Abstract
Anti-Helicobacter pylori activities were determined by agar dilution, confocal laser scanning microscopy, and cell proliferation assays following treatment with various grape extracts. Muscadine grape skin possessed the strongest activity, followed by grape synergy (skin and seed) and seed, suggesting that higher phenolic levels do not necessarily determine overall anti-H. pylori efficacy.
Helicobacter pylori is considered the etiological agent of peptic ulcers and gastritis and is associated with mucosa-associated lymphoid tissue lymphoma and gastric cancer (4). Although treatment is usually effective, it can result in side effects, such as antibiotic resistance development (14) and relapse due to low compliance (2). Therefore, alternative methods should be explored to treat H. pylori infection.
Studies have reported many natural plant extracts with anti-H. pylori activity, including garlic, broccoli, cranberries, and green tea (5, 12, 16, 26). Grapes (Vitis vinifera), well known for their high levels of antioxidants and polyphenols, have also shown promise as novel antimicrobial agents. A few studies have already reported the anti-H. pylori activities of grape seed and wine, including an active chemical constituent (e.g., resveratrol, a stilbene from red wine) (13). However, no effort has been made to evaluate the grape skin or different grape types (e.g., table and muscadine grapes). For example, muscadines (Vitis rotundifolia) contain significantly higher levels of phenolics than commercial table grapes in addition to possessing some unique forms of these compounds (23). However, little is currently known about the antibacterial properties these fruits possess, making them prime candidates for study. In addition, it is believed that the high complexity of bioactive compounds present in these products and their broad range of activity over a number of microorganisms may make it difficult for microbes to acquire resistance during treatment (26).
The objectives of this study were to investigate the effects of various grape extracts against H. pylori and to determine any correlations between anti-H. pylori activity and extract phenolic content.
Five H. pylori strains (G2-1, 26695, WV 99, NB2-1, and 1324P-1) were obtained from Douglas Berg (Washington University, St. Louis, MO), and five clinical H. pylori isolates (D5251, D5131, D5178, D5136, and D5135) were obtained from Ben Gold (Emory University and Centers for Disease Control and Prevention, Atlanta, GA). H. pylori P1pDH80, a green fluorescent protein (GFP)-labeled strain, was provided by Rainer Haas (Max von Pettenkofer Institut fur Hygiene und Medizinische Mikrobiologie, Munchen, Germany). H. pylori SS1, a mouse-adapted strain, was provided by Kathryn Eaton (Department of Veterinary Biosciences, Ohio State University, Columbus, OH). For the entire study, bacteria were grown on brain heart infusion agar (Difco Laboratories, Detroit, MI) (pH 7.4 ± 0.2) supplemented with 10% horse serum (HS) (Sigma Chemical Co., St. Louis, MO) at 37°C for 72 h under microaerophilic conditions (5% O2, 10% CO2, and 85% N2) in a GasPak jar (BBL Microbiology Systems, Cockeysville, MD) (8). Liquid cultures were grown in brucella broth (Difco) (pH 7.0 ± 0.2) supplemented with 10% HS and incubated, with shaking (60 rpm), under the same conditions. Cultures were transferred from frozen stock (brucella broth-HS-10% glycerol, −80°C), subcultured twice, and then grown overnight for antimicrobial susceptibility testing.
Fresh grapes were purchased from a local supermarket. Skins from red, white, and black table grapes were dried at 70°C for 3 days in a gravity convection oven (Fisher Scientific, Gaithersburg, MD). Muscadine powder was provided by Muscadine Products Corporation (Wray, GA). Samples were defatted in petroleum ether (VWR International, West Chester, PA) and extracted in acetone-water-acetic acid (90:9.5:0.5) at 60°C for 8 h using a Soxhlet apparatus (7). The crude extracts were concentrated under vacuum, resuspended in methanol, and filter sterilized (0.2 μm; VWR). All extracts were stored at −20°C in the dark and used within 1 week. The total phenolic content of each extract was determined by the Folin-Singleton colorimetric method (21) and expressed as mg gallic acid equivalents (GAE)/g extract dry weight (dw). Major phenolics were measured by high-performance liquid chromatography as previously described (20) and reported as mg/g extract dw.
