Abstract
Six Leptolyngbya strains, isolated from the archaeological surfaces of hypogean sites, were phenotypically and genetically characterized by light and electron microscopy and 16S rRNA gene and 16S-23S internally transcribed spacer (ITS) sequencing. Three phycoerythrin-rich (red) and three phycocyanin-rich (green) isolates were assigned to different operational taxonomic units (OTUs). Among the green isolates, one strain showed an OTU intraspecific variation due to differences in the ITS sequences and genomic polymorphism. Within the ITS sequence, variable regions, conserved domains and tRNAIle and tRNAAla genes showed high sequence identity among the phylotypes. Together, these data indicated a relatedness of the six strains to other Leptolyngbya from subaerophytic and geothermal environments and allowed the definition of novel Leptolyngbya OTUs.
Archaeological hypogea, such as Roman catacombs and the necropolis, are man-made underground sites illuminated from natural openings to the outside and/or from artificial lights that permit visits by tourists. The low-light conditions (photosynthetic photon flux density of <2 μmol m−2 s−1), however, still sustain the development of phototrophic biofilms on calcareous substrata (2, 3, 16). These microbial communities are composed of chroococcal and filamentous cyanobacteria, a few green algae, diatoms, mosses, and bacterial populations, which are all embedded in common extracellular polymeric secretions (9, 10, 17, 26, 47, 55). The development and metabolic activity of these biofilms play a significant role in the biodeterioration of the art works of great historical and artistic value that are present in hypogea. Aesthetic damage to stuccoes, frescoes, and marble is the result of the colored patinas of microbial origin. Structural damage is due to the ability of microbial biofilms to mobilize constituent elements from mineral substrata (21, 48).
The phototrophic communities of Roman hypogea are dominated by abundant populations of subaerophytic epilithic cyanobacteria belonging to the genera Eucapsis, Leptolyngbya, Scytonema, and Fischerella that have been identified on the basis of their cytological and ultrastructural features (8, 10). Some of these taxa were never recorded outside of these habitats and for this reason were defined as “troglobitic,” i.e., obligate cavernicole taxa unable to survive outside of caves or other low-light environments (27). Subaerophytic (35) troglobitic cyanobacteria belonging to the genus Leptolyngbya are particularly abundant in phototrophic biofilms present in Roman hypogea. To date, different Leptolyngbya species have been detected in all the hypogea investigated, but their phenotypic simplicity made their correct identification difficult (6, 34), and molecular data are scarce. The 16S rRNA gene sequences of only two troglobitic Leptolyngbya isolates, strains VRUC135 and VRUC184, have been reported (15, 39). The genus Leptolyngbya was first described by Anagnostidis and Komárek (11). This genus includes groups of filamentous cyanobacteria in LPP group B that exhibit very thin trichomes (<3 μm), exemplified by Lyngbya, Plectonema, and Phormidium (45). However, the heterogeneity of this genus has since been questioned (7, 35). Molecular data are needed to clarify its phylogenetic position. In the last few years, the 16S rRNA gene sequences of several Leptolyngbya strains isolated from desert, marine, and freshwater environments have become available (57). Leptolyngbya species are very common and yet difficult to identify because of the controversial position of this genus among Cyanobacteria. It has been recently proposed to divide the genus Leptolyngbya into at least two genera, each with six ecological groups, for easier identification, since the majority of the 91 species so far described have distinct and delimited ecological requirements (35). A polyphasic approach combining morphological, ultrastructural, molecular, and ecophysiological studies is the most progressive method in the modern taxonomic study of cyanobacteria (19, 20, 29, 35, 38, 56). This approach is especially suitable for the taxonomy of species belonging to genera such as Leptolyngbya, which are morphologically very simple.
In the present study, six strains of Leptolyngbya isolated from five Roman hypogea, namely, the catacombs of St. Callistus, St. Sebastian, Domitilla, and Priscilla and the Domus Aurea (2), all located in Rome (Italy), were investigated by means of a polyphasic approach. The cytomorphological features were analyzed by light microscopy and transmission electron microscopy (TEM), while the genetic diversity was investigated by 16S rRNA gene and 16S-23S internally transcribed spacer (ITS) sequencing. Molecular approaches to the study of cyanobacterial systematics have focused primarily on the analysis of 16S rRNA gene sequences (57); however, this gene is highly conserved, and its use in estimating relationships at the subgeneric level is limited (24). On the other hand, the ITS region, which is more variable than the 16S rRNA gene and less subject to selection pressure, is considered a useful tool in cyanobacterial taxonomy (12, 14, 18, 23, 37, 43). All these data allowed the successful identification of two phylotypes among six members of the genus Leptolyngbya and highlighted their intraspecific genetic variation.
MATERIALS AND METHODS
Cyanobacterial strains.
Six nonaxenic strains of Leptolyngbya belonging to the Vergata Rome University Culture Collection (VRUC) and isolated from biofilms present in five Roman hypogea were studied (Table 1). All strains were cultured in liquid BG11 medium (45) at 20 ± 1°C, 60% relative humidity, and a photosynthetic photon flux density of 5 μmol photons m−2 s−1 (measured with a Licor LI-185B quantum/radiometer/photometer equipped with an LI-190SB quantum sensor) provided by fluorescent cool-white lamps (Philips HPL-N150W) with a dark-light cycle of 12:12 h.
TABLE 1.
Leptolyngbya strains used in this study
| Strain designation | Isolation date | Isolation site |
|---|---|---|
| VRUC184 CSC7-1/Albertano et Bruno | 1994 | Catacombs of St. Callistus, on tufa |
| VRUC201 CD2/Kovacik et Albertano | 1992 | Catacombs of Domitilla, on plaster |
| VRUC206 CSC8/Kovacik et Albertano | 1992 | Catacombs of St. Callistus, on tufa |
| VRUC192 CP9-1/Albertano | 1992 | Catacombs of Priscilla, on tufa |
| VRUC198 CSS6-3/Albertano | 1992 | Catacombs of St. Sebastian, on tufa |
| VRUC135/Albertano | 1985 | Domus Aurea, on frescoes |
Microscopy.
Microscopic investigations of fresh samples were performed using a Zeiss Axioskop light microscope equipped with differential interference contrast. Images were taken using a Nikon Coolpix 5000 digital camera. Drawings were made after light microscopy observations in order to illustrate the key characteristics of each strain. For the morphological characterization of cyanobacterial strains, the diacritic traits used for description of botanical species were considered (35). In order to characterize cell size, at least 50 measurements were made for each biometrical characteristic. The six Leptolyngbya strains were also observed by TEM after fixation in 0.2 M phosphate buffer (pH 7.2) containing 2.5% glutaraldehyde and subsequent postfixation in 1% osmium tetroxide, dehydration in ethanol series, and embedding with an 812 resin kit (Multilab, England). Ultrathin sections were stained with uranyl acetate and lead citrate (44) and observed using a Zeiss CEM 902 electron microscope at 80 kV.
