referenced to residual solvent signals with resonances dH/C 3.31/49.15 (MeOH-d4).
Synthesis of phenethylamide metabolites.
Isovaleric acid or isobutyric acid (39.8 mmol, 1 eq) in 50 ml acetonitrile was treated with 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (47.7 mmol, 1.2 eq), 1-hydroxybenzotriazole (47.7 mmol, 1.2 eq), diisopropylethylamine (59.6 mmol, 1.5 eq), and phenethylamine (39.8 mmol, 1 eq). The reaction mixture was stirred overnight at ambient temperature, concentrated in vacuo, and then partitioned between EtOAc and 0.5 N HCl. The organic phase was separated, sequentially extracted with saturated NaHCO3 and H2O, dried over Na2SO4, and evaporated to dryness. Desired products were purified by silica gel column chromatography (Selecto Scientific) using 30% EtOAc in TMP, followed by recrystallization from TMP and EtOAc. Products were analyzed by NMR and mass spectrometry as described above and were identical to the purified natural products.
Detection of lactonase genes.
Amplification of the aiiA lactonase gene by PCR was performed as described by Dong et al. (8) with the following modification. PCR was performed using optimized conditions consisting of an initial denaturation step of 5 min at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 44.9°C, and 1 min at 72°C and a final extension time of 7 min at 72°C. Bacillus cereus was used as a positive control for the lactonase gene.
RESULTS
Bacterial isolate C42 was cultivated from a sea grass sample collected from a Rhode Island estuary. Attempts to culture the bacterium on MB agar plates prepared with fresh H2O resulted in no growth, indicating that the organism is obligate marine. A BLAST search (NCBI, 1 November 2007) of the amplified 16S rRNA gene sequence (1,353 bases, deposited with GenBank under accession no. EU259061) clearly placed this isolate in the genus Halobacillus, with the nearest characterized species being H. salinus strain HSL-3 (99% similarity).
Isolate C42 demonstrated the ability to inhibit bioluminescence of V. harveyi in a cocultivation experiment. Colonies of C42 were cultivated on a marine agar plate for 48 h and then covered with 12,000- to 14,000-MWCO dialysis membranes. V. harveyi seeded in soft marine agar was poured over the membrane, and the plate was incubated for 12 to 16 h. Upon conclusion of the assay, distinct dark zones of approximately 25 mm surrounded the established 5-mm C42 bacterial colony (Fig. 2a). No visible growth inhibition of V. harveyi was observed. The assay was repeated with 3,000- to 4,000-MWCO dialysis membranes, and the same results were recorded.
FIG. 2.
Inhibition of V. harveyi bioluminescence by marine Halobacillus isolate C42. (a) Strain C42 was cultivated on marine agar for 48 h and then overlaid with dialysis membrane (molecular weight cutoff, 12,000 to 14,000). Soft agar inoculated with V. harveyi BB120 was poured over the surface of the plate and incubated for 16 h at 28°C. The picture is a light image showing decreased bioluminescence surrounding a colony of isolate C42. (b) Light image of a disc diffusion assay of the bacterial (left) and synthetic (right) metabolite 2. The growth of V. harveyi was not visibly inhibited surrounding the C42 colony or the discs.
The above experiment suggested that small molecules might be diffusing across the dialysis membrane and interfering with luminescence by V. harveyi. Experiments were conducted to test if secreted cell signaling antagonists were responsible for the observed activity. A large-scale cultivation of C42 was undertaken on 100 trays of marine agar for 48 h at 25°C. The whole cultures were extracted with EtOAc, yielding 2 g of crude extracts. Disc diffusion assays against V. harveyi BB120 demonstrated inhibition of bioluminescence without any visible effects on bacterial growth (ZOI, 63 mm; 500 μg/disc). Bioassay-guided fractionation of the active agents was accomplished using silica gel chromatography followed by reversed-phase high-pressure liquid chromatography purification. These efforts resulted in the purification of two active metabolites. Compound 1 displayed a 15-mm ZOI and compound 2 displayed a 22-mm ZOI against V. harveyi BB120 in disc diffusion assays (500 μg/disc). The pure metabolites were identified as N-(2′-phenylethyl)-isobutyramide (1) and 3-methyl-N-(2′-phenylethyl)-butyramide (2) based on comparison of 1H and 13C NMR and mass spectral data with literature values (30). De novo synthesis of the identified metabolites confirmed the predicted structures and measured bioactivities (Fig. 2b and see below) of these metabolites.