The anti-H. pylori activities of grape extracts (range, 256 to 2,048 μg/ml) and the pure compounds resveratrol, ellagic acid, and myricetin (range, 6.25 to 50 μg/ml) (Sigma) were evaluated by the agar dilution method in duplicate (14a). Each bacterial suspension was spot inoculated (ca. 1 × 105 CFU/spot) onto brain heart infusion-HS plates containing each antimicrobial, and the MIC was determined following incubation at 37°C for 3 days under microaerophilic conditions.
AGS cells (ATCC CRL-1739; American Type Culture Collection, Manassas, VA) were grown in Ham's F-12K medium supplemented with 10% fetal bovine serum (HyClone Laboratories, South Logan, UT) and incubated at 37°C in a 5% CO2 incubator with 100% humidity to 90% confluence. Cells were washed with phosphate-buffered saline (PBS; pH 7.4) and detached using 0.25% trypsin-EDTA (Sigma). Cell concentrations were determined using trypan blue and a hemocytometer.
For cell viability assays, overnight cultures of H. pylori strains 26695, P1pDH80, and SS1 at final concentrations of 5 × 105 CFU/ml were treated with grape extract or pure compounds for different times (see Table 2). Following treatment, H. pylori was pelleted by centrifugation (3,500 × g for 10 min), washed twice with sterile PBS, and resuspended in fresh cell culture medium. AGS cells were grown as described previously, distributed into 96 cell culture wells (Corning Costar Corp., Cambridge, MA), and incubated overnight to allow for adherence. Extract-treated H. pylori cultures (26695, P1pDH80, and SS1) were distributed to each well at a bacterium-to-AGS-cell ratio of 100:1 (based on the control, i.e., AGS cells infected with H. pylori without treatment). This was necessary since AGS cell growth was sensitive to both extracts and constituent compounds in preliminary experiments (data not shown). Following overnight incubation, the culture medium was replaced with RPMI 1640 (HyClone Laboratories) after washing with sterile PBS to remove nonadherent H. pylori cells. AGS cell proliferation was then determined by adding 3-(4,5-dimethyl-thiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)- 2H-tetrazolium (MTS) solution (CellTiter 96 AQueous one-solution proliferation assay; Promega, Madison, WI) following the manufacturer's directions. Absorbances were read at 490 nm using a BioTek μ-Quant microplate reader (Bio-Tek Instruments, Inc., Winooski, VT) and standardized against the AGS cell control.
TABLE 2.
AGS cell proliferation following infection with H. pylori 26695, SS1, and GFP-labeled P1pDH80 treated with muscadine grape skin, seed, and synergy extracts
| Extract | Concentration (μg/ml) | % AGS cell proliferation at treatment time (h)a:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
2
|
6
|
12
|
24
|
||||||||||||
| 26695 | P1 | SS1 | 26695 | P1 | SS1 | 26695 | P1 | SS1 | 26695 | P1 | SS1 | 26695 | P1 | SS1 | ||
| Skin | 0 | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 b | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 a |
| 512 | 44.6 a | 48.3 a | 54.5 a | 45.4 a | 50.5 b | 50.8 a | 47.0 a | 62.3 b | 73.9 b | 73.2 b | 86.8 b | 85.3 b | 89.4 b | 89.8 b | 90.8 b | |
| 1,024 | 44.6 a | 48.3 a | 54.5 a | 45.3 a | 47.7 a | 55.8 b | 55.4 b | 72.8 c | 84.2 c | 84.7 c | 92.4 c | 90.7 c | 94.4 b | 94.2 c | 95.4 c | |
| 2,048 | 44.6 a | 48.3 a | 54.5 a | 46.7 b | 49.4 a | 55.0 b | 71.6 c | 77.5 d | 86.2 c | 90.6 d | 93.1 c | 91.8 c | 98.4 c | 97.3 d | 97.3 d | |