Amplification of the 16S rRNA gene and ITS.
Genomic DNAs were extracted from Leptolyngbya cells as described previously (15). The amplification of the 16S-23S rRNA operons from the six Leptolyngbya strains was performed using primer 1 (5′-AGAGTTTGATCCTGGCTCAG-3′ [59]), corresponding to nucleotides 8 to 27 of the 16S rRNA gene in Synechocystis sp. strain PCC 6301, and primer 18m (5′-TCTGTGTGCCTAGGTATCC-3′ [49]), corresponding to nucleotides 26 to 45 of the 23S rRNA gene in Synechocystis sp. strain PCC 6301. PCRs were carried out in 25-μl aliquots containing approximately 100 ng DNA, a deoxynucleoside triphosphate mixture (0.2 mM each), buffer (1/10 volume of the supplied 10× buffer) supplemented to give a final concentration of 3 mM MgCl2, 1 U of Taq polymerase (Amersham, Pharmacia), and 0.5 pmol of each primer. Reactions were cycled with a Mastercycler gradient (Eppendorf) as follows: 1 cycle at 94°C for 3 min; 30 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 3 min; and a final step at 72°C for 7 min. PCRs resulted in products of ∼2,000 bp, which were purified on an agarose gel with a Qiaquick gel extraction kit (Qiagen) and used as templates to amplify the 16S rRNA gene and the ITS region.
The 16S rRNA genes were amplified using as the forward primer the cyanobacterial specific primer CYA359 (41) and the universal primer C (5′-ACGGGCGGTGTGTAC-3′) corresponding to Escherichia coli positions 1406 to 1392. Final PCR concentrations were as described above. Amplifications were run in a GeneAmp PCR system 2700 (Applied Biosystem) as follows: 1 cycle of 2 min at 94°C; 30 cycles of 1 min at 94°C, 40 s at 45°C, and 1 min at 72°C; and a final elongation step of 7 min at 72°C. After purification from the agarose gel using a Qiaquick gel extraction kit (Qiagen), the PCR products (∼1,100 bp) were cloned into pGEM-T Easy vector (Promega) and sequenced using primers CYA359, C, AC552F (5′-CAGCCGCGGTAATAC-3′), and AC552R (5′-GTATTACCGCGGCTG-3′).
PCR amplifications of the ITS regions were performed using the forward primer 16S3′F (49) and reverse primer 18m (58). Amplifications were run in a GeneAmp PCR system 2700 (Applied Biosystem) as follows: 1 cycle of 3 min at 94°C; 30 cycles of 1 min at 94°C, 40 s at 45°C, and 1 min at 72°C; and a final elongation step of 7 min at 72°C. PCR products of about 600 bp were purified with a Qiaquick gel extraction kit (MinElute gel extraction kit; Qiagen) and commercially sequenced independently on both strands using primers 16S3′F, ALAF (5′-GGTTTAGCTCAGTTGGT-3′), and 18m. Conserved domains and tRNAs were searched along the ITS sequences for the six strains using the cyanobacterial ITS alignment proposed by Iteman et al. (32) as a reference.
Phylogenetic analysis.
16S rRNA gene and ITS sequences obtained in this study were first analyzed by a similarity search using the BLAST software (www.ncbi.nlm.nih.gov/blast), and then the two most similar sequences were selected. Next the sequences were aligned with a representative data set of sequences of other Leptolyngbya strains available from GenBank, both manually and with the ClustalW program (www.ebi.ac.uk/clustalw). Maximum-parsimony trees were generated using a heuristic search constrained by random sequence addition, steepest descent, and tree-bisection-reconnection branch swapping using the PAUP* 4.0 b10 software package (51). Bootstrap values were obtained from 500 replicates with one random sequence addition to jumble the data using the PAUP* software. A maximum-likelihood tree was constructed using MODELTEST v 3.7 (42) with corrected invariable sites (I) and gamma shaper parameters (G). Distance analysis using the HKY85 distance method was also performed.
The 16S rRNA gene sequence of E. coli K-12 was used as an outgroup for the construction of trees with the 16S rRNA gene sequences obtained in this study and the corresponding sequences of several Leptolyngbya strains available in GenBank. The ITS sequences were aligned with the closest related strains available from GenBank for which the alignment with our ITS sequences seemed meaningful. Oscillatoria sp. strain PCC 9240 was arbitrarily defined as an outgroup. The trees were edited using TREEVIEW version 1.6.6 (R. D. M. Page, distributed by the author at http://Taxonomy.zoology.gla.ac.uk/rod/rod.html.).
PositionsThe 405 to 780 of the E. coli 16S rRNA gene were used to find operational taxonomic units (OTUs) using a threshold of 97.5% identity among our strains (53). Furthermore, the identified OTUs were divided in two categories (52): “new” OTUs, composed of only our sequences exhibiting less than 97.5% identity with GenBank sequences, and “cosmopolitan” OTUs, including our sequences and others from different environments. The OTUs defined were compared with near-complete 16S rRNA gene and ITS region sequences to show phylogenetic relationships within the trees.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences were deposited in the GenBank database under accession numbers AY769961 (VRUC184) (an update of a shorter sequence), DQ295207 (VRUC198), DQ295208 (VRUC192), DQ295209 (VRUC201), and DQ295210 (VRUC206). ITS sequences were deposited with access codes EF560651 (VRUC135), EF560652 (VRUC192), EF560653 (VRUC198), EF560654 (VRUC184), EF560655 (VRUC206), and EF560656 (VRUC201).
RESULTS
Morphology and ultrastructure.
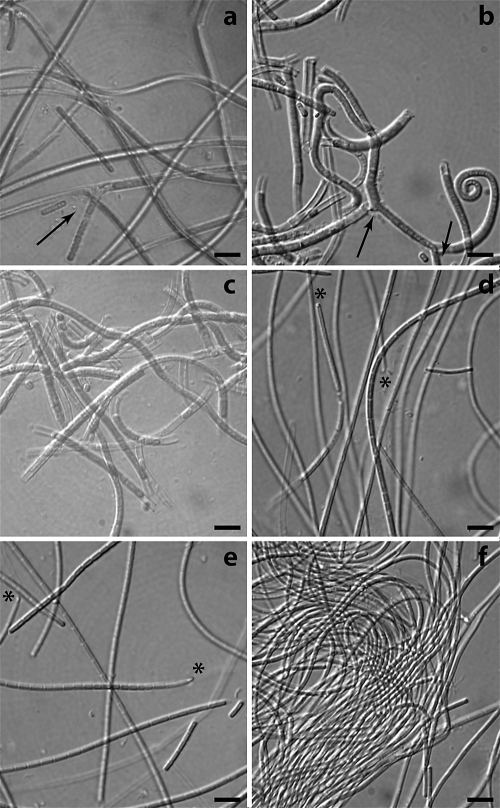
The six Leptolyngbya strains were observed by light and electron microscopy. On the basis of their pigmentation (Fig. 1) the strains could be divided into two phenotypes: strains VRUC184, VRUC201, and VRUC206 were green or blue-green in color, while VRUC135, VRUC192, and VRUC198 were red, due to a high phycoerythrin content. All the strains appeared as long filaments, surrounded by uncolored sheaths open at each end, and were able to form hormogonia as reproductive cells (Fig. 2). The three green strains had isodiametric cells and trichome fragmentation by randomly occurring death of individual cells within a filament (Table 2), while two of them, VRUC184 and VRUC201, showed false branching (Fig. 1a to c). The trichomes of the red strains were thinner than those of the green strains (Fig. 1d and e; Table 2), with evident constrictions at the cross walls, cells which were longer than wide, and a conical apical cell characterized by an orange spot at the tip (4). Cells of the green strains varied between 2.0 and 2.3 μm in width and 1.9 and 2.7 μm in length, and those of the red strains varied between 1.3 and 1.5 μm in width and 3.5 and 5.8 μm in length.
