Abstract
For insects, the prevalence of numerous vertically transmitted viruses can be high in their host populations. These viruses often have few, if any, pathological effects on their hosts, and consequently, many of them can remain unnoticed for long periods, despite their potential role in the evolution of the host phenotype. Some females of Leptopilina boulardi, a solitary parasitoid of Drosophila larvae, are infected by an inherited virus (LbFV) that manipulates the behavior of the wasp by increasing its tendency to lay eggs in a host that is already parasitized (superparasitism). This behavioral alteration allows horizontal transmission of the virus within superparasitized Drosophila larvae. Using suppressive subtractive hybridization with infected and uninfected lines, we identified one putative viral sequence. Based on this sequence, we developed a simple PCR test. We tested the correlation between the superparasitism phenotype and PCR amplification of the putative viral marker using several experimental conditions (including horizontal transfers) and several parasitoid genotypes. All of the results revealed that there was a perfect match between the superparasitism phenotype and the amplification profile, which validated use of the molecular marker as a tool to track the presence of the virus and provided the first genomic data for this fascinating virus. The results also show that there was very efficient horizontal and vertical transmission of LbFV, which probably explains its high prevalence in the French populations that we sampled (67 and 70% of infected females). This manipulative virus is likely to play a major role in the ecology and evolution of its parasitoid host.
Viruses are obligate intracellular parasites that inevitably disturb the normal functioning of the cellular machinery of their hosts. Although most of the viruses commonly studied cause severe damage to their hosts, potentially killing them, some of these viruses have few, if any, detectable effects on the host phenotype or have effects only under very specific environmental conditions. Some examples are the Sigma virus which infects Drosophila melanogaster and is responsible for mortality only under anoxic conditions (7, 16), a picorna-like virus in the fire ant Solenopsis invicta (32), deformed wing virus in honey bee colonies (10), and the salivary gland hypertrophy virus found in Glossina pallidipes (1). These viruses are usually involved in long-standing associations with their hosts through parent-to-offspring transmission (vertical transmission), and their prevalence in host populations may be high (8, 9, 31, 32). As a consequence of their low impact on host fitness, these viruses can remain unnoticed for long periods, despite the fact that they may be a crucial element of the host's phenotype determinism and affect the dynamics and evolution of host populations and communities.
During the last few decades, genomic or transcriptomic approaches have revealed (often fortuitously) new associations and have been shown to be powerful tools for discovering asymptomatic viruses or viral particles that have unexpected effects on the host phenotype. Differential display screening for RNAs and sequencing expression libraries have allowed the identification of persistent viruses in several insect species, such as the recently described noravirus of Drosophila characterized as an iflavirus belonging to the picorna-like virus family, which have no pathological effects on their hosts (20). Iflaviruses were also fortuitously detected by cDNA-amplified fragment length polymorphism analysis in the parasitoid wasp Venturia canescens (25). Endoparasitoid wasps constitute one of the most diverse groups of insects, and there are tens of thousands of species of these wasps which develop inside the bodies of other insects and have established frequent and sometimes obligatory associations with viruses or virus-like entities (15). The best-known example of parasitoid-associated viruses is the polydnaviruses, which are injected by female wasps into the host larvae together with their eggs. Polydnaviruses neutralize host immune defenses, allowing successful development of the larvae of parasitoids until they emerge as free-living adults (15, 29). Uncharacterized viruses known as virus-like particles or viruses belonging to other families, such as the Reoviridae or Ascoviridae, play a similar role in other parasitoid species (14, 28).
The Drosophila parasitoid wasp Leptopilina boulardi (Hymenoptera: Figitidae) is a solitary species; that is, only one parasite larva per host can complete development to the adult stage. Accordingly, females generally lay a single egg in a Drosophila larva. However, observations of naturally caught individuals revealed that a number of females superparasitized larvae; that is, they deposited their eggs in hosts that were already parasitized despite the fact that secondary parasites most often loose the within-host competition that occurs. Analyses of this unusual behavior revealed that superparasitism is induced by viral particles designated LbFV (L. boulardi filamentous virus) observed in the ovaries of L. boulardi females, where virus replication takes place in the nuclei of cells surrounding the oviduct lumen (34, 37). The virus is vertically transmitted through the maternal lineage and can be horizontally transferred when both uninfected and infected larvae are present in the same Drosophila host (34, 35). Besides induction of superparasitism, infected females do not have major fitness costs (33, 36). Theoretical models indicate that induction of superparasitism can be adaptive for the virus (18). Therefore, this phenomenon is best interpreted as host behavioral manipulation. To date, LbFV has not been molecularly characterized because it is difficult to purify viral particles, which probably are present in insects at a low density. The only method used to diagnose infection is based on the clear-cut behavioral modification that is expressed by infected females in an experimental procedure in which only a few hosts are available for parasitization. Using infected strains with the superparasitizing phenotype (S phenotype) and uninfected strains with the nonsuperparasitizing phenotype (NS phenotype) having the same nuclear background, we performed a gene expression analysis using suppressive subtractive hybridization (SSH) and mirror orientation selection techniques in order to obtain viral sequences. From the library obtained, we selected one viral sequence with which we developed a simple PCR-based test for infection. We performed horizontal transmission experiments to test the reliability of the molecular marker for detecting the virus. Finally, we used the PCR-based tool to detect and measure the prevalence of LbFV in natural populations of L. boulardi, in the related species Leptopilina heterotoma, and in their common host D. melanogaster.
MATERIALS AND METHODS
Insect strains and rearing procedures.