| 4,096 | 44.6 a | 48.3 a | 54.5 a | 48.3 b | 52.4 b | 55.9 b | 73.5 c | 78.3 d | 90.8 d | 95.9 e | 94.2 d | 95.2 d | 99.4 c | 97.8 d | 98.8 d | |
| Seed | 0 | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 a |
| 512 | 44.6 a | 48.3 a | 54.5 a | 41.8 a | 48.2 a | 59.0 b | 45.0 a | 52.4 b | 63.0 b | 60.0 b | 57.6 b | 80.3 b | 65.7 b | 62.5 b | 85.0 b | |
| 1,024 | 44.6 a | 48.3 a | 54.5 a | 43.2 a | 48.6 a | 58.2 b | 47.3 a | 73.9 c | 61.3 b | 73.9 c | 89.9 c | 82.1 c | 85.8 c | 93.2 c | 86.4 b | |
| 2,048 | 44.6 a | 48.3 a | 54.5 a | 46.7 b | 54.2 b | 58.9 b | 47.8 b | 75.0 c | 74.8 c | 73.1 c | 92.4 c | 84.5 c | 90.7 d | 94.5 c | 92.5 c | |
| 4,096 | 44.6 a | 48.3 a | 54.5 a | 47.5 b | 55.4 b | 59.0 b | 56.4 c | 75.1 c | 81.3 d | 80.4 d | 93.1 c | 89.5 d | 92.6 d | 95.3 c | 95.0 c | |
| Synergy | 0 | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 a | 44.6 a | 48.3 a | 54.5 a |
| 512 | 44.6 a | 48.3 a | 54.5 a | 43.5 a | 48.4 a | 55.0 a | 50.1 b | 58.3 b | 55.8 a | 76.1 b | 87.9 b | 84.2 b | 84.5 b | 92.2 b | 85.5 b | |
| 1,024 | 44.6 a | 48.3 a | 54.5 a | 43.5 a | 48.5 a | 55.5 a | 53.7 b | 67.5 c | 61.8 b | 80.9 c | 92.2 c | 88.4 c | 88.2 c | 97.2 c | 93.1 c | |
| 2,048 | 44.6 a | 48.3 a | 54.5 a | 43.9 a | 48.4 a | 56.8 a | 60.8 c | 76.4 d | 74.0 c | 88.2 d | 94.7 c | 88.6 c | 92.9 c | 98.2 c | 95.1 c | |
| 4,096 | 44.6 a | 48.3 a | 54.5 a | 40.8 a | 51.9 b | 51.7 b | 68.8 d | 77.2 d | 80.9 d | 89.2 d | 93.5 c | 90.1 d | 95.9 c | 98.8 c | 96.8 c | |
The same letter represents no significant difference (P > 0.05) between treatment concentrations for each H. pylori strain within each column for each extract. Eight replicates per extract concentration and time combination were used for each H. pylori strain.
The effects of muscadine grape extracts on H. pylori attachment to AGS cells were also determined by confocal laser scanning microscopy. Overnight cultures of GFP-labeled H. pylori P1pDH80 were treated with different concentrations of muscadine grape extracts for 1, 2, 4, 6, 12, and 24 h under microaerophilic conditions. Following treatment, H. pylori cells were washed in sterile PBS and incubated for 1 h with overnight cultures of AGS cells attached to eight-well Labtek chamber slides (Fisher Scientific, Norcross, GA) at low density. Following the removal of nonadherent cells, the attachment of GFP-labeled H. pylori to host cells was examined using a Zeiss LSM-510 laser scanning confocal microscope (Carl Zeiss, Inc., Germany).
During storage, total phenolic levels in grape extracts declined significantly, with up to 46% of the phenolic content lost during 3-month storage at 4°C and −15°C (data not shown). However, phenolic levels for grape extracts stored at −80°C (with or without N2) did not significantly decrease (P > 0.05) within the first month. Due to the rapid loss of total phenolic compounds in grape extracts, all the experiments were carefully implemented as to maximize the retention of these bioactive compounds.
The MIC results indicate that the grape extracts and pure compounds have significant but differing effects against H. pylori growth (Table 1). Muscadine grape skin extract was most effective (MIC range, 256 to 512 μg/ml), followed by muscadine seed (MIC range, 256 to 1,024 μg/ml) and synergy extracts (skin and seed) (MIC range, 512 to 1,024 μg/ml). Resveratrol and ellagic acid also inhibited H. pylori (MIC range, 6.25 to 50 μg/ml); however, myricetin had no effect and was not tested further (Table 1).