FIG. 1.
Photomicrographs illustrating the morphological features of the six Leptolyngbya strains. On the basis of their pigmentation they could be divided into phycocyanin-rich strains (green) VRUC184 (a), VRUC201 (b), and VRUC206 (c) and phycoerythrin-rich strains (red) VRUC192 (d), VRUC198 (e), and VRUC135 (f). Arrows indicate false branching (a and b), and asterisks indicate the eyespot-like structure at the tip of the apical cell (d to f). Bars, 5 μm.
FIG. 2.
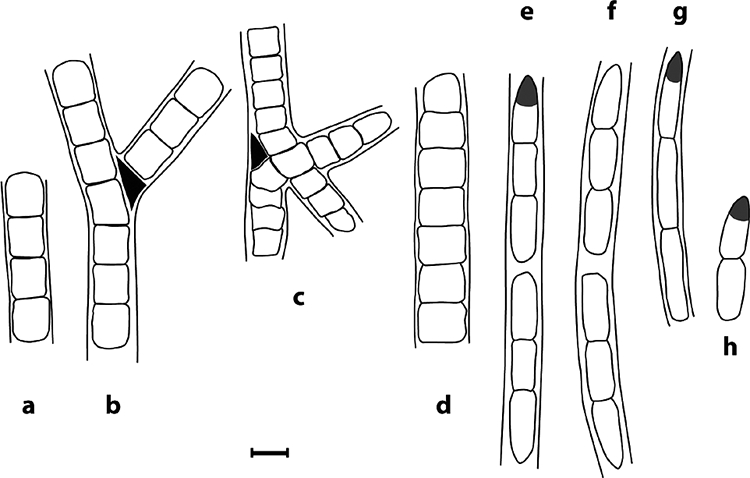
Line images of the six Leptolyngbya strains, showing the most relevant morphological features. All strains exhibited trichome fragmentation or hormogonia (a and h). Strains VRUC184 (a and b) and VRUC201 (c) formed false branching in correspondence with the position of necridial cells, while VRUC206 (d) lacked them. VRUC192 (e), VRUC198 (f), and VRUC135 (g and h) had cells longer than wide and an orange spot at the tip of the apical cell. Bar, 2 μm.
TABLE 2.
Morphological features of the six Leptolyngbya strains observed with a light microscopea
| Strain | Trichome fragmentation/ hormongonia | Necridic cells | False branching | Constrictions | Sheath | Filament diam (μm), mean ± SD | Cell width (μm), mean ± SD | Cell length (μm), mean ± SD | Apical cell shape | Pigmentation |
|---|---|---|---|---|---|---|---|---|---|---|
| VRUC184 | + | + | + | + | + | 2.4 ± 0.4 | 2.2 ± 0.4 | 2.7 ± 0.6 | Rounded | Green |
| VRUC201 | + | + | + | − | + | 2.2 ± 0.3 | 2.0 ± 0.4 | 1.9 ± 0.3 | Rounded | Blue-green |
| VRUC206 | + | + | − | + | + | 2.4 ± 0.3 | 2.3 ± 0.4 | 2.2 ± 0.5 | Rounded | Blue-green |
| VRUC192 | + | − | − | ++ | + | 2.1 ± 0.5 | 1.4 ± 0.2 | 3.5 ± 0.7 | Conical | Red |
| VRUC198 | + | − | − | ++ | + | 1.7 ± 0.4 | 1.5 ± 0.3 | 4.0 ± 0.7 | Conical | Red |
| VRUC135 | + | − | − | ++ | + | 1.8 ± 0.2 | 1.3 ± 0.2 | 5.8 ± 0.9 | Conical | Red |
−, absent; +, present; ++, abundant.
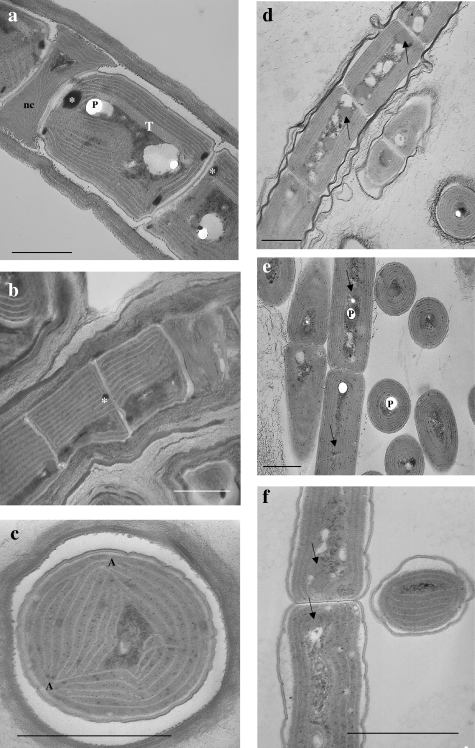
The six Leptolyngbya isolates showed similar ultrastructural features typical of the genus (Fig. 3). The number of thylakoids varied from 5 to 6 in all the red strains, from 6 to 8 in the green strains VRUC201 and VRUC206, and from 6 to 10 in the green strain VRUC184. The latter had inner thylakoids which were more densely packed than the peripheral ones. The thylakoidal arrangement was parietal in all six strains, although in the green strain VRUC201 the thylakoids had anchorage points attaching them to the cell membrane. The nucleoplasm was characterized by the presence of carboxysomes, cyanophycin, and lipid globules, and interthylakoidal glycogen granules were also observed. Ultrastructural differences between the strains included the structure and the thickness of the envelopes. In fact, the green-pigmented strains VRUC201, VRUC206, and VRUC184 (Fig. 3a, b, and c) all possessed a multistratified compact sheath with fibrils parallel to the long axis of the filament. In contrast, the red strains VRUC192, VRUC198, and VRUC135 (Fig. 3d, e, and f) had a thin, bilayered sheath; the internal layer was made up of fibrils running parallel to the long axis of the filament, while the external layer was formed by disordered fibrils that were sometimes grouped into bundles. Several cells were observed to be actively dividing by centripetal invagination of the cell wall. More than one necridic cell was observed in the trichomes of strains VRUC184, VRUC201, and VRUC206. Coccoid or rod-shaped bacteria were sometimes visible in the cultures, often associated with the outermost sheath layers.