Two reference lines of L. boulardi, designated NSref and Sref, that had the same nuclear genetic background and differed only in their infection status were used for most experimental procedures. NSref is an inbred uninfected line (obtained by brother-sister mating that resulted in an estimated homozygosity greater than 82%) that originated from Siena, Italy. Females of this line lay only one egg per host according to the results of a standard experimental procedure (34), and this phenotype is referred to in this paper as the NS phenotype. The Sref line, infected by LbFV, was derived from the NSref line and was obtained after artificial injection of viral particles (37). This newly infected line proved to be stable for generations for both virus infection and superparasitism behavior, and the mean number of eggs per host ranged roughly from two to four.
We also used wild-caught L. boulardi strains that had the S or NS phenotype. Seventeen lines from Gotheron (near Valence, France) known for coexistence of the S and NS phenotypes and two lines from Epinouze (near Lyon, France) (S phenotype) were collected in 2007. We also used one Portuguese line from the island of Madeira (NS phenotype, collected in 2001), a Brazilian line from Rio de Janeiro (S phenotype, collected in 2007), and two Spanish lines from Palma de Mallorca (one S phenotype line and one NS phenotype line, collected in 2006).
In the laboratory, parasitoids were reared with a photoperiod consisting of 12 h of light and 12 h of darkness at 25°C, using as the host a strain of D. melanogaster that originated from Sainte-Foy-lès-Lyon (Rhône, France) and was fed on a standard diet (11).
Subtractive hybridization.
Total RNA was prepared from L. boulardi Sref and NSref ovaries, since viral replication was observed in this tissue. Ovaries were immersed in a dispersion buffer (buffer D; Evrogen, Russia) and stored at −20°C until RNA was extracted (446 pairs of ovaries for the Sref line and 407 pairs of ovaries for the NSref line). The total RNA of each line was extracted by using the procedure suggested by Evrogen (http://www.evrogen.com/technologies/RNA-isolation.shtml).
Aliquots of total RNA from both infected and uninfected ovaries were sent to Evrogen (Russia) for cDNA subtraction. SSH in only one direction (i.e., subtraction of NS phenotype from S phenotype [forward subtraction]) was performed as described previously (12, 13) in order to identify genes specifically expressed in the Sref line. In order to reduce the number of false-positive clones in the SSH-generated library, the SSH technology was combined with a mirror orientation selection procedure (24). The subtracted cDNA samples enriched with differentially expressed sequences were used for library construction. cDNA was then cloned into the pAL16 vector (Evrogen, Russia) and used for Escherichia coli transformation. Four 96-well plates containing individual colonies were used for differential dot blot screening to further identify S phenotype-specific sequences. Finally, the whole subtraction procedure revealed 128 S phenotype-specific expressed clones that were sequenced by GATC Biotech (France). The sequence data were analyzed using the BLASTx algorithm with SpTrembl, the tBLASTx algorithm with NCBI Unigen for arthropods, and the NCBI nonredundant database.
DNA extraction and PCR-based detection of putative viral sequences.
Because electron microscopy revealed viral particles replicating in the nuclei of the insect cells, we hypothesized that LbFV has a DNA genome. Thus, based on a putative viral sequence obtained from the subtracted library, we designed PCR primers that were used for amplification starting with simple DNA extracts. The results of our PCR assays were consistent with the hypothesis described above. DNA extraction was performed using individual females that were crushed in 150 μl of 5% (wt/vol) Chelex (Bio-Rad, United States) and 5 μl of proteinase K (20 mg/ml; Eurobio, France) and incubated overnight at 56°C. After 20 min of incubation at 95°C, samples were centrifuged, and 2-μl portions of the supernatants were used for PCRs. Two sets of primers were designed. The first set of primers, primers I1CL1-F and I1CL1-R, amplified a 345-bp fragment (Table 1) and was used in a uniplex PCR performed with a 25-μl (final volume) mixture containing 2 μl of DNA template, 1.5 mM MgCl2, each deoxynucleoside triphosphate (dNTP) at a concentration of 50 μM, each primer at a concentration of 200 nM, and 0.5 U of Taq polymerase (EuroBlueTaq; Eurobio, France). The second set of primers, primers 102-F and 500-R, was designed to perform a multiplex PCR that amplified the putative viral sequence and also an insect gene as a control for the quality of DNA extraction. The insect sequence was obtained from a previous subtracted library (not shown) and putatively encodes a ribosomal protein (RPS2; E value for ribosomal protein S2 from D. melanogaster, 5 × 10−76). Primer sets 102-F/500-R and RPS2-F/RPS2-R amplified 399- and 162-bp fragments, respectively (Table 1). Multiplex PCRs were performed with a 25-μl (final volume) reaction mixture containing 2 μl of DNA template, 1.5 mM MgCl2, each dNTP at a concentration of 50 μM, each of the four primers at a concentration of 200 nM, and 0.5 U of Taq polymerase (EuroBlueTaq; Eurobio, France). The following cycling program was used for both uniplex and multiplex PCRs: 1 min at 95°C, 30 s at 56°C, and 30 s at 72°C for 30 cycles, followed by 10 min of elongation at 72°C (PTC-100; MJ Research, United States). Five microliters of amplification products was separated by electrophoresis on a 2% agarose gel at 100 V and visualized by ethidium bromide staining. All primers were designed using the Primer3 software (27; http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) and were synthesized by Eurogentec (France).
TABLE 1.