TABLE 1.
MICs of grape extracts and compounds on H. pylori growth by agar dilution assay
| Extract/compounda | MIC (μg/ml) of H. pylori strainb:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G2-1 | 26695 | WV99 | NB2-1 | 1324P-1 | D5251 | D5131 | D5178 | D5136 | D5135 | SS1 | P1pDH80 | |
| RGS | 1,024 | 512 | 512 | 512 | 512 | 1,024 | 512 | 512 | 512 | 512 | − | − |
| WGS | 1,024 | 512 | 1,024 | 512 | 512 | 1,024 | 512 | 512 | 512 | 512 | − | − |
| BGS | 1,024 | 512 | 1,024 | 1,024 | 512 | 2,048 | 512 | 512 | 512 | 512 | − | − |
| MSN | 256 | 256 | 256 | 512 | 256 | 256 | 512 | 512 | 256 | 512 | 512 | 512 |
| MSD | 512 | 1,024 | 512 | 256 | 256 | 256 | 1,024 | 1,024 | 512 | 1,024 | 512 | 1,024 |
| MSY | 512 | 512 | 1,024 | 512 | 1,024 | 512 | 512 | 1,024 | 512 | 1,024 | 512 | 512 |
| RSV | − | 12.5 | − | − | − | − | − | − | − | − | 6.25 | 12.5 |
| ELA | − | 50 | − | − | − | − | − | − | − | − | 12.5 | 12.5 |
| MYR | − | >50 | − | − | − | − | − | − | − | − | >50 | >50 |
RGS, red grape skin; WGS, white grape skin; BGS, black grape skin; MSN, muscadine grape skin; MSD, muscadine grape seed; MSY, muscadine grape synergy; RSV, resveratrol; ELA, ellagic acid; MYR, myricetin.
Average MIC at 72 h by agar dilution assay. Each experiment was performed in duplicate. −, not determined.
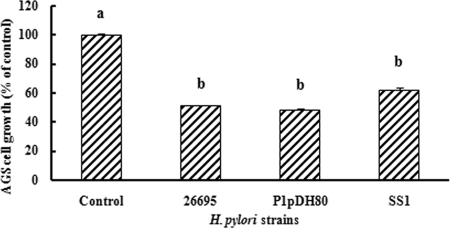
Figure 1 shows that there were substantial reductions in AGS cell viabilities following 24-h H. pylori infection with all three tested strains as determined by MTS assay. However, following treatment of the cells with grape extracts, average AGS cell growth was significantly higher than the controls (Table 2) and directly proportional to both extract concentration (P < 0.05) and treatment time (P < 0.05). Muscadine grape skin extract had the highest anti-H. pylori activity following 24 h treatment (AGS cell growth range, 97.8 to 99.4% at 4,096 μg/ml), followed by muscadine grape synergy and seed extract (Table 2). Treatment of H. pylori 26695 and SS1 with resveratrol (6.25 to 50 μg/ml) and ellagic acid (6.25 to 50 μg/ml) was also effective (data not shown); however, no significant difference (P > 0.05) was found between AGS cell growth following treatment of these two H. pylori strains with these compounds.
FIG. 1.
AGS cell proliferation following 24-h exposure to H. pylori. Error bars represent the standard errors of eight replicates. The same letter represents no significant difference (P > 0.05) between H. pylori strains.
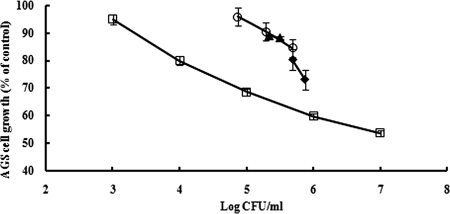
Because bacteria were treated in the absence of AGS cells, H. pylori populations used for MTS assays varied. Therefore, AGS cells were exposed to different concentrations of H. pylori 26695 without muscadine grape extract treatment for 24 h and measured by MTS assay. AGS cell growth with extract-treated H. pylori (12 h) was then compared to a standard curve. Figure 2 shows that AGS cell growth was higher for extract-treated H. pylori 26695 at the same cell populations as controls, indicating that additional factors, besides reduced bacterial numbers, may affect H. pylori following treatment.