FIG. 3.
TEM micrographs of strains VRUC184 (a), VRUC201 (b), VRUC206 (c), VRUC192 (d), VRUC198 (e), and VRUC135 (f). Note the presence of parietal thylakoids, polyphosphates (P), cyanophycin granules (asterisks), and carboxysomes (arrows) in the cytoplasm. In strain VRUC206 (c) the thylakoids were connected to the cell membrane (A), while in VRUC184 (a) the central thylakoids (T) were more densely packed than the peripheral ones. In the latter, a necridic cell (nc) is also visible. Bars, 1 μm.
Phylogenetic analysis of the partial sequences of the 16S rRNAs and ITSs.
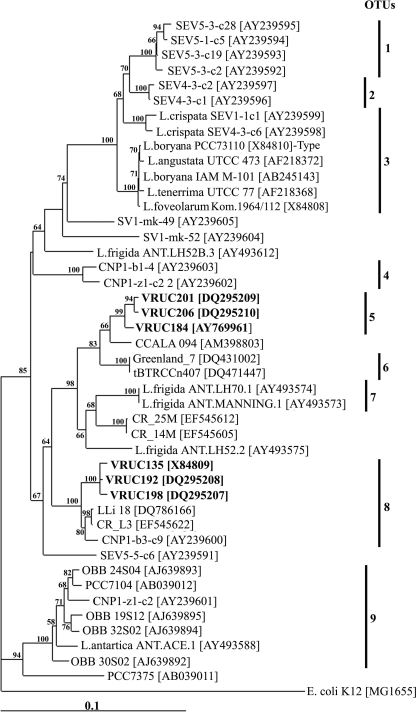
The 16S rRNA gene sequence (∼1,025 bases) was obtained for four Leptolyngbya strains, namely, VRUC192, VRUC198, VRUC201, and VRUC206. The 16S rRNA gene sequence of strain VRUC135 was previously reported (39), while a shorter 16S rRNA sequence of strain VRUC184 (15) has now been extended up to 1,027 bp. The red Leptolyngbya strains, VRUC135, VRUC192, and VRUC198, showed high DNA sequence identity (99.3% to 99.6%), as did the green strains VRUC184, VRUC201, and VRUC206 (98.4% to 99.5%). The sequence identity between the red and the green strains was about 92%. The neighbor-joining trees (Fig. 4) based on a data set of 45 16S rRNA gene sequences had the same topology of the trees inferred by the maximum-likelihood and the maximum-parsimony methods (not shown). As previously described (19), the well-supported clade of aquatic Leptolyngbya taxa, including the type species L. boryana (Gom.) Anagn. and Kom. PCC 73110 was sister to a clade containing Leptolyngbya strains from desert soils. In another paraphyletic branch of the phylogenetic tree, the red and green isolates of subaerophytic Leptolyngbya formed two different clusters whose near-terminal branches were supported by high bootstrap values. Notably, the green strains clustered into a group that was sister to a clade formed by two strains of Leptolyngbya, one epilithic on granite in Nepal (CCALA 094, GenBank accession no. AM398803 [38]) and one benthic in Artic hot springs (Greenland_7, GenBank accession no. DQ431002 [46]), plus an unidentified filamentous strain from thermal springs in Jordan (tBTRCCn407, GenBank accession no. DQ471447 [30]). The first of these strains showed 97.4 to 97.6% pairwise identity with the green strains, while the second and the third had less than 95% identity. The red strains were sister to a clade formed by three strains of Leptolyngbya sp.: two from geothermal waters of Costa Rica (LLi 18, GenBank accession no. DQ786166 [K. Finsinger and W. R. Hess, unpublished]; CR_L3, GenBank accession no. EF545622 [S. Morales et al., unpublished]) and one from Canyonland National Park (Utah) (CNP1-b3-c9, GenBank accession number AY239600 [19]) whose ecology was not disclosed. The pairwise identity of these three strains with the red strains ranged from 97.5% to 98.0%.
FIG. 4.
Neighbor-joining tree inferred from 45 16S rRNA gene sequences (∼ 916 bp). The numbers at the nodes indicate bootstrap values as percentages greater than 50% obtained using distance as an optimality criterion with 500 replicates. Numbers 1 to 9 indicate the OTUs inferred from partial 16S rRNA gene sequences (E. coli positions 405 to 780; threshold value, 97.5% identity). All the strains are Leptolyngbya sp. except where the species name is given. The sequences determined in the present study are indicated in bold. The E. coli K-12 sequence was designated as an outgroup. GenBank accession numbers are in brackets. The scale marker represents 0.1 nucleotide substitution per sequence position.
Based on the alignment of shorter 16S rRNA sequences, corresponding to positions 405 to 780 of the E. coli 16S rRNA gene, nine OTUs were found (Fig. 4) using a threshold of 97.5% identity. The red strains grouped in OTU8 with strains LLi 18, CR_L3, and CNP1-b3-c9 (pairwise identity of 97.8 to 98.3%); thus, we define this as a “cosmopolitan OTU” because it included our sequences and others from different environments. The green strains grouped in OTU5; strain CCALA 094 was near the threshold value (pairwise identity of 97 to 97.5%) for assignment to this OTU. The sequence identity within each OTU varied from 97 to 100% for the longer sequence of the 16 rRNA gene sequences (∼916 nucleotides) and from 97.5 to 100% for the OTUs (data not shown). OTU7 corresponded to OTU9 determined in a previous study (52).
The PCR amplification of the ITS regions yielded one dominant band of the expected size (Fig. 5): 600 nucleotides for the three red strains, 564 nucleotides for strain VRUC184, 630 nucleotide for strain VRUC206, and 631 nucleotides for strain VRUC201. This corresponded to the length of the ITS plus 22 bp of the 16S rRNA gene and 44 bp of the 23S rRNA gene, since the first primer recognition site is located ∼50 bp before the 3′ end of the 16S rRNA gene and the second is ∼50 bp after the 5′ end of the 23S rRNA gene. Sometimes minor bands were obtained, similar to those of cyanobacterial strains in which the presence of heteroduplex formation and multiple operons had been shown (32, 33). The sequences obtained for the 600-bp PCR product showed that the ITS regions of each of the six Leptolyngbya strains contained both tRNAAla and tRNAIle genes and the conserved domains D1, D1′, D2, D3, box A, D4, and D5, as described by Iteman et al. (32) (Fig. 6). In all six strains we found 100% sequence identity within these conserved regions, except for box A and D5, where only a few gaps and nucleotide substitutions were present. This distinguished the strains belonging to OTU5 from the strains belonging to OTU8. The polymorphism was found in the more variable regions (V2, box B, and V3) with some different nucleotide substitutions. The red strains VRUC192, VRUC198, and VRUC135 showed high pairwise identity (99.6 to 100%) and were readily separated from the green strains VRUC184, VRUC201, and VRUC206, with lower than 74% identity. Two green strains, VRUC201 and VRUC206 (with 98.2% pairwise identity), were distinct from strain VRUC184 with 80% sequence identity.