Primers used in this study
| Primer | Sequence | Length of amplicon (bp) |
|---|---|---|
| I1CL1-F | 5′-GCAGCGGCTTTATTATTTGC-3′ | 345 |
| I1CL1-R | 5′-TGAAGAAATCGCTTTTGCAG-3′ | |
| 102-F | 5′-TGTGGCGTTTATTCTTCTTTAGG-3′ | 399 |
| 500-R | 5′-TGATGATGATGCTGCTGAAA-3′ | |
| RPS2-F | 5′-TGCTATTGGAGACAGCAACG-3′ | 162 |
| RPS2-R | 5′-AAACGCACCTGAACTGAACC-3′ | |
| I1CL1-inv1-F | 5′-AAACGCCACATTCGTGTTTA-3′ | |
| I1CL1-inv1-R | 5′-TTTTATTGAATAGTTAGTTGGCTTTGA-3′ | |
| I1CL1-F2 | 5′-ATGCAAACACGCATTAATTT-3′ | 929 |
| I1CL1-R2 | 5′-AAAAACTAATTTTAGCATCACAAA-3′ | |
| ITS2-F | 5′-TGTGAACTGCAGGACACATG-3′ | 596 (L. heterotoma), 385 (D. melanogaster) |
| ITS2-R | 5′-AATGCTTAAATTTAGGGGGTA-3′ |
Correlation between the viral marker and the S phenotype.
Because the candidate sequence showed no homology with any known viral sequence in public databases, we examined whether the marker was a viral marker by performing a set of experiments to determine the association between the S phenotype and PCR amplification in naturally and artificially infected S phenotype individuals after horizontal transfers. The S phenotype was estimated by using the protocol described by Varaldi et al. (34). Briefly, females (1 or 2 days old) were placed from 5 p.m. to 10 a.m. on 10 first-instar D. melanogaster larvae in a petri dish containing an agar layer and a thin spot of yeast. About 48 h later, four or five hosts obtained from each petri dish were dissected, and the number of immature parasites (eggs and larvae) was determined. The superparasitism behavior of each female was estimated by determining the mean number of parasites per parasitized Drosophila host.
(i) Correlation in S phenotype and NS phenotype L. boulardi females.
In the first analysis, the correlation between putative viral sequence amplification (multiplex PCR) and superparasitizing behavior was tested using Sref and NSref stocks. We analyzed 148 females of the Sref line for which positive PCR amplification was expected and 186 NSref females for which no amplification was expected. For a subset of females, superparasitism behavior was individually estimated before the PCR test (42 NSref females and 36 Sref females).
In the second set of experiments, we used isofemale lines established using wild-collected insects from different localities (France, Portugal, Brazil, and Spain) to correlate molecular marker amplification and superparasitism behavior. Each isofemale line was established with only one founding female and was maintained by mass rearing for several generations. For each line, four to six females were analyzed for superparasitism using the standard procedure, and a separate set of females was tested with the molecular diagnostic tool using 23 isofemale lines.
Finally, we separately tested amplification of the putative viral marker from heads, thoraxes, and abdomens of Sref and NSref females.
(ii) Horizontal transfer of the virus and correlation with the putative viral sequence.
To further test the reliability of the putative viral marker, we horizontally transferred the behavior-modifying agent. Intraspecific horizontal transfer of LbFV was attempted by letting two L. boulardi females lay eggs in a single Drosophila host; one of these females was a member of an NS phenotype uninfected line that was PCR negative for the viral marker and fertilized (recipient), and the other was a member of an S phenotype infected line that was PCR positive and a virgin (donor). Since L. boulardi has a haplodiploid mode of reproduction, daughters arise only from fertilized eggs (diploids), whereas sons emerge from unfertilized eggs (haploids). Thus, because we used fertilized females for the NS phenotype recipient line and virgin females for the S phenotype donor line, we were assured that the emerging females (F1) were daughters of the NS phenotype recipient females. We expected that after transfer, recipient females that had the S phenotype would also be positive for PCR amplification of the putative viral sequence. Horizontal transfer experiments were performed with 13 different donor lines originating from Epinouze, Gotheron, and Palma de Mallorca populations. In all cases, we used the uninfected PCR-negative NSref line as the recipient. Experiments were performed by providing 50 D. melanogaster larvae (strain Sainte-Foy-lès-Lyon from France) to a fertilized L. boulardi NSref female for 24 h (at 21°C, with a photoperiod consisting of 12 h of light and 12 h of darkness). The NSref female was then replaced by virgin S phenotype females and incubated for 24 h at 25°C (to ensure virginity, single parasitized host pupae were isolated before emergence of the parasitoids). Note that for the first 24 h the temperature used was lower to slow host development and thereby provide young larvae to S phenotype females. At the end of the experiment, all females were stored in alcohol (100% ethanol) at −80°C before DNA extraction. Emerging F1 females were fertilized by NSref males, transferred to unparasitized Drosophila hosts for 24 h, and screened for putative viral infection by PCR. The newly established lines for which PCR tests were positive were kept for rearing. Four to fifteen generations after the transfer, superparasitism behavior and PCR tests were performed with each newly infected line using four to nine individuals per line.
iPCR.
To increase the size of the putative viral genomic sequence, we performed an inverse PCR (iPCR). Genomic DNA (0.75 μg) from S and NS phenotype strains were restricted with 20 U of RsaI, MboI, EcoRI, or XbaI (Promega, France) in 25-μl (final volume) mixtures according to the manufacturer's instructions. Intramolecular ligation was performed with 10 μl of digest and 2 U of T4 DNA ligase (Invitrogen, France) in a 400-μl (final volume) mixture for 16 h at 4°C. Ligated DNA was precipitated and resuspended in 40 μl of TE buffer (10 mM Tris-HCl, pH 7.5; 0.1 mM EDTA, pH 8.7). PCR were performed using EuroBlue Taq polymerase (EuroBlueTaq; Eurobio, France) according to the manufacturer's instructions with primers I1CL1-inv1-F and I1CL1-inv1-R (Table 1) and 3 μl of ligated and precipitated DNA. The PCR conditions were 3 min at 95°C, followed by 30 cycles of 30 s at 94°C, 45 s at 55°C, and 4 min at 72°C and then 10 min of elongation at 72°C. The PCR fragment obtained for the S phenotype strains was directly sequenced with the I1CL1-inv1-L primer. Using this sequence, we designed two primers (I1CL1-F2 and I1CL1-R2) (Table 1) to obtain the total DNA fragment. The PCR product (929 bp) was sequenced on positive and negative senses with the I1CL1-F2 and I1CL1-R2 primers.