Some members of the genus Bacillus are reported to quench quorum sensing by enzymatic degradation of the lactone signaling molecules (9). Isolate C42 was analyzed for the presence of lactonase genes; however, molecular analysis provided no evidence for the presence of previously reported lactonase genes. C42 did not detectably degrade autoinducers in an AHL degradation assay (data not shown [8]). Therefore, enzymatic degradation was not a contributing factor in the observed inhibition of bioluminescence by isolate C42.
Metabolites inhibit multiple quorum sensing-regulated phenotypes.
Since bioluminescence is a quorum sensing-regulated phenotype, the metabolites were further tested for activity against other quorum sensing reporter strains. Disc diffusion assays were conducted against C. violaceum CV026, a bacterial strain that produces the purple pigment violacein in response to threshold concentrations of exogenously supplied HHL (31). Both compounds inhibited violacein production without visible growth inhibition. ZOIs of 10 mm and 13 mm were observed for compounds 1 and 2, respectively, when tested at 500 μg/disc. Both compounds were additionally tested against the wild-type strain C. violaceum ATCC 12472. This bacterium requires no exogenous addition of autoinducer and is thus more ecologically relevant. Compound 2 demonstrated increased activity with a zone of 20 mm at 500 μg/disc, while compound 1 retained the same activity against the wild-type strain.
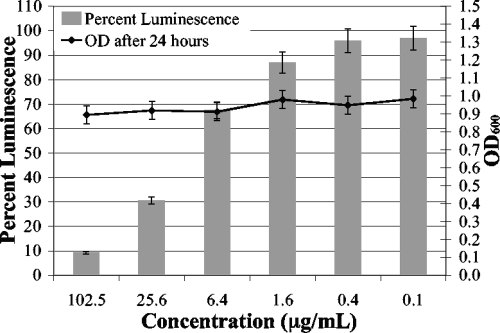
The metabolites inhibited bioluminescence of planktonically growing V. harveyi BB120. The treated cultures were grown in microtiter plate wells in the presence of a compound or control for 4 h, and then luminescence was measured with a Packard Lumicount microtiter plate reader. Compound 2 inhibited bioluminescence with an EC50 of 9 μg/ml (Fig. 3). Compound 1 had no notable activity in this assay. The cultures were then shaken for an additional 14 h, and bacterial growth was measured (OD600) to test for antibiotic activity. Neither of the two compounds demonstrated growth inhibition at the highest concentrations tested (100 μg/ml).
FIG. 3.
Concentration-dependent inhibition of bioluminescence by planktonic V. harveyi. V. harveyi strain BB120 regulates bioluminescence via AHL-mediated quorum sensing. Serial dilutions of metabolite 2 were added to microtiter plate wells containing dilutions of V. harveyi BB120 and incubated for 4 h. Relative fluorescence units (RLU) were normalized for growth (OD600). No bacterial growth inhibition, noted by optical density values 14 h after the end point, was observed over the concentration range of the assay (dark line).
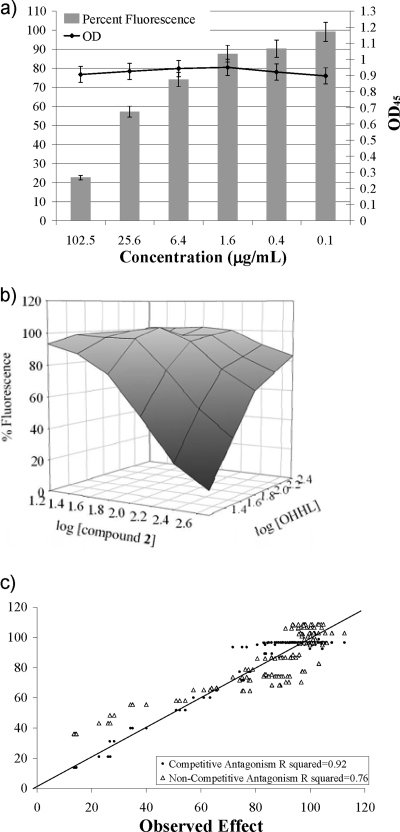
The similar molecular sizes and structures of the inhibitors and AHL autoinducers suggested that the phenethylamides might be AHL structural mimics and compete for receptor binding. The mutant E. coli JB525 strain was used to test this hypothesis. This sensor strain produces the LuxR AHL receptor of Vibrio fischeri, and once activated by exogenously supplied autoinducer, LuxR positively affects expression of a luxI promoter that then initiates production of an unstable GFP (1). Thus, AHL binding to LuxR can be measured as a function GFP fluorescence. Since the strain can sense but not produce AHL signals, concentrations of agonist (OHHL) and antagonist can be manipulated to investigate agonist-antagonist relationships (27). Similar dose-response, competition methods have been used to investigate the potential mechanisms of action of synthetic quorum sensing antagonists (20, 34). Metabolite 1 had no notable activity in this assay, while metabolite 2 displayed concentration-dependent inhibition of GFP (Fig. 4a, EC50 = 19 μg/ml at 32 nM OHHL). No E. coli growth inhibition was measured (OD450) at 200 μg/ml of metabolite 2 after 14 h, the highest concentration tested. Next, compound 2 was tested in serial dilutions against rising concentrations of OHHL (16 to 512 nM). Increased OHHL surmounted the inhibitory effects of 2 (Fig. 4b), consistent with an agonist-antagonist relationship. To better understand the nature of this antagonism, the measured effects of OHHL and compound 2 were plotted against values predicted by pharmacodynamic models for competitive and noncompetitive antagonism (Fig. 4c) (22). The mathematical model for competitive antagonism (equation 1) provided an excellent correlation with the experimentally observed responses, with an R2 value of 0.92 indicating that 92% of the variability in the effect can be explained from the concentration of agonist and antagonist and by the model used. This competitive antagonist model also yielded good precision for Emax (122 ± 6 μg/ml) and EC50 (28 ± 7 μg/ml) values estimated as judged by low standard error values. In contrast, the model for noncompetitive antagonism provided a much poorer fit (R2 = 0.77) and higher values of standard error (Emax = 74 ± 22 μg/ml and EC50 = 106 ± 62 μg/ml).
FIG. 4.
Antagonism of OHHL-mediated GFP production by E. coli JB525. E. coli strain JB525 produces the LuxR of V. fischeri and makes an unstable GFP in response to exogenously added AHL autoinducers. (a) H. salinus metabolite 2 blocks GFP production in a concentration-dependent manner. The graph shows assay results with OHHL supplied at 32 nm. No growth inhibition of the bacterium was observed over the concentration range of the assay (dark line) at 14 h. (b) Surface plot showing GFP production (fluorescence) at various concentrations of antagonist (compound 2) and agonist (OHHL). The inhibitory activity of compound 2 is surmounted by higher concentrations of OHHL agonist, consistent with an antagonist-agonist relationship. The concentration of compound 2 is micromolar and that of OHHL is nanomolar before conversion to log scale. (c) Plot of measured versus predicted responses using the stated pharmacodynamic model. Predicted responses were calculated using equations that model competitive and noncompetitive antagonism. The solid line is the line of unity. Response (fluorescence) is normalized to 100%. Measured values correlate better with those predicted for competitive antagonism (□, R2 = 0.92) than with those predicted for noncompetitive antagonism (▴, R2 = 0.76).
Enhanced antagonism effects of additional bacterial metabolites.
Crude extracts from the Halobacillus cultures were more active in disc diffusion assays than the isolated metabolites. However, the bioassay-guided fractionation led only to phenethylamides 1 and 2 as possessing the desired bioactivities. Therefore, it was hypothesized that another compound(s) might work in concert with the identified metabolites to augment the activity. During the silica gel chromatography step of the bioassay-guided fractionation, the fourth fraction (80% EtOAc in TMP) was composed almost entirely of metabolites 1 and 2. This material was retested in the V. harveyi BB120 disc diffusion assay in combination with the other fractions (250 μg/disc per fraction). It was determined that the second fraction (40% EtOAc in TMP) significantly enhanced bioluminescence inhibition when assayed in combination (ZOI = 75 mm). Interestingly, this fraction exhibited no observable activity of its own at 500 μg/disc. 1H NMR spectra of this material suggested that it was composed of lipophilic compounds possessing long alkyl chains. Unfortunately, further chromatographic separation of fraction 2 resulted in loss of the enhanced inhibitory effects.