FIG. 5.
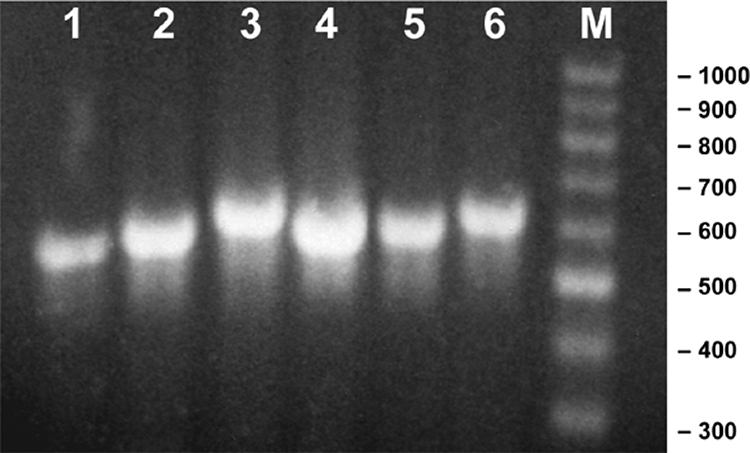
Agarose gel showing the PCR products obtained by amplification of the ITS regions with the six Leptolyngbya strains. One principal band was obtained for all the strains. Lanes: 1, VRUC184; 2, VRUC192; 3, VRUC201; 4, VRUC135; 5, VRUC198; 6, VRUC206; M, 1-kb molecular size ladder.
FIG. 6.
Nucleotide sequence alignments of full-length ITSs of the eight Leptolyngbya strains (six catacomb strains and strains LLi 18 and CCALA 094). The conserved (D1, D1′, D2, D3, D4, and D5) and variable (V2 and V3) domains, the antiterminator (box B and box A), and the tRNAIle and tRNAAla genes are indicated. One hundred percent sequence identity is indicated in grey within the conserved domains and is shaded within the variable regions.
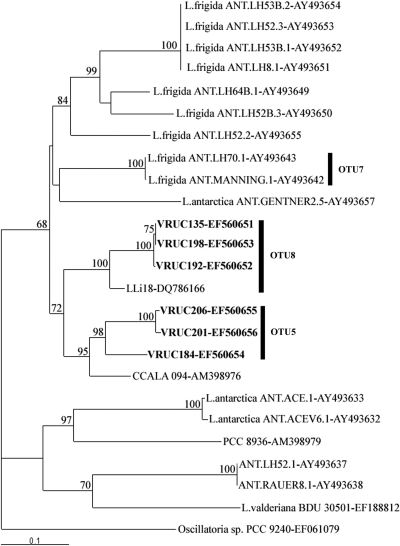
When the ITS sequences of these six strains were compared to a selection of Leptolyngbya ITS sequences available from GenBank, the results (Fig. 7) were comparable with the grouping inferred from the 16S rRNA gene sequences. The epilithic Leptolyngbya strain CCALA 094 (GenBank accession no. AM398976) clustered with the green strains; they had 73.7 to 77.1% sequence identity for the full-length ITS, but 100% sequence identity was observed within the conserved domains (Fig. 6). The thermal Leptolyngbya strain LLi 18 (GenBank accession no. DQ786166), belonging to OTU8, clustered with the red strains, sharing 80% sequence identity for the full-length ITS, with 100% sequence identity in the conserved domains. In both cases the degree of dissimilarity was higher in the polymorphic regions and in particular in region V2. Unfortunately, no ITS sequences are available for the other two strains, CNP1-b3-c9 and CR_L3, belonging to the same OTU. The ITS sequences of the two strains of Leptolyngbya frigida belonging to OTU7 clustered together.
FIG. 7.
Distance tree (HKY85) constructed from an alignment of the ITS region sequences obtained for the six Leptolyngbya strains (in bold) along with other ITS sequences (Leptolyngbya sp. except where the species name is given) available from GenBank. Oscillatoria sp. strain PCC 9240 was arbitrarily used as an outgroup. Numbers above branches indicate parsimony bootstrap values (percentages) of greater than 50% based on 500 replicates. Three clusters represent the same strains as in the tree obtained with the 16S rRNA gene sequences and the definition of OTU5, -7, and -8. The GenBank accession number is indicated after the strain name. The scale marker represents 0.1 nucleotide substitution per sequence position.
DISCUSSION
In this study, we report on the cytomorphological and genetic diversity of six subaerophytic troglobitic Leptolyngbya strains isolated from five Roman hypogea, as revealed by combining morphological and ultrastructural observations with 16S rRNA gene and ITS sequencing.
Six strains of Leptolyngbya could be separated into two morphotypes based on pigmentation and cell diameter. However, within the same morphotype, the differences observed, i.e., the presence or absence of false branching, cell sizes, and constrictions at the cross wall, did not allow a net distinction among the strains. Furthermore, the six Leptolyngbya strains also shared common ultrastructural features. In all cases, the parietal arrangement of thylakoids was typical of this genus, as it is in Pseudoanabaenaceae (11). Most of the morphological and ultrastructural features of subaerophytic Leptolyngbya strains have been previously studied, because of their ecological relevance in Roman hypogea (1, 4, 5, 6, 8). In fact, these are the most common cyanobacteria in extreme low-light environments and are the major agents of biodeterioration of underground archaeological sites attributable to the presence of acidic and sulfated groups in the heteropolysaccharides that form their sheaths, which have the ability to remove cations from stone substrata (13).
The current deficient state of cyanobacterial taxonomy makes a reevaluation of diagnostic traits based on a combination of thylakoidal patterns and molecular phylogenetic analyses timely (28, 36). In this respect, the thylakoid arrangement in our Leptolyngbya strains is in agreement with the position of this taxon in the phylogenetic tree of cyanobacteria. The 16S rRNA gene sequence identity among the green and the red strains was 92%. This would support a tentative assignment of the three green strains to the genus “Leptolyngbya” Komárek and Anagnostidis sensu stricto (35) and of the red strains to a different taxon.
Phylogenetic analysis based on longer (∼916 nucleotides) and shorter (∼400 nucleotides) sequences of the 16S rRNA gene confirmed the well-known polyphyletic nature of the genus Leptolyngbya (19, 31, 40, 54). In fact, different clusters and nine OTUs were obtained when 44 Leptolyngbya sequences were aligned. The type species, L. boryana (Gom.) Anagn. et Kom. PCC 73110 and L. foveolarum Komárek 1964/112, assigned to the genus “Leptolyngbya” (35), were present in a paraphyletic branch and in a different OTU than the green strains although they were assigned to the same genus. This highlights the heterogeneity of this genus and the likely presence of more generic entities still to be defined.