FIG. 2.
AGS cell proliferation following 24-h exposure to various concentrations of H. pylori 26695 (⋄) or H. pylori 26695 treated with muscadine skin (o), seed (⧫), or synergy (▴) extracts for 12 h (one representative experiment with three replicates). Error bars represent standard errors.
Treatment with muscadine grape skin extract resulted in a significant decrease in H. pylori attachment to AGS cells compared to that of controls (Fig. 3). All extracts (range, 1,024 to 4,096 μg/ml) reduced H. pylori attachment with effects observed at the lowest concentration (data not shown). The majority of the GFP-labeled H. pylori cells were coccoidal in form and appeared to clump at higher extract concentrations and longer treatment times, especially at 12 and 24 h, indicating a decline in attachment efficiency (Fig. 3).
FIG. 3.
Confocal laser scanning microscopy images of GFP-labeled H. pylori attached to AGS cells following treatment for 0, 12, and 24 h with muscadine grape skin extract at 4,096 μg/ml. Excitation wavelength, 488 nm; emission wavelength, 510 to 530 nm. All images were acquired and analyzed by the LSM-510 software package (Zeiss LSM 510, version 2.8).
Phenolic compounds in grape products play important roles for antioxidant, anti-inflammatory, and antimicrobial effects (13, 18, 20). In this study, muscadine grape seed extract contained the highest total phenolic content (645.5 mg GAE/g dw), followed by muscadine synergy and skin extracts (381.9 and 135.0 mg GAE/g dw, respectively). Total phenolic concentrations in muscadine seed extracts were approximately five times higher than its skin counterpart, a finding consistent with that previously reported by Pastrana-Bonilla et al. (18). High-performance liquid chromatography results were also in agreement with the previous study for the major phenolics ellagic acid, myricetin, quercetin, and resveratrol with respective average concentrations of 77.3, 22.5, 3.4, and 1.4 mg/g extract dw.
In this study, muscadine grape skin extract was most effective against H. pylori in all tests performed. Although muscadine grape seed contained significantly higher total phenolic levels than other extracts, its efficacy against H. pylori was lower than or similar to that of muscadine skin and synergy extracts (Tables 1 and 2), suggesting that higher phenolic levels do not necessarily correlate with increased anti-H. pylori activity but rather the type and concentrations of compounds present in these extracts. Resveratrol, the least-abundant major phenolic in muscadine grape skin, has been shown to inhibit cagA-positive H. pylori strains (6). Ellagic acid, the most-abundant phenolic, has also been reported to possess anti-H. pylori activity (15). In addition, this compound has been found to inhibit cancer, alter bacterial protein conformations, and affect ion pump function (11, 22); however, it is not known if a similar mode of action against H. pylori exists. Because these phenolics are partially hydrophobic, this may allow them to interact with the bacterial cell wall and lipopolysaccharide interfaces more effectively by decreasing membrane stability (25). Therefore, further research on individual compounds and their interactions in the fruit itself is necessary to elucidate the possible synergistic/antagonistic mechanism(s) by which these compounds exert their effects.
Bacterial attachment to stomach epithelium is considered the initial step for H. pylori pathogenesis. Several recent studies have evaluated the antiadhesive properties of various natural compounds (1, 9, 17). Confocal microscopy revealed fewer bacterial cells present on host cell surfaces following treatment with grape extracts (Fig. 3), in agreement with AGS cell proliferation assays. Two possible explanations are that these compounds may interfere with H. pylori's ability to increase the expression of AGS cell adhesion molecules (10) or inhibit sialic acid-specific adhesions to epithelial cells (1).