DISCUSSION
Antagonism of quorum sensing has previously been observed between closely related species of bacteria. Antagonist activity arises from slight structural variations between similar autoinducers. For example, quorum sensing inhibition has been observed between subgroups of S. aureus isolates, with structurally distinct autoinducing peptides from one subgroup able to chemically antagonize the signaling systems of others (21). Inhibition of quorum sensing also occurs between the closely related species S. aureus and Staphylococcus epidermidis (1). In gram-negative bacteria, AHL receptor proteins are activated most efficiently by their native autoinducers. Small alterations in the AHL structure can disrupt proper signaling, either diminishing or abolishing subsequent gene transcription. To illustrate, decanoyl homoserine lactone, the native autoinducer for Burkholderia pseudomallei (41), potently inhibits the HHL quorum sensing system of C. violaceum (31).
Differing from the studies discussed above, here we observed inhibition of quorum sensing phenotypes between distantly related bacteria. The structures for the two bioactive metabolites were previously identified from a limnic Bacillus species. Neither molecule demonstrated toxicity when tested against a panel of microalgae, bacteria, and fungi at concentrations up to 200 μg/ml (30). The quorum sensing-inhibitory properties of these metabolites are reported here for the first time.
Pharmacodynamic modeling demonstrated that the experimentally measured responses in the E. coli JB525 assay are consistent with those predicted for competitive antagonism. Competitive antagonists lack intrinsic efficacy but bind to the same receptor site as does the native agonist. Like AHLs, the H. salinus metabolites possess a ring system with a side chain connected via an amide bond. In synthetic studies that have targeted the creation of quorum sensing antagonists based on AHL structural motifs, inhibitors have been designed with a phenyl ring appended to either the end of the acyl chain or replacing the lactone ring. For example, 4-phenylbutanoyl homoserine lactone inhibits the binding of OHHL in E. coli containing LuxR and also antagonizes LuxR in V. fischeri (12, 37). 3-Oxo-C12-(2-aminophenol) was found to inhibit GFP production in a Pseudomonas aeruginosa strain constructed to express GFP in its quorum sensing circuit (39), as well as TraR in Agrobacterium tumefaciens (13). The phenethylamide metabolites described here represent a new chemotype of quorum sensing inhibitors from nature.
Lipophilic metabolites appear to work in concert with the identified metabolites for maximum attenuation of quorum sensing. This result is not entirely unexpected, since some lipids can enhance the sensitivity of bacteria to antibiotics by modifying cell permeability (23). Attempts to purify the activity-enhancing compounds were unsuccessful, suggesting that several lipophilic molecules might be acting in concert to exert this effect. Further investigation is under way to elucidate the nature of this combination result. Nevertheless, the pure phenethylamide metabolites demonstrated inhibition of quorum sensing-regulated behaviors in an array of assays, and synthetically prepared compounds reproduced these activities identically.
The ubiquitous marine bacterium V. harveyi was selected as an assay strain since it could be cocultured with H. salinus on seawater agar plates and has a well-studied quorum sensing system. Chemical communication by V. harveyi is accomplished via three parallel pathways that utilize the autoinducers HAI-1, CAI-1, and AI-2. HAI-1 is 3-hydroxybutanoyl-l-homoserine lactone and facilitates the species-specific AHL pathway (2). CAI-1 has recently been established as (S)-3-hydroxytridecan-4-one (16, 19) and may serve as an intergenus communication molecule. AI-2 is a furanosyl borate diester that has been proposed to play a role in interspecies communication (2). In addition to bioluminescence (35, 40), these three parallel quorum sensing systems regulate type III secretion (17), siderophore and polysaccharide production (24), and metalloprotease production (32). Inhibition of the HAI-1 pathway by the phenethylamide metabolites is consistent with a strong decrease in V. harveyi bioluminescence. Genetic knockouts of the HAI-1 pathway lead to a 99.9% reduction in bioluminescence (16).
Several eukaryotes, including plants and fungi, have been shown to produce secondary metabolites that interfere with quorum sensing pathways of gram-negative bacteria (29, 36, 38, 42). Cell signaling antagonists are proposed to have a protective effect against pathogens and microbial fouling (14). The H. salinus isolate studied here suggests that some gram-positive bacteria have also developed this chemical skill, perhaps as a mechanism to compete within microbial assemblages in their natural environments. Prevention of coordinated bacterial behaviors such as swarming motility, antibiotic secretion, and biofilm production could greatly impede the success of a bacterial community in competing for space and other resources. Indeed, quorum sensing antagonists have been proposed as mechanistically novel antimicrobial drugs (18). Further investigations are currently under way to survey the prevalence of such nontoxic interactions in the marine environment and also to better understand the ecological significance of quorum sensing antagonism in bacterial competition.