The phylogenetic analyses also indicated that the three troglobitic red strains clustered with three strains of Leptolyngbya sp. from subaerophytic and geothermal environments and belonged to the same OTU8, sharing more than 97.5% sequence identity (in the region corresponding to E. coli positions 405 to 780). Based on these data and on the species definition of Stackebrandt and Goebel (50), in which sequence identity among strains of less than 97.5% indicates that they represent different species, all these six strains may belong to the same species.
The phylogenetic relationships deduced from the 16S rRNA gene sequencing were in agreement with the ITS grouping as well with the grouping of the different OTUs. The analysis of the ITS sequences of the six strains identified three clusters: the first containing the red strains VRUC135, VRUC192, and VRUC198; the second the green strains VRUC201 and VRUC206; and the third the green strain VRUC184. Indeed, the ITS sequencing not only supported the high morphological and genetic identity shown for the red strains but discriminated among the green strains.
Complete sequence identity was found in the conserved domains in all six strains along with the Leptolyngbya strains CCALA 094 from Nepal and LLi 18 from Costa Rica. These domains are important sites for folding of the rRNA transcripts or for the transcriptional antitermination and the encoding of tRNAIle and tRNAAla (32). High identity in the conserved domains and polymorphisms in the variable regions of the ITS were also found in benthic and pelagic Microcystis colonies from a French storage reservoir (29). Nonetheless, the more variable regions (V2, box B, and V3) facilitated the discrimination among the six strains, defining three clusters, one with the red strains, one with the two green isolates VRUC201 and VRUC206, and another one with the green strain VRUC184. It appears that the secondary structure of these variable regions is more important than the primary sequence (32), and according to secondary structure predictions, variations are mostly confined to regions corresponding to loops or hairpin structures (22, 32). Furthermore, these variable regions showed the close relationship of the red strains with strain LLi 18 (92% sequence identity). This finding together with the 16S rRNA gene sequence data indicated that the two strains belong to the same species, although more knowledge of the morphological characters of the LLi 18 strain are needed to confirm this. The ITS relatedness of strains CNP1-b3-c9 and CR_L3 cannot be determined because of a lack of the corresponding sequences. However, because of the high sequence identity of the 16S rRNA gene sequences of these two strains with the LLi 18 strain (>98%), we could infer that these strains may also belong to the same species of the red Leptolyngbya strains.
OTU5 represents a new phylotype, because our sequences did not exhibit more than 97.5% identity with GenBank sequences. The genetic comparison of the 16S rRNA and ITS sequences of CCALA 094 with those of the green strains showed sequence identities of less than 97.5% and 77%, respectively. This strain also has a different cytomorphology, with cells shorter than wide and peripheral ondulating thylakoids (38). Based on these data, CCALA 094 was excluded from OTU5, and thus this should be considered a “new” OTU.
All these results demonstrate the utility of the polyphasic approach in cyanobacterial taxonomic studies. The ITS pattern configuration identified in the epilithic troglobitic Leptolyngbya strains, containing both tRNAIle and tRNAAla, has been reported as the most common for cyanobacteria. It was found also in almost all plastids investigated to date and is probably the same which the rRNA operon of the cyanobacterial ancestor may have possessed (14). This is in accordance with the hypothesis of an early origin of the Oscillatoriales and Chroococcales with respect to the heterocystous cyanobacteria (57) and confirms that the 16S-23S region represents a potentially powerful tool for studies of phylogeny and molecular evolution of cyanobacteria (14, 25, 43).
The comparison with other sequences available in the GenBank database showed that some genotypes are conserved in troglobitic Leptolyngbya strains as well as in subaerophytic and geothermal isolates such as CCALA 094 from Nepal, CNP1-b3-c9 from Utah, and LLi 18 and CR_L3 from Costa Rica, probably because of the extreme environmental conditions of their habitats.
In this study, we found a good resolution of the genetic variability among these strains using ITS domain sequencing. Since ITS differences reflect the geographic distribution of cyanobacteria, as has been reported for aquatic strains (18, 52), more ITS sequences of subaerophytic Leptolyngbya strains are needed to better understand their evolution and biogeography. To our knowledge, this is the first study in which a polyphasic approach, combining morphological and ultrastructural observations with 16S rRNA gene and ITS sequencing, was employed to resolve the diversity of Leptolyngbya strains and to assess intraspecific variation.
Acknowledgments
This work was partly supported by the EU program Energy, Environment and Sustainable Development, project “CATS—-Cyanobacteria attack rocks,” contract EVK4-CT2000-00028; by the Italian Ministry of University and Research, project PRIN 2001, 2003; and by the Italian Ministry of Foreign Affairs (Direzione Generale per la Promozione e Cooperazione Culturale).
We thank Maria Lo Ponte for the English revision of the manuscript, Giuliana Allegrucci for useful suggestions on the phylogenetic analysis, and Roberto Targa for line images.
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Albertano, P. 1991. Effects of monochromatic lights on four species of Leptolyngbya. Arch. Hydrobiol. Algolog. Studies 64:199-214. [Google Scholar]
- 2.Albertano, P. 1993. Epilithic algal communities in hypogean monument environment. Giorn. Bot. Ital. 127:385-392. [Google Scholar]
- 3.Albertano, P. 2003. Methodological approaches to the study of stone alteration caused by cyanobacterial biofilms in hypogean environments, p. 302-315. In R. J. Koestler, V. R. Koestler, A. E. Charola and F. E. Nieto-Fernandez (ed.), Art, biology, and conservation: biodeterioration of works of art. Metropolitan Museum of Art, New York, NY.
- 4.Albertano, P., L. Barsanti, L. Passatelli, and P. Gualtieri. 2000. A complex photoreceptive structure in the cyanobacterium Leptolyngbya sp. Micron 31:27-34. [DOI] [PubMed] [Google Scholar]
- 5.Albertano, P., and S. Bellezza. 2001. Cytochemistry of cyanobacterial exopolymers in biofilms from Roman hypogea. Nova Hedwigia 123:501-518. [Google Scholar]
- 6.Albertano, P., and M. Grilli Caiola. 1988. Structural and ultrastructural characters of a red biodeteriorating Lyngbya sp. in culture. Arch. Hydrobiol. Algolog. Studies 50:55-57. [Google Scholar]
- 7.Albertano, P., and L. Kováčik. 1994. Is the genus Leptolyngbya an homogeneous taxon? Arch. Hydrobiol. Algolog. Studies 75:37-51. [Google Scholar]
- 8.Albertano, P., L. Kováčik, and M. Grilli Caiola. 1994. Preliminary investigations on epilithic cyanophytes from a Roman necropolis. Arch. Hydrobiol. Algolog. Studies 75:71-74. [Google Scholar]
- 9.Albertano, P., L. Kovacik, P. Marvan, and M. Grilli Caiola. 1995. A terrestrial epilithic diatom from Roman catacombs, p. 11-21. In D. Marino and M. Montresor (ed.), Proceedings of the Thirteenth International Diatom Symposium. Biopress Limited, Bristol, United Kingdom.