Following treatment with muscadine grape extracts or compounds, AGS cell proliferation was similar to that of the controls without H. pylori (Table 2), suggesting that extracts either reduce or inhibit H. pylori from binding to and/or damaging AGS cells. Figure 2 reveals that approximately 2 log CFU H. pylori/ml in extract-treated populations lost the ability to damage host cells, suggesting that some unknown factor(s), in addition to lower H. pylori numbers, may be responsible for higher cell proliferation levels. The loss of attachment potential for H. pylori following grape extract treatment could be due to many levels of damage and injury to the microorganism, including the inhibition of basic energy production and/or virulence factors. Because H. pylori VacA toxin is known to cause gastric injury via damage to host stomach cells, the increased AGS cell proliferation may be attributed to the inhibition of vacA-induced ion channel formation, urea conduction, and cell vacuolization (24). The polyphenol compounds may form aggregates with the toxin, in turn preventing its receptor binding and internalization into the host cell (27). A recent finding by Ruggiero et al. (19) found that red wine was able to prevent H. pylori-induced gastric epithelium damage in mice, suggesting that VacA inhibition may be involved. However, further studies are needed to determine the specific effect(s) (e.g., loss of attachment mechanisms, virulence factors, host membrane integrity, etc.) these extracts have on the organism's overall pathogenesis.
In this study, grape extracts and their compounds were effective at inhibiting H. pylori in vitro, with highest efficacy by muscadine grape skin extract. Although anti-H. pylori activities by individual compounds were reported, it is believed that a synergistic mode of action is more likely responsible for the extract's antimicrobial activity (26). However, these complex interactions among compounds found in these extracts have yet to be determined. Our results suggest that anti-H. pylori activity does not necessarily correlate with higher phenolic content as previously thought. This study supports the need for further research to fully evaluate the in vivo potential of grape extracts and constituent compounds for use in the dietary management of H. pylori infection.
Acknowledgments
This research was partially supported by a grant from the Institute for Nutraceutical Research at Clemson University and the South Carolina Research Authority.
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Burger, O., I. Ofek, M. Tabak, E. Weiss, N. Sharon, and I. Neeman. 2000. A high molecular mass constituent of cranberry juice inhibits Helicobacter pylori adhesion to human gastric mucus. FEMS Immunol. Med. Microbiol. 29:295-301. [DOI] [PubMed] [Google Scholar]
- 2.Canducci, F., A. Armuzzi, F. Cremonini, G. Cammarota, F. Bartolozzi, P. Pola, G. Gasbarrini, and A. Gasbarrini. 2000. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment. Pharmacol. Ther. 14:1625-1629. [DOI] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Cover, T., and M. Blaser. 1995. Helicobacter pylori: a bacterial cause of gastritis, peptic ulcer disease, and gastric cancer. ASM News 61:21-26. [Google Scholar]
- 5.Fahey, J. W., X. Haristoy, P. M. Dolan, T. W. Kensler, I. Scholtus, K. K. Stephenson, P. Talalay, and A. Lozniewski. 2002. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 99:7610-7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaby, A. 2001. Helicobacter pylori eradication: are there alternatives to antibiotics? Altern. Med. Rev. 6:355-366. [PubMed] [Google Scholar]
- 7.Jayaprakasha, G., T. Selvi, and K. Sakariah. 2003. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 36:117-122. [Google Scholar]
- 8.Jiang, X., and M. P. Doyle. 2000. Growth supplements for Helicobacter pylori. J. Clin. Microbiol. 38:1984-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, C., and A. Jaworski. 1987. Fractionation and HPLC determination of grape wine. J. Agric. Food Chem. 35:257-259. [Google Scholar]