Acknowledgments
This research was supported by NSF grant MCB 04538743 and NIH grant P20 RR016457 from the BRIN Program of the National Center for Research Resources. It was further made possible by the use of Rhode Island INBRE research core facilities supported jointly by NCRR/NIH grant no. P20 RR016457 and the network institutions.
We thank M. Givskov for the use of E. coli JB525.
Footnotes
Published ahead of print on 5 December 2008.
REFERENCES
- 1.Andersen, J. B., A. Heydorn, M. Hentzer, L. Eberl, O. Geisenberger, B. B. Christensen, S. Molin, and M. Givskov. 2001. gfp-based N-acyl homoserine-lactone sensor systems for detection of bacterial communication. Appl. Environ. Microbiol. 67:575-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassler, B. L., E. P. Greenberg, and A. M. Stevens. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 179:4043-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassler, B. L., and R. Losick. 2006. Bacterially speaking. Cell 125:237-246. [DOI] [PubMed] [Google Scholar]
- 4.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blunt, J. W., B. R. Copp, M. H. G. Munro, P. T. Northcote, and M. R. Prinsep. 2006. Marine natural products. Nat. Prod. Rep. 23:26-78. [DOI] [PubMed] [Google Scholar]
- 6.Chhabra, S. R., P. Stead, N. J. Bainton, G. P. C. Salmond, G. S. A. B. Stewart, P. Williams, and B. W. Bycroft. 1993. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-l-homoserine lactone. J. Antibiot. (Tokyo) 43:441-454. [DOI] [PubMed] [Google Scholar]
- 7.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 8.Dong, Y. H., A. R. Gusti, Q. Zhang, J. L. Xu, and L. H. Zhang. 2002. Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl. Environ. Microbiol. 68:1754-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong, Y. H., J. L. Xu, X. Z. Li, and L. H. Zhang. 2000. AiiA, an enzyme that inactivates the acyl homoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 97:3526-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 11.George, E. A., and T. W. Muir. 2007. Molecular mechanisms of agr quorum sensing in virulent staphylococci. Chembiochem 8:847-855. [DOI] [PubMed] [Google Scholar]
- 12.Geske, G. D., J. C. O'Neill, D. M. Miller, R. J. Wezeman, M. E. Mattmann, Q. Lin, and H. E. Blackwell. 2008. Comparative analyses of N-acylated homoserine lactones reveal unique structural features that dictate their ability to activate or inhibit quorum sensing. Chembiochem 9:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geske, G. D., R. J. Wezeman, A. P. Siegel, and H. E. Blackwell. 2005. Small molecule inhibitors of bacterial quorum sensing and biofilm formation. J. Am. Chem. Soc. 127:12762-12763. [DOI] [PubMed] [Google Scholar]
- 14.Givskov, M., R. De Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg, E. P. 2003. Bacterial communication and group behavior. J. Clin. Investig. 112:1288-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henke, J. M., and B. L. Bassler. 2004. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 186:6902-6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henke, J. M., and B. L. Bassler. 2004. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 186:3794-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hentzer, M., and M. Givskov. 2003. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 112:1300-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins, D. A., M. E. Pomianek, C. M. Kraml, R. K. Taylor, M. F. Semmelhack, and B. L. Bassler. 2007. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature 450:883-886. [DOI] [PubMed] [Google Scholar]
- 20.Janssens, J. C. A., K. Metzger, R. Daniels, D. Ptacek, T. Verhoeven, L. W. Habel, J. Vanderleyden, D. E. De Vos, and S. C. J. De Keersmaecker. 2007. Synthesis of N-acyl homoserine lactone analogues reveals strong activators of SdiA, the Salmonella enterica serovar Typhimurium LuxR homologue. Appl. Environ. Microbiol. 73:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji, G., R. Beavis, and R. R. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 22.Jonker, D. M., S. A. G. Visser, P. H. van der Graaf, R. A. Voskuyl, and M. Danhof. 2005. Towards a mechanism-based analysis of pharmacodynamic drug-drug interactions in vivo. Pharmacol. Ther. 106:1-18. [DOI] [PubMed] [Google Scholar]