- 10.Albertano, P., and C. Urzì. 1999. Structural interactions among epilithic cyanobacteria and heterotrophic microorganisms in Roman hypogea. Microb. Ecol. 38:244-252. [DOI] [PubMed] [Google Scholar]
- 11.Anagnostidis, K., and J. Komàrek. 1988. Modern approach to classification system of cyanophytes. 3. Oscillatoriales. Arch. Hydrobiol. Algolog. Studies 53:327-472. [Google Scholar]
- 12.Baurain, D., L. Renquin, S. Grubisic, and P. Scheldeman. 2002. Remarkable conservation of internally transcribed spacer sequences of Arthrospira (‘Spirulina') (Cyanophyceae, Cyanobacteria) strains from four continents and of recent and 30-year-old dried samples from Africa. J. Phycol. 38:384-393. [Google Scholar]
- 13.Bellezza, S., R. De Philippis, G. Paradossi, and P. Albertano. 2003. Leptolyngbya sp. strains from Roman hypogea: cytochemical and physico-chemical characterisation of exopolysaccharides. J. Appl. Phycol. 15:193-200. [Google Scholar]
- 14.Boyer, S. L., V. R. Flechtner, and J. R. Johansen. 2001. Is the 16S-23S rRNA internal transcribed spacer (ITS) region a good tool for use in molecular systematic and population genetics? A case study in cyanobacteria. Mol. Biol. Evol. 18:1057-1069. [DOI] [PubMed] [Google Scholar]
- 15.Bruno, L., D. Billi, and P. Albertano. 2005. Optimization of molecular techniques applied to the taxonomy of epilithic Leptolyngbya strains. Arch. Hydrobiol. Algolog. Studies 117:197-207. [Google Scholar]
- 16.Bruno, L., S. Piermarini, and P. Albertano. 2001. Characterisation of spectral emission by cyanobacterial biofilms in the Roman catacombs of Priscilla in Rome (Italy). Nova Hedwigia 123:229-236. [Google Scholar]
- 17.Bruno, L., C. Urzi', D. Billi, and P. Albertano. 2006. Genetic characterization of epilithic cyanobacteria and their associated bacteria. Geomicrobiol. J. 23:293-299. [Google Scholar]
- 18.Cadel-Six, S., C. Peyraud-Thomas, L. Brient, N. Tandeau de Marsac, R. Rippka, and A. Méjean. 2007. Different genotypes of anatoxin-producing cyanobacteria coexist in the Tarn River, France. Appl. Environ. Microbiol. 73:7605-7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casamatta, D. A., J. R. Johansen, M. L. Vis, and S. T. Broadwater. 2005. Molecular and morphological characterization of ten polar and near polar strains within the Oscillatoriales (Cyanobacteria). J. Phycol. 41:421-438. [Google Scholar]
- 20.Castenholz, R. W., and T. B. Norris. 2005. Revisionary concepts of species in the cyanobacteria and their applications. Arch. Hydrobiol. Algolog. Studies 117:53-69. [Google Scholar]
- 21.Ciferri, O. 1999. Microbial degradation of paintings. Appl. Environ. Microbiol. 65:879-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa, J.-L., P. Paulsrud, and P. Lindblad. 2002. The cyanobacterial tRNALeu (UAA) intron: evolutionary patterns in a genetic marker. Mol. Biol. Evol. 19:850-857. [DOI] [PubMed] [Google Scholar]
- 23.Ernst, A., S. Becker, U. I. A. Wollenzien, and C. Postius. 2003. Ecosystem-dependent adaptive radiations of picocyanobacteria inferred from 16S rRNA and ITS-1 sequence analysis. Microbiology 149:217-228. [DOI] [PubMed] [Google Scholar]
- 24.Fox, G. E., J. D. Wisotzkey, and P. Jurtshuk. 1992. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 42:166-170. [DOI] [PubMed] [Google Scholar]
- 25.Gugger, M., R. Molica, B. Le Berre, P. Dufour, C. Bernard, and J.-F Humbert. 2005. Genetic diversity of Cylindrospermopsis strains (Cyanobacteria) isolated from four continents. Appl. Environ. Microbiol. 71:1097-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez-Mariné, M., E. Clavero, and M. Roldan. 2003. Why there is such luxurius growth in the hypogean environments? Arch. Hydrobiol. Algologi. Studies 109:229-240. [Google Scholar]
- 27.Hoffmann, L. 2002. Caves and other low-light environments: aerophytic photoautotrophic microorganisms, p. 171-177. In G. Bitton (ed.), Encyclopedia of environmental microbiology. John Wiley & Sons, New York, NY.
- 28.Hoffmann, L., J. Komárek, and J. Kaštoský. 2005. System of cyanoprokaryotes (cyanobacteria). Arch. Hydrobiol. Algolog. Studies 117:95-115. [Google Scholar]
- 29.Humbert, J. F., D. Duris-Latour, B. Le Berre, H. Giraudet, and M. J. Salençon. 2005. Genetic diversity in Microcystis populations of a French storage reservoir assessed by sequencing of the 16S-23S rRNA intergenic spacer. Microb. Ecol. 49:308-314. [DOI] [PubMed] [Google Scholar]
- 30.Ionescu, D., A. Oren, Y. Hindiyeh, and H. I. Malkawi. 2007. The thermophilic cyanobacteria of the Zerka Ma'in thermal springs in Jordan, p. 413-424. In J. Seckbach (ed.), Algae and cyanobacteria in extreme environments. Springer, Dordrecht, The Netherlands.
- 31.Ishida, T., M. M. Watanabe, J. Sugiyama, and A. Yokota. 2001. Evidence for polyphyletic origin of the members of the orders of Oscillatoriales and Pleurocapsales as determined by 16S rDNA analysis. FEMS Microbiol. Lett. 201:79-82. [DOI] [PubMed] [Google Scholar]
- 32.Iteman, I., R. Rippka, N. Tandeau de Marsac, and M. Herdman. 2000. Comparison of conserved structural and regulatory domains within divergent 16S rRNA-23S rRNA spacer sequences of cyanobacteria. Microbiology 146:1275-1286. [DOI] [PubMed] [Google Scholar]
- 33.Iteman, I., R. Rippka, N. Tandeau de Marsac, and M. Herdman. 2002. rDNA analyses of planktonic heterocystous cyanobacteria, including members of the genera Anabaenopsis and Cyanospira. Microbiology 148:481-496. [DOI] [PubMed] [Google Scholar]
- 34.Komárek, J. 2003. Problem of the taxonomic category ‘species’ in cyanobacteria. Arch. Hydrobiol. Algolog. Studies 109:281-297. [Google Scholar]
- 35.Komárek, J., and K. Anagnostidis. 2005. Cyanoprokaryota. Part 2. Oscillatoriales, p. 1-759. In B. Büdel, G. Gärtner, L. Krienitz, and M. Schagerl (ed.) Süusswasserflora von Mitteleuropa Band, 19/2. Gustav Fischer, Jena, Germany.