- 10.Lim, J. W., H. Kim, and K. H. Kim. 2003. Cell adhesion-related gene expression by Helicobacter pylori in gastric epithelial AGS cells. Int. J. Biochem. Cell Biol. 35:1284-1296. [DOI] [PubMed] [Google Scholar]
- 11.Lin, Y. T., Y. I. Kwon, R. G. Labbe, and K. Shetty. 2005. Inhibition of Helicobacter pylori and associated urease by oregano and cranberry phytochemical synergies. Appl. Environ. Microbiol. 71:8558-8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabe, K., M. Yamada, I. Oguni, and T. Takahashi. 1999. In vitro and in vivo activities of tea catechins against Helicobacter pylori. Antimicrob. Agents Chemother. 43:1788-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahady, G. B., S. L. Pendland, and L. R. Chadwick. 2003. Resveratrol and red wine extracts inhibit the growth of CagA+ strains of Helicobacter pylori in vitro. Am. J. Gastroenterol. 98:1440-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer, J. M., N. P. Silliman, W. Wang, N. Y. Siepman, J. E. Sugg, D. Morris, J. Zhang, H. Bhattacharyya, E. C. King, and R. J. Hopkins. 2002. Risk factors for Helicobacter pylori resistance in the United States: the Surveillance of Helicobacter pylori Antimicrobial Resistance Partnership (SHARP) study, 1993-1999. Ann. Intern. Med. 136:13-24. [DOI] [PubMed] [Google Scholar]
- 14a.National Committee for Clinical Laboratory Standards. 1997. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard, 2nd ed. NCCLS document M31-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 15.Nohynek, L. J., H. Alakomi, M. P. Kähkönen, M. Heinonen, I. M. Helander, K. Oksman-Caldentey, and R. H. Puupponen-Pimiä. 2006. Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 54:18-32. [DOI] [PubMed] [Google Scholar]
- 16.O'Gara, E. A., D. J. Hill, and D. J. Maslin. 2000. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 66:2269-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Mahony, R., H. Al-Khtheeri, D. Weerasekera, N. Fernando, D. Vaira, J. Holton, and C. Basset. 2005. Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J. Gastroenterol. 11:7499-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastrana-Bonilla, E., C. C. Akoh, S. Sellappan, and G. Krewer. 2003. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 51:5497-5503. [DOI] [PubMed] [Google Scholar]
- 19.Ruggiero, P., G. Rossi, F. Tombola, L. Pancotto, L. Lauretti, G. Del Giudice, and M. Zoratti. 2007. Red wine and green tea reduces Helicobacter pylori- or VacA-induced gastritis in a mouse model. World J. Gastroenterol. 13:349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi, J., J. Yu, J. Pohorly, and Y. Kakuda. 2003. Polyphenolics in grape seeds—biochemistry and functionality. J. Med. Food 6:291-299. [DOI] [PubMed] [Google Scholar]
- 21.Singleton, V. L., and J. A. Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16:144-158. [Google Scholar]
- 22.Smith, S. H., P. L. Tate, G. Huang, J. B. Magee, K. M. Meepagala, D. E. Wedge, and L. L. Larcom. 2004. Antimutagenic activity of berry extracts. J. Med. Food 7:450-455. [DOI] [PubMed] [Google Scholar]
- 23.Talcott, S. T., and J. H. Lee. 2002. Ellagic acid and flavonoid antioxidant content of muscadine wine and juice. J. Agric. Food Chem. 50:3186-3192. [DOI] [PubMed] [Google Scholar]
- 24.Tombola, F., S. Campello, L. De Luca, P. Ruggiero, G. Del Giudice, E. Papini, and M. Zoratti. 2003. Plant polyphenols inhibit VacA, a toxin secreted by the gastric pathogen Helicobacter pylori. FEBS Lett. 543:184-189. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya, H., M. Sato, Y. Kameyama, N. Takagi, and I. Namikawa. 1987. Effect of lidocaine on phospholipid and fatty acid composition of bacterial membranes. Lett. Appl. Microbiol. 4:141-144. [Google Scholar]
- 26.Vattem, D. A., Y.-T. Lin, R. Ghaedian, and K. Shetty. 2005. Cranberry synergies for dietary management of Helicobacter pylori infections. Proc. Biochem. 40:1583-1592. [Google Scholar]
- 27.Yahiro, K., D. Shirasaka, M. Tagashira, A. Wada, N. Morinaga, F. Kuroda, O. Choi, M. Inoue, N. Aoyama, M. Ikeda, T. Hirayama, J. Moss, and M. Noda. 2005. Inhibitory effects of polyphenols on gastric injury by Helicobacter pylori VacA toxin. Helicobacter 10:231-239. [DOI] [PubMed] [Google Scholar]