- 23.Krogfelt, K. A., M. Utley, H. C. Krivan, D. C. Laux, and P. S. Cohen. 2000. Specific phospholipids enhance the activity of β-lactam antibiotics against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 46:377-384. [DOI] [PubMed] [Google Scholar]
- 24.Lilley, B. N., and B. L. Bassler. 2000. Regulation of quorum sensing in Vibrio harveyi by LuxO and sigma-54. Mol. Microbiol. 36:940-954. [DOI] [PubMed] [Google Scholar]
- 25.Long, R. A., A. Qureshi, D. J. Faulkner, and F. Azam. 2003. 2-n-Pentyl-4-quinolinol produced by a marine Alteromonas sp. and its potential ecological and biogeochemical roles. Appl. Environ. Microbiol. 69:568-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long, R. A., D. C. Rowley, E. Zamora, J. Liu, D. H. Bartlett, and F. Azam. 2005. Antagonistic interactions among marine bacteria impede the proliferation of Vibrio cholerae. Appl. Environ. Microbiol. 71:8531-8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manefield, M., R. De Nys, N. Kumar, R. Read, M. Givskov, P. Steinberg, and S. Kjelleberg. 1999. Evidence that halogenated furanones from Delisa pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 145:283-291. [DOI] [PubMed] [Google Scholar]
- 28.Manny, A. J., S. Kjelleber, N. Kumar, R. de Nys, R. W. Read, and P. Steinberg. 1997. Reinvestigation of the sulfuric acid-catalysed cyclization of brominated 2-alkyllevulinic acids to 3-alkyl-5-methylene-2(5H)-furanones. Tetrahedron 53:15813-15826. [Google Scholar]
- 29.Martinelli, D., G. Grossmann, U. Sequin, H. Brandl, and R. Bachofen. 2004. Effects of natural and chemically synthesized furanones on quorum sensing in Chromobacterium violaceum. BMC Microbiol. 4:25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maskey, R. P., R. N. Asolkar, E. Kapaun, I. Wagner-Dobler, and H. Laatsch. 2002. Phytotoxic arylethylamides from limnic bacteria using a screening with microalgae. J. Antibiot. (Tokyo) 55:643-649. [DOI] [PubMed] [Google Scholar]
- 31.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. A. B. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 32.Mok, K. C., N. S. Wingreen, and B. L. Bassler. 2003. Vibrio harveyi quorum sensing: a coincidence detector for two autoinducers controls gene expression. EMBO J. 22:870-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morohoshi, T., M. Kato, K. Fukamachi, N. Kato, and T. Ikeda. 2008. N-Acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC 12472. FEMS Microbiol. Lett. 279:124-130. [DOI] [PubMed] [Google Scholar]
- 34.Morohoshi, T., T. Shiono, K. Takidouchi, M. Kato, N. Kato, J. Kato, and T. Ikeda. 2007. Inhibition of quorum sensing in Serratia marcescens AS-1 by synthetic analogs of N-acylhomoserine lactone. Appl. Environ. Microbiol. 73:6339-6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nealson, K. L. 1977. Autoinduction of bacterial luciferase. Arch. Microbiol. 112:73-79. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen, T. B., M. E. Skindersoe, T. Bjarnsholt, R. K. Phipps, K. B. Christensen, P. O. Jensen, J. B. Anderson, B. Koch, T. O. Larsen, M. Hentzer, L. Eberl, N. Hoiby, and M. Givskov. 2005. Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology 151:1325-1340. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer, A. L., B. L. Hanzelka, A. Eberhard, and E. P. Greenberg. 1996. Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J. Bacteriol. 178:2897-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skindersoe, M. E., P. Ettinger-Epstein, T. B. Rasmussen, T. Bjarnsholt, R. De Nys, and M. Givskov. 2008. Quorum sensing antagonism from marine organisms. Mar. Biotechnol. 10:56-63. [DOI] [PubMed] [Google Scholar]
- 39.Smith, K. M., Y. Bu, and H. Suga. 2003. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol. 10:563-571. [DOI] [PubMed] [Google Scholar]
- 40.Stewart, G. S. A. B., and P. Williams. 1992. lux genes and the applications of bacterial bioluminescence. J. Gen. Microbiol. 138:1289-1300. [DOI] [PubMed] [Google Scholar]
- 41.Ulrich, R. L., D. DeShazer, E. E. Brueggemann, H. B. Hines, P. C. Oyston, and J. A. Jeddeloh. 2004. Role of quorum sensing in the pathogenicity of Burkholderia pseudomallei. J. Med. Microbiol. 53:1053-1064. [DOI] [PubMed] [Google Scholar]
- 42.Vattem, D. A., K. Mihalik, S. H. Crixell, and R. J. C. McLean. 2007. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 78:302-310. [DOI] [PubMed] [Google Scholar]