- 36.Komárek, J., and J. Kaštovsky. 2003. Coincidences of structural and molecular characters in evolutionary lines of cyanobacteria. Arch. Hydrobiol. Algolog. Studies 109:305-325. [Google Scholar]
- 37.Laloui, W., K. A. Palinska, R. Rippka, F. Partensky, N. Tandeau de Marsac, M. Herdman, and I. Iteman. 2002. Genotyping of axenic and non-axenic isolates of the genus Prochlorococcus and the OMF-Synechococcus clade by size sequence analysis or RFLP of the internal transcribed spacer of the ribosomal operon. Microbiology 148:453-465. [DOI] [PubMed] [Google Scholar]
- 38.Marquardt, J., and K. A. Palinska. 2007. Genotypic and phenotypic diversity of cyanobacteria assigned to the genus Phormidium (Oscillatoriales) from different habitats and geographical sites. Arch. Microbiol. 187:397-413. [DOI] [PubMed] [Google Scholar]
- 39.Nelissen, B., R. De Baere, A. Wilmotte, and R. De Wachter. 1996. Phylogenetic relationships of nonaxenic filamentous cyanobacterial strains based on 16S rRNA sequence analysis. J. Mol. Evol. 42:194-200. [DOI] [PubMed] [Google Scholar]
- 40.Nelissen, B., A. Wilmotte, J.-M. Neefs, and R. De Wachter. 1994. Phylogenetic relationships among filamentous helical cyanobacteria investigated on the basis of 16S ribosomal RNA gene sequence analysis. Syst. Appl. Microbiol. 17:206-210. [Google Scholar]
- 41.Nübel, U., F. Garcia-Pichel, and G. Muyzer. 1997. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 63:3327-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 43.Premanandh, J., B. Priya, I. Teneva, B. Dzhambazov, D. Prabaharan, and L. Uma. 2006. Molecular characterization of marine cyanobacteria from the Indian subcontinent deduced from sequence analysis of the phycocyanin operon (cpcB-IGS-cpcA) and 16S-23S ITS region. J. Microbiol. 44:607-616. [PubMed] [Google Scholar]
- 44.Reynolds, C. S. 1963. The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J. Cell Biol. 17:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rippka, R., J. Deruelles, B. Waterbury, M. Herdman, and R. Y. Stanier. 1979. Generic assignments, strains histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1-61. [Google Scholar]
- 46.Roeselers, G., T. B. Norris, R. W. Castenholz, S. Rysgaard, R. N. Glud, M. Kuhl, and G. Muyzer. 2007. Diversity of phototrophic bacteria in microbial mats from Arctic hot springs (Greenland). Environ. Microbiol. 9:26-38. [DOI] [PubMed] [Google Scholar]
- 47.Saarela, M., H-L. Halakomi, M-L. Suihko, L. Maunuksela, L. Raaska, and T. Mattila-Sandholm. 2004. Heterotrophic microorganisms in air and biofilm samples from Roman catacombs, with special emphasis on actinobacteria and fungi. Int. Biodeterior. Biodegrad. 54:27-37. [Google Scholar]
- 48.Sànchez-Moral, S., J. C. Cañaveras, L. Laiz, C. Saiz-Jimenez, J. Bedoya, and L. Luque. 2003. Biomediated precipitation of calcium carbonate metastable phases in hypogean environments. Geomicrob. J. 20:491-500. [Google Scholar]
- 49.Scheldeman, P., D. Baurain, R. Bouhy, M. Scott, M. Muhling, B. A. Whitton, A. Belay, and A. Wilmotte. 1999. Arthrospira (Spirulina) strains from four continents are resolved into only two clusters, based on amplified ribosomal DNA restriction analysis of the internally transcribed spacer. FEMS Microbiol. Lett. 172:213-222. [DOI] [PubMed] [Google Scholar]
- 50.Stackebrandt, E., and B. M. Goebel. 1994. Taxonomical note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 51.Swofford, D. L. 2001. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), version 4. Sinauer Associates, Sunderland, MA.
- 52.Taton, A., S. Grubisic, D. Ertz, D. A. Hodgson, R. Piccardi, N. Biondi, M. R. Tredici, M. Mainini, D. Losi, F. Marinelli, and A. Wilmotte. 2006. Polyphasic study of antartic cyanobacterial strains. J. Phycol. 42:1257-1270. [Google Scholar]
- 53.Taton, A., S. Grubisic, E. Brambilla, R. de Wit, and A. Wilmotte. 2003. Cyanobacterial diversity in natural and artificial microbial mats of lake Fryxell (Dry Valleys, Antarctica): a morphological and molecular approach. Appl. Environ. Microbiol. 69:5157-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner, S., K. M. Pryer, V. P. W. Miao, and J. D. Palmer. 1999. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol. 46:327-338. [DOI] [PubMed] [Google Scholar]
- 55.Urzì, C., P. Donato, C. Lo Passo, and P. Albertano. 2002. Occurrence and biodiversity of Streptomyces strains isolated from Roman hypogea, p. 269-272. In E. Galan and F. Zezza (ed.), Protection and conservation of the cultural heritage of the Mediterranean cities. Balkema Publishers, Lisse, The Netherlands.
- 56.Willame, R., C. Boutte, S. Grubisic, A. Wilmotte, J. Komárek, and L. Hoffmann. 2006. Morphological and molecular characterization of planktonic cyanobacteria from Belgium and Luxembourg. J. Phycol. 42:1312-1332. [Google Scholar]
- 57.Wilmotte, A., and M. Herdman. 2001. Phylogenetic relationships among the cyanobacteria based on 16S rRNA sequences, p. 487-599. In G. M. Garrity, D. R. Boone, and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology. Springer-Verlag, New York, NY.
- 58.Wilmotte, A., J. M. Neefs, and R. De Wachter. 1994. Evolutionary affiliation of the marine nitrogen-fixing cyanobacterium Trichodesmium sp. strain NIBB 1067, derived by 16S ribosomal RNA sequence analysis. Microbiology 140:2159-2164. [DOI] [PubMed] [Google Scholar]
- 59.Wilmotte, A., G. Van der Auwer, and R. De Wachter. 1993. Structure of the 16S ribosomal RNA of the thermophilic cyanobacterium Chlorogloeopsis HTF (‘Mastigocladus laminosus HTF') strain PCC7518, and phylogenetic analysis. FEBS Lett. 317:96-100. [DOI] [PubMed] [Google Scholar]