Detection and prevalence in host-parasitoid communities.
Using the viral primers 102-F and 500-R, we detected the virus and estimated its frequency in different parasitoid and host species for one location in the south of France (Annonay). In September 2007, 10 traps baited with split bananas were placed in an apple orchard. They were exposed for 15 days for natural colonization and brought back to the laboratory before insects emerged. After incubation, emerging D. melanogaster, the major Drosophila species during the sampling period, and two species of emerging parasitoids, L. boulardi and L. heterotoma, the two main Drosophila larval parasitoid in the area, were collected and stored in alcohol (100% ethanol) at −80°C before DNA extraction. To control for the quality of DNA extraction, we used the multiplex reaction for L. boulardi and PCR amplified the internal transcribed spacer 2 (ITS2) region for L. heterotoma and D. melanogaster. A PCR for ITS2 was performed by using a 25-μl (final volume) reaction mixture containing each dNTP at a concentration of 200 μM, each ITS2 primer (ITS2-F and ITS2-R) at a concentration of 200 nM, 0.5 U EuroBlue Taq polymerase (EuroBlueTaq; Eurobio, France), and 2 μl of DNA. The PCR conditions were 2 min at 95°C, followed by 35 cycles of 30 s at 95°C, 45 s at 55°C, and 1.5 min at 72°C and then 10 min of elongation at 72°C. The same set of primers was used to amplify the ITS2 regions of L. heterotoma and D. melanogaster (Table 1) (2). We also estimated the prevalence of LbFV in the L. boulardi population of Avignon (France), sampled in September 2007, using the same sampling procedure.
We also determined the presence of LbFV in strains initiated by using individuals originating from Sicily (Italy, sampled in 2000), La Martinique (the West Indies, sampled in 1999), and Lamto (Ivory Coast, sampled in 1996). For these locations, the numbers of initial samples were very low (less than three founding females) and the strains were mass reared, which precluded obtaining estimated prevalences for the populations.
RESULTS
Putative viral marker identification.
The whole subtraction procedure revealed 128 S phenotype-specific clones. The sequences clustered in 12 multisequence contigs and 38 singlets, yielding an estimated 50 unique sequences. The analysis revealed that 24 sequences displayed significant matches with entries in the databases and corresponded to insect sequences. The other 26 sequences had no significant matches with viral sequences in the databases, and we decided to focus our investigations on the contig containing the highest number of individual sequences (n = 13). This 809-bp contig was used to design two sets of primers (see Materials and Methods) (Table 1). The reliability of these primers for amplifying a viral sequence was then tested by analyzing the association between the S phenotype and PCR amplification in different sets of experiments.
Correlation between S phenotype behavior and PCR amplification. (i) Correlation using S and NS phenotype L. boulardi females.
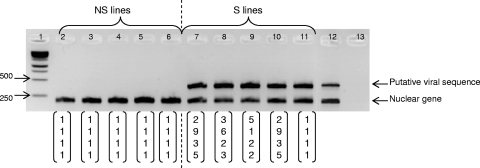
A total of 186 L. boulardi females of the NSref line and 148 females of the Sref line were tested by performing PCR with the putative viral marker. We observed no amplification for females belonging to the NSref line, and we amplified the putative viral sequence for all females belonging to the Sref line (Fig. 1A); thus, there was a perfect correlation between PCR amplification and superparasitism behavior. Heads, thoraxes, and abdomens were all PCR positive for the Sref line and negative for the NSref line (not shown).
FIG. 1.
Correlation between the egg-laying behavior and the molecular test of viral infection for the Sref and NSref lines (A) and for S and NS phenotype isofemale lines originating from various populations of L. boulardi (B). The boxes indicate the mean number of parasitoid eggs per parasitized host calculated for each female, and the fraction above each box indicates the number of PCR-positive females/number of females tested. M, Madeira line; P1 and P2, Palma de Mallorca lines; R, Rio de Janeiro line; G1 to G17, Gotheron lines. a, uniplex PCRs; b, multiplex PCRs.
We also tested the putative marker of LbFV for various L. boulardi genotypes using isofemale laboratory strains founded by NS phenotype females from Gotheron (n = 8), Madeira (n = 1), and Palma de Mallorca (n = 1) and by S phenotype females from Gotheron (n = 9), Epinouze (n = 2), Rio de Janeiro (n = 1), and Palma de Mallorca (n = 1) (Fig. 1B). We detected no PCR amplification for females belonging to the NS phenotype lines of the different populations (0/16 PCRs). On the other hand, the molecular tests for all tested individuals belonging to the different S phenotype lines were positive (77/77 PCRs); thus, there was a perfect correlation between putative viral amplification and superparasitism behavior.
(ii) Correlation using horizontal transfer experiments.
As previously reported (34, 35), intraspecific horizontal transfer of LbFV occurs between L. boulardi individuals sharing the same superparasitized host. We tried to infect the NSref line with several virus isolates originating from 13 different S phenotype donor lines in superparasitism experiments. As expected, all NSref females used in the experiment initially were PCR negative for the putative viral marker. Furthermore, the NSref line clearly had the typical NS phenotype (Table 2 and Fig. 1A). However, after sharing hosts with the S phenotype lines, some of the offspring were PCR positive for the putative viral marker, indicating that there was horizontal transmission of the putative viral sequence. Horizontal transfer was successful for 9 of the 13 donor lines. The PCR-positive females were used to found lines, and their offspring also were PCR positive and showed clear signs of LbFV infection since superparasitism was abundant (Table 2). To summarize, we successfully transformed nine independent NS phenotype lines into nine S phenotype lines through horizontal transfer of LbFV; these lines were PCR negative before the transfer and positive after the transfer. This established that there was a perfect correlation between superparasitism behavior and the molecular tool in the horizontal transfer experiments.
TABLE 2.
Horizontal cotransmission of the S phenotype and the putative viral markera
| Donor
|
One generation after transfer (emerging F1)
|
Later after transfer
|
||||||
|---|---|---|---|---|---|---|---|---|
| Lineb | Phenotype (n)c | No. of positive PCR tests/no. of individuals tested | No. of females tested | PCR results | n | No. of generations | Phenotypec | No. of positive PCR tests/no. of individuals tested |
| G9 | 3.3 ± 0.75 (6) | 3/3d | 3 | + | 1 | 15 | 3.60 ± 0.33 | 4/4e |
| − | 2 | |||||||
| G10 | 3.9 ± 0.52 (6) | 3/3d | 11 | + | 6 | 15 | 3.35 ± 0.22 | 5/5e |
| − | 5 | |||||||
| G11 | 5.3 ± 0.81 (6) | 3/3d | 3 | + | 0 | |||
| − | 3 | |||||||
| G12 | 5.7 ± 1.17 (6) | 3/3d | 2 | + | 2 | 15 | 3.31 ± 0.30 | 5/5e |
| − | 0 | |||||||
| G13 | 7.1 ± 1.25 (6) | 3/3d | 5 | + | 0 | |||
| − | 5 | |||||||
| G14 | 7.2 ± 0.61 (6) | 3/3d | 14 | + | 0 | |||
| − | 14 | |||||||
| G15 | 8 ± 1.62 (6) | 3/3d | 4 | + | 2 | 15 | 2.68 ± 0.34 | 5/5e |
| − | 2 | |||||||
| G16 | 9.3 ± 1.93 (6) | 3/3d | 6 | + | 3 | 15 | 3.55 ± 0.12 | 5/5e |
| − | 3 | |||||||
| G17 | 11.9 ± 1.80 (6) | 3/3d | 6 | + | 3 | 15 | 3.57 ± 0.07 | 5/5e |
| − | 3 | |||||||
| G18 | 5.88 ± 1.51 (5) | 18/18e | 21 | + | 3 | 4 | 2.51 ± 0.11 | 9/9e |
| − | 18 | |||||||
| Ep1 | 6.48 ± 0.89 (5) | 4/4e | 5 | + | 1 | 4 | 2.19 ± 0.07 | 9/9e |
| − | 4 | |||||||
| Ep2 | 8.18 ± 1.05 (5) | 11/11e | 10 | + | 0 | |||
| − | 10 | |||||||
| P2 | 2.89 ± 0.72 (6) | 18/18e | 18 | + | 7 | 4 | 2.29 ± 0.10 | 9/9e |
| − | 11 | |||||||
The recipient lines were founded by females belonging to the NSref line. In each case before transfer the recipient had the NS phenotype (1.1 ± 0.01 parasitic larvae per parasitized host), and a PCR was negative.
The donor lines were S phenotype lines from different geographical areas. G9 to G18, Gotheron lines; Ep1 and Ep2, Epinouze lines; P2, Palma de Mallorca line.
The phenotype is expressed as the number of parasitic larvae per parasitized host (mean ± standard error).
Uniplex PCR.
Multiplex PCR.
Incomplete penetrance of the virus-induced phenotype.
Thirty-six Sref females and 42 NSref females were individually tested to determine their superparasitism behavior before molecular diagnosis of infection. All of the NSref females had an NS phenotype, and the PCR tests for these females were negative. However, although the PCR tests were positive for all of the Sref females, only 29/36 had the typical S phenotype behavior, which indicates that the virus does not always induce behavioral alteration (Fig. 2).
FIG. 2.
Multiplex PCR amplification for 10 individuals belonging to the NSref and Sref lines. Data for superparasitism behavior are indicated below the gel and show the number of L. boulardi larvae counted in each of the four dissected hosts. Lane 1, 1-kb DNA ladder (Fermentas, St. Leon-Rot, Germany) used as a molecular weight marker; lanes 2 to 6, NSref line individuals; lanes 7 to 11, Sref line individuals; lanes 12 and 13, positive and negative controls.
Molecular structure of the viral gene.
Analysis of the mRNA revealed the position of a potential open reading frame, encoding 206 amino acids (Fig. 3), that did not exhibit significant matches with entries in the databases. iPCR was successful only with the RsaI enzyme, and the results allowed us to extend the DNA viral sequence for 230 bp to obtain a total length of 1,039 bp (GenBank accession number FM876312). We found a short 77-bp intron sequence at position 887 of the viral DNA sequence (Fig. 3). However, an analysis of this sequence did not result in further information for molecular characterization of LbFV because this sequence did not display significant hits with entries in public databases.
FIG. 3.
Schematic diagram of the viral sequence and putative open reading frame. The arrow indicates the position and direction of the potential open reading frame. The upper line shows the mRNA sequence, and the lower line indicates the DNA sequence. The dotted line indicates the position of the intron. The positions of the primers are indicated on the DNA sequence, and their designations are indicated below the sequence. The numbers in parentheses are the positions of the primers.
Prevalence in a host-parasitoid community.
The prevalence of LbFV was estimated for one location in southeastern France (Annonay) using the newly developed molecular marker for L. boulardi, its relative L. heterotoma, and their common host D. melanogaster. None of the tests with the 34 L. heterotoma individuals tested and the 35 D. melanogaster individuals tested were positive. In contrast, 20 of 30 (67%) L. boulardi individuals tested were positive for the virus. We estimated the frequency of LbFV only for L. boulardi at Avignon, where 16 of 23 (70%) individuals were positive for the virus.
We also detected viral infection in Sicily (1/1), La Martinique (4/4), and Lamto (3/3), indicating that LbFV has a broad geographic distribution.
DISCUSSION
A subtracted cDNA library was constructed to compare mRNAs of two lines of L. boulardi (S and NS phenotype lines) having the same nuclear genotype but having contrasting superparasitism behaviors due to the presence of a virus (LbFV) in the S phenotype line. In this subtracted library, we identified a sequence specific to the S phenotype line. This sequence had no homology with any sequence in public databases. Based on this sequence, we developed a simple PCR test for DNA templates. The results indicated that PCR amplification occurred with DNA extracts from S phenotype individuals but not with DNA extracts from NS phenotype individuals, ruling out the possibility that the difference was due to a differentially expressed insect gene. We also showed that this molecular tool reliably tracked the presence of the virus inducing superparasitism and consequently that the sequence is the first published sequence of LbFV. This result indicates that LbFV has at least an intermediate DNA stage during replication, and probably a DNA genome, as previously hypothesized based on its apparent replication in the nuclei of insect cells (37).
Reliability.
We tested the reliability of the PCR diagnosis in two ways. First, we tested both the S phenotype and the PCR amplification profile of numerous parasitoid lines originating from different locations. All assays indicated that the lines having the NS phenotype were PCR negative, whereas the S phenotype lines were PCR positive. Furthermore, we demonstrated that the DNA sequence examined is part of an infectious element since the sequence was horizontally transferred from an S phenotype line to an NS phenotype line. Indeed, initially NS phenotype PCR-negative lines were transformed into S phenotype PCR-positive lines after development under superparasitism conditions in competition with S phenotype PCR-positive lines. The horizontal cotransmission of the S phenotype and the DNA sequence observed in nine independent experiments supported the conclusion that this sequence is part of the virus genome.
Penetrance.
We also found that although in LbFV-infected lines most females had the typical S phenotype, some females did not (7/36 females in our assay). This indicates that the penetrance of the virus extended phenotype was incomplete. One potential explanation for this is that the density of the virus must exceed a certain threshold to manipulate wasp behavior, as suggested previously for other host-symbiont interactions (4, 5). According to this hypothesis, the nonmanipulated infected wasps would have lower viral densities. This emphasizes the importance of using molecular detection of LbFV instead of a phenotypic test to estimate viral prevalence. Notably, this observation is consistent with the results of previous experiments in which behavioral manipulation was shown to be transiently silent over generations in an infected line (35).
Epidemiology.
LbFV seems to be very widespread because we detected it in populations from different continents, including Europe (France, Italy, Spain, and Portugal), Africa (Ivory Coast), and South America (Brazil and La Martinique). Furthermore, in the populations sampled in France, we found very high prevalence of the virus. The key factors affecting the epidemiology of a symbiont with a mixed mode of transmission (vertical and horizontal) are the rates of vertical and horizontal transmission, the direct effects on host fitness, and the benefits from any potential manipulation. Our results show that vertical transmission is very efficient since each of the nine newly infected lines that were constructed stably transmitted the virus until the assay was performed (4 or 15 generations depending on the line), confirming data obtained previously (37). Moreover, all 148 Sref line females tested were PCR positive. However, vertical transmission is known to be incomplete since one infected line and one uninfected line were obtained previously from the progeny of a single wild-caught female (35). From a theoretical point of view, any costly symbiont with imperfect vertical transmission cannot be maintained in a host population unless it compensates by manipulation of host reproduction (e.g., reproductive parasites, such as Wolbachia bacteria [23]) or by horizontal transmission (3). Our results show that the prevalence of LbFV remains high in natural populations (67% at Annonay and 70% at Avignon) despite the slight fitness cost (33), which can be accounted for by the efficiency of horizontal transmission and of the manipulative process. For both of these locations, the estimates of LbFV prevalence obtained using the molecular marker are consistent with previous estimates based on phenotypic tests done in the same location but 3 years earlier (at Annonay, 15 of 35 S phenotype isofemale lines tested [P = 0.081, Fisher's exact test]; at Avignon, 24 of 38 S phenotype isofemale lines tested [P = 0.782, Fisher's exact test] [S. Patot, unpublished]). A high prevalence of vertically transmitted virus infecting parasitoids has been reported previously for other species (19). Some of these viruses are considered mutualists since they provide immune protection to the parasitoid egg inside the host (26, 30). However, for other viruses, no evidence of immune protection has been found (30), and the mechanisms that allow them to reach high prevalence are not understood. Occasional horizontal transfer, potentially associated with behavioral manipulation, provides a plausible and testable explanation. In the field, L. boulardi and the related species L. heterotoma attack the same hosts, mostly D. melanogaster at the location studied. The evidence for interspecific competition is strong, and it is likely that cases of multiparasitism (Drosophila larvae parasitized by both species) occur in the field (17), which would provide a means for interspecific horizontal transfer. However, we did not find evidence of LbFV infection in L. heterotoma females, even though LbFV is common in the sampled populations of L. boulardi (67 and 70%). Although this requires further investigation, the results suggest that L. heterotoma may be somehow refractory to LbFV infection. Interestingly, in contrast to L. boulardi, L. heterotoma is infected by symbiotic Wolbachia bacteria (38), and interactions between symbionts may explain the observed pattern, as suggested by recent data for interactions between herpesvirus and pathogenic bacteria in humans (6) or between Wolbachia and RNA viruses in the fly D. melanogaster (21). Adults of the common host D. melanogaster also were not infected by LbFV. The presence of Wolbachia in D. melanogaster (22) may also be involved in this pattern or may simply be explained by the inability of LbFV to infect fly cells due to the phylogenetic distance between L. boulardi and D. melanogaster. Alternatively, LbFV may be able to infect D. melanogaster cells, but Drosophila may be unable to get rid of the developing parasitoid (that will kill it in most cases). In addition, the virus may impose a strong cost on LbFV-infected D. melanogaster (cleared of parasitoid infestation), preventing it from reaching the adult stage and transmitting the infection to offspring. Laboratory experiments controlling for exposure to the virus are necessary to further test these hypotheses.
Conclusion.
Using a transcriptomic approach, we developed a marker and obtained the first molecular data on the behavior-manipulating virus infecting the parasitoid wasp L. boulardi. A simple PCR test to detect infection confirmed previous phenotypic data showing that the virus is common in natural populations and thus is an important factor in the host-parasitoid interaction. Furthermore, the molecular data obtained pave the way for future wide-scale epidemiological studies and provide an entry point for analysis of the viral DNA genome. Additional genomic information could provide a way to identify the closest relatives of this virus and investigate the proximal mechanisms mediating its fascinating manipulation effects.
Acknowledgments
We are grateful to Roland Allemand for his assistance with the field work and for helping with insect determination. We thank Sylvain Gandon and Nicolas Ris for providing some samples. We thank Jérôme Briolay for technical support. We also thank Sylvain Charlat for useful comments on the manuscript.
This work was financially supported by the Centre National de la Recherche Scientifique (CNRS UMR 5558) and by the French Ministery Education Nationale, de l'Enseignement Supérieur et de la Recherche, Fond National de la Science, ACI Jeunes Chercheurs 2004, JC 10013 (Virus symbiotiques manipulateurs du comportement des insectes parasites).
Footnotes
Published ahead of print on 5 December 2008.
REFERENCES
- 1.Abd-Alla, A. M., F. Cousserans, A. G. Parker, J. A. Jehle, N. J. Parker, J. M. Vlak, A. S. Robinson, and M. Bergoin. 2008. Genome analysis of a Glossina pallidipes salivary gland hypertrophy virus reveals a novel, large, double-stranded circular DNA virus. J. Virol. 82:4595-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allemand, R., C. Lemaître, F. Frey, M. Boulétreau, F. Vavre, G. Nordlander, J. van Alphen, and Y. Carton. 2002. Phylogeny of six African Leptopilina species (Hymenoptera: Cynipoidea, Figitidae), parasitoids of Drosophila, with description of three new species. Ann. Soc. Entomol. Fr. 38:319-332. [Google Scholar]
- 3.Altizer, S. M., and D. J. Augustine. 1997. Interactions between frequency-dependent and vertical transmission in host-parasite systems. Proc. Biol. Sci. 264:807-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anbutsu, H., and T. Fukatsu. 2003. Population dynamics of male-killing and non-male-killing spiroplasmas in Drosophila melanogaster. Appl. Environ. Microbiol. 69:1428-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anbutsu, H., and T. Fukatsu. 2006. Tissue-specific infection dynamics of male-killing and nonmale-killing spiroplasmas in Drosophila melanogaster. FEMS Microbiol. Ecol. 57:40-46. [DOI] [PubMed] [Google Scholar]
- 6.Barton, E. S., D. W. White, J. S. Cathelyn, K. A. Brett-McClellan, M. Engle, M. S. Diamond, V. L. Miller, and H. W. T. Virgin. 2007. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447:326-329. [DOI] [PubMed] [Google Scholar]
- 7.Brun, G. P. N. 1980. The viruses of Drosophila, p. 625-702. In M. W. Ashburner (ed.), The genetics and biology of Drosophila. Academic Press, New York, NY.
- 8.Carpenter, J. A., D. J. Obbard, X. Maside, and F. M. Jiggins. 2007. The recent spread of a vertically transmitted virus through populations of Drosophila melanogaster. Mol. Ecol. 16:3947-3954. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Y. A., J. S. Pettis, A. Collins, and M. F. Feldlaufer. 2006. Prevalence and transmission of honeybee viruses. Appl. Environ. Microbiol. 72:606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y. P., J. A. Higgins, and M. F. Feldlaufer. 2005. Quantitative real-time reverse transcription-PCR analysis of deformed wing virus infection in the honeybee (Apis mellifera L.). Appl. Environ. Microbiol. 71:436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David, J. 1962. A new medium for rearing Drosophila in axenic condition. Drosophila Info. Serv. 36:128. [Google Scholar]
- 12.Diatchenko, L., Y. F. Lau, A. P. Campbell, A. Chenchik, F. Moqadam, B. Huang, S. Lukyanov, K. Lukyanov, N. Gurskaya, E. D. Sverdlov, and P. D. Siebert. 1996. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc. Natl. Acad. Sci. USA 93:6025-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diatchenko, L., S. Lukyanov, Y. F. Lau, and P. D. Siebert. 1999. Suppression subtractive hybridization: a versatile method for identifying differentially expressed genes. Methods Enzymol. 303:349-380. [DOI] [PubMed] [Google Scholar]
- 14.Feddersen, I., K. Sander, and O. Schmidt. 1986. Virus-like particles with host protein-like antigenic determinants protect an insect parasitoid from encapsulation. Experientia 42:1278-1281. [Google Scholar]
- 15.Federici, B. A., and Y. Bigot. 2003. Origin and evolution of polydnaviruses by symbiogenesis of insect DNA viruses in endoparasitic wasps. J. Insect Physiol. 49:419-432. [DOI] [PubMed] [Google Scholar]
- 16.Fleuriet, A. 1976. Presence of hereditary rhabdovirus sigma and polymorphism for a gene for resistance to this virus in natural population in Drosophila melanogaster. Evolution 30:735-739. [DOI] [PubMed] [Google Scholar]
- 17.Fleury, F., N. Ris, R. Allemand, P. Fouillet, Y. Carton, and M. Bouletreau. 2004. Ecological and genetic interactions in Drosophila-parasitoids communities: a case study with D. melanogaster, D. simulans and their common Leptopilina parasitoids in south-eastern France. Genetica 120:181-194. [DOI] [PubMed] [Google Scholar]
- 18.Gandon, S., A. Rivero, and J. Varaldi. 2006. Superparasitism evolution: adaptation or manipulation? Am. Nat. 167:E1-E22. [DOI] [PubMed] [Google Scholar]
- 19.Graham, R. I., S. Rao, R. D. Possee, S. M. Sait, P. P. Mertens, and R. S. Hails. 2006. Detection and characterisation of three novel species of reovirus (Reoviridae), isolated from geographically separate populations of the winter moth Operophtera brumata (Lepidoptera: Geometridae) on Orkney. J. Invertebr. Pathol. 91:79-87. [DOI] [PubMed] [Google Scholar]
- 20.Habayeb, M. S., S. K. Ekengren, and D. Hultmark. 2006. Nora virus, a persistent virus in Drosophila, defines a new picorna-like virus family. J. Gen. Virol. 87:3045-3051. [DOI] [PubMed] [Google Scholar]
- 21.Hedges, L. M., J. C. Brownlie, S. L. O'Neill, and K. N. Johnson. 2008. Wolbachia and virus protection in insects. Science 322:702. [DOI] [PubMed] [Google Scholar]
- 22.Mercot, H., and S. Charlat. 2004. Wolbachia infections in Drosophila melanogaster and D. simulans: polymorphism and levels of cytoplasmic incompatibility. Genetica 120:51-59. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill, S. L., A. A. Hoffmann, and J. H. Werren. 1997. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, New York, NY.
- 24.Rebrikov, D. V., O. V. Britanova, N. G. Gurskaya, K. A. Lukyanov, V. S. Tarabykin, and S. A. Lukyanov. 2000. Mirror orientation selection (MOS): a method for eliminating false positive clones from libraries generated by suppression subtractive hybridization. Nucleic Acids Res. 28:E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reineke, A., and S. Asgari. 2005. Presence of a novel small RNA-containing virus in a laboratory culture of the endoparasitic wasp Venturia canescens (Hymenoptera: Ichneumonidae). J. Insect Physiol. 51:127-135. [DOI] [PubMed] [Google Scholar]
- 26.Renault, S., S. Bigot, M. Lemesle, P. Y. Sizaret, and Y. Bigot. 2003. The cypovirus Diadromus pulchellus RV-2 is sporadically associated with the endoparasitoid wasp D. pulchellus and modulates the defence mechanisms of pupae of the parasitized leek-moth, Acrolepiopsis assectella. J. Gen. Virol. 84:1799-1807. [DOI] [PubMed] [Google Scholar]
- 27.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365-386. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt, O., U. Theopold, and M. Strand. 2001. Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. Bioessays 23:344-351. [DOI] [PubMed] [Google Scholar]
- 29.Shelby, K. S., and B. A. Webb. 1999. Polydnavirus-mediated suppression of insect immunity. J. Insect Physiol. 45:507-514. [DOI] [PubMed] [Google Scholar]
- 30.Stasiak, K., S. Renault, B. A. Federici, and Y. Bigot. 2005. Characteristics of pathogenic and mutualistic relationships of ascoviruses in field populations of parasitoid wasps. J. Insect Physiol. 51:103-115. [DOI] [PubMed] [Google Scholar]
- 31.Tentcheva, D., L. Gauthier, N. Zappulla, B. Dainat, F. Cousserans, M. E. Colin, and M. Bergoin. 2004. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 70:7185-7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valles, S. M., C. A. Strong, P. M. Dang, W. B. Hunter, R. M. Pereira, D. H. Oi, A. M. Shapiro, and D. F. Williams. 2004. A picorna-like virus from the red imported fire ant, Solenopsis invicta: initial discovery, genome sequence, and characterization. Virology 328:151-157. [DOI] [PubMed] [Google Scholar]
- 33.Varaldi, J., M. Bouletreau, and F. Fleury. 2005. Cost induced by viral particles manipulating superparasitism behaviour in the parasitoid Leptopilina boulardi. Parasitology 131:161-168. [DOI] [PubMed] [Google Scholar]
- 34.Varaldi, J., P. Fouillet, M. Ravallec, M. Lopez-Ferber, M. Bouletreau, and F. Fleury. 2003. Infectious behavior in a parasitoid. Science 302:1930. [DOI] [PubMed] [Google Scholar]
- 35.Varaldi, J., S. Gandon, A. Rivero, S. Patot, and F. Fleury. 2006. A newly discovered virus manipulates superparasitism behavior in a parasitoid wasp, p. 119-139. In K. Bourtzis and T. A. Miller (ed.), Insect symbiosis, vol. 2. CRC Press, Boca Raton, FL. [Google Scholar]
- 36.Varaldi, J., S. Petit, M. Bouletreau, and F. Fleury. 2006. The virus infecting the parasitoid Leptopilina boulardi exerts a specific action on superparasitism behaviour. Parasitology 132:747-756. [DOI] [PubMed] [Google Scholar]
- 37.Varaldi, J., M. Ravallec, C. Labrosse, M. Lopez-Ferber, M. Bouletreau, and F. Fleury. 2006. Artificial transfer and morphological description of virus particles associated with superparasitism behaviour in a parasitoid wasp. J. Insect Physiol. 52:1202-1212. [DOI] [PubMed] [Google Scholar]
- 38.Vavre, F., F. Fleury, J. Varaldi, P. Fouillet, and M. Bouletreau. 2000. Evidence for female mortality in Wolbachia-mediated cytoplasmic incompatibility in haplodiploid insects: epidemiologic and evolutionary consequences. Evolution 54:191-200. [DOI] [PubMed] [Google Scholar]