Abstract
Since meat from poultry colonized with Campylobacter spp. is a major cause of bacterial gastroenteritis, human exposure should be reduced by, among other things, prevention of colonization of broiler flocks. To obtain more insight into possible sources of introduction of Campylobacter into broiler flocks, it is essential to estimate the moment that the first bird in a flock is colonized. If the rate of transmission within a flock were known, such an estimate could be determined from the change in the prevalence of colonized birds in a flock over time. The aim of this study was to determine the rate of transmission of Campylobacter using field data gathered for 5 years for Australian broiler flocks. We used unique sampling data for 42 Campylobacter jejuni-colonized flocks and estimated the transmission rate, which is defined as the number of secondary infections caused by one colonized bird per day. The estimate was 2.37 ± 0.295 infections per infectious bird per day, which implies that in our study population colonized flocks consisting of 20,000 broilers would have an increase in within-flock prevalence to 95% within 4.4 to 7.2 days after colonization of the first broiler. Using Bayesian analysis, the moment of colonization of the first bird in a flock was estimated to be from 21 days of age onward in all flocks in the study. This study provides an important quantitative estimate of the rate of transmission of Campylobacter in broiler flocks, which could be helpful in future studies on the epidemiology of Campylobacter in the field.
Campylobacter spp. are a common cause of diarrhea in humans, and many cases of campylobacteriosis are associated with the handling and consumption of contaminated poultry meat (5). Studies of the epidemiology of Campylobacter have resulted in the implementation of biosecurity and hygienic measures on poultry farms and slaughterhouses with the ultimate goal of reducing human exposure (6, 16). These measures likely contributed to a reduction in the number of Campylobacter-positive broiler flocks. Nevertheless, contaminated meat is still on the market (10), and a further reduction in the prevalence of Campylobacter-positive flocks is considered necessary by public health authorities in many countries (4).
Clearly, more knowledge concerning the mechanism of introduction of Campylobacter into a flock is essential for improving the current control programs. This, in turn, requires an estimate of the moment that a flock becomes colonized. However, it does not seem feasible to detect the first bird that is colonized in a commercial broiler flock because of flock size and the necessary sampling frequency.
An alternative approach is to determine the transmission rate (β) of Campylobacter within a flock. β, which is defined as the number of secondary infections caused by one colonized bird per day, determines the rate of increase in the number of colonized birds over time. It can be used to determine the moment of introduction from field data for increasing Campylobacter prevalence over time. The estimates for β that have been obtained in experimental studies (15, 17) are 1.04 to 1.13 per day. However, experimental conditions differ substantially from the field situation, which implies that the β in commercial flocks should also be estimated.
A series of field studies in Australia were carried out between 1999 and 2004 in which broiler flocks were sampled daily to weekly. The aim of these studies was to develop an understanding of the epidemiology of Campylobacter in Australian broiler flocks. We analyzed data from the unique data set obtained in these studies to estimate the rate of Campylobacter transmission in commercial broiler flocks. Additionally, we estimated the moment that the first bird in a flock was colonized with Campylobacter (for reasons of convenience, we refer to this event as the moment of introduction of Campylobacter into a flock) and assessed how accurately moments of introduction can be estimated.
MATERIALS AND METHODS
Data set.
Three longitudinal studies were carried out in southeast Queensland, Australia, between 1999 and 2004. A subset of the full data set was selected for this study; for flocks to be included in this study, they had to have been sampled at least twice, and at least one Campylobacter-positive dropping had to be detected. Flocks with migration barriers separating groups of birds within a shed and flocks whose sampling age was not stated were excluded. A total of 42 flocks met all the inclusion criteria (see the supplemental material for details).
At each sampling age randomly selected individual fecal or cecal droppings were collected immediately after the droppings were produced. Birds that produced these droppings were not marked, as the likelihood of sampling a single bird more than once a day was considered limited for the large populations used. Care was taken to ensure that each sample was collected without any contaminating material. The samples were collected with a sterile swab, which was immediately placed in a sterile container. Samples were kept on ice during transport to the laboratory and were streaked on Karmali Campylobacter agar base (Oxoid CM935; Oxoid, Melbourne, Australia) containing Campylobacter selective supplement (Oxoid SR167E) immediately after arrival in the laboratory. Agar plates were incubated at 42°C for 48 h in an incubator with an atmosphere consisting of 85% N2, 10% CO2, and 5% O2. Colony morphology and cell motility as determined by phase-contrast microscopy were used to confirm identification to the genus level. Single colonies from a number of positive samples from each positive flock were subcultured on sheep blood agar and incubated as described above before they were identified to the species level by oxidase, catalase production, and hippurate hydrolysis tests.
The final data set consisted of data for sampling events for each flock, including the age of the flock (t), sample size (nt), flock size at the start of the rearing period (N), and number of positive samples (xt) (see the supplemental material for details).
Modeling of Campylobacter transmission in broiler flocks.
It is generally assumed that following the onset of Campylobacter colonization of the gut, broilers shed these bacteria for the rest of their lives (1). Therefore, we assumed that birds were either susceptible (noncolonized) or infectious (colonized) and that an increase in prevalence could be described by a susceptible-infectious type of mathematical model (12, 17). In this model, susceptible chicks can be colonized upon contact with an infectious chick, which occurs at rate βi(t), where i(t) is the proportion of infectious chicks in the shed. β is considered the mean number of chickens that can be colonized by one infectious chicken per day in a susceptible population.
In the large populations of broilers usually present in commercial flocks, the change in i(t) can be approximated by the deterministic differential equation:
 |
(1) |
whose the solution is the logistic curve described by
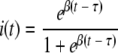 |
(2) |
in which τ is the time at which 50% of the birds are infected. From τ, β, and N, the time when transmission starts (t0) is calculated as follows:
 |
(3) |
Estimation of β in the study population.
Because the increase in i(t) follows a logistic curve, β is estimated by a logistic regression analysis of xt. However, this is possible only if for at least two samples only some of the swabs are positive, because only then is the steepness of the increase in prevalence (determined by β) observed. Eight flocks met this criterion. The susceptible-infectious model was fitted for each of these eight flocks by logistic regression of the binomially distributed xt (and nt) with t as covariate and exp(τ) as the intercept. The model fits resulted in eight estimates for β and τ, from which t0 could be estimated by using equation 3. Confidence intervals for β and t0 were derived by profile likelihood analysis (3), which was carried out using Mathematica (version 6.0; Wolfram Research, Inc. [http://www.wolfram.com]).
Because flocks with low β have been more likely to meet the inclusion criteria for the logistic regression analysis than flocks with high β, bias would be introduced if the eight estimates were considered representative of all commercial broiler flocks. Therefore, in the next step, we estimated the mean (μβ) and standard deviation (σβ) of a normal distribution of β among all flocks by maximizing the likelihood function
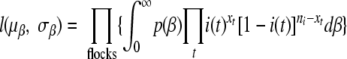 |
(4) |
where p(β) is the density of the normal distribution of β, i(t) is the logistic curve for equation 2, and t, xt, and nt are the data for all flocks. This maximum likelihood estimation method does not result in any flock-specific estimates for β or t0; estimation results are limited to μβ and σβ, with 95% confidence intervals derived by profile likelihood (3). Although β cannot be negative, the assumption of a normal distribution is valid, because the distribution is well above zero (see Results). The analysis was carried out using Mathematica.
Estimation of the t0 for the study population.
Because no flock-specific estimates of the moment of introduction (t0) could be obtained with the analysis described above, we used Markov chain Monte Carlo integration to obtain Bayesian posterior distributions of β and τ, and therefore t0, for each flock separately (7). The prior distribution of τ was uninformative (flat), whereas the prior distribution of β was the normal distribution resulting from maximum likelihood estimation (with μβ = 2.37 day−1 and σβ = 0.295 day−1). The likelihood function was
 |
(5) |
We used the maximum likelihood results for all 42 flocks as input for estimation of t0 in the same flocks, but it would have been more correct to divide the data into two mutually exclusive subsets and use each subset only once. However, the approach which we used resulted in more precise estimates of μβ and σβ and estimates of t0 for all flocks instead of only a subset. The possible errors resulting from our approach were minor, as determined by a separate sensitivity analysis of estimation of t0 to correctness of the prior distribution of β (see below).
The posterior distribution was sampled 10,000 times by single-component Metropolis-Hastings sampling, after 100 samples were used for burn-in (8). The means of the (normal) proposal distributions for the (i + 1)th samples of both β and τ were the ith samples. The standard deviation of the proposed distribution of β was 0.295 day−1; the standard deviation of the proposal for τ was determined from the data set, and it was one-quarter of the time interval between the last 0% prevalence sample and first 100% prevalence sample. This resulted in means and 95% credible intervals for t0 for 40 of the 42 flocks (for 2 flocks, the flock size was not known). The sampling algorithm was programmed using Mathematica.
Accuracy of t0 estimation.
Although Bayesian estimation of t0 does provide 95% credible intervals, these intervals are based on a logistic curve which does not take account of the stochastic nature of transmission in the early phase of a Campylobacter outbreak. Therefore, we assessed the accuracy of the method by estimating t0 using simulated outbreaks in which the estimates could be compared to the real value.
We simulated 10,000 outbreaks in flocks consisting of 20,000 chicks with values for β sampled from the estimated distribution (μβ = 2.37 day−1; σβ = 0.295 day−1), with a t0 of 0. Simulations were carried out as described previously (17) with three sample sizes (10, 20, and 100 birds) and three sampling frequencies (once every day, every third day, or every seventh day). This resulted in 10,000 simulated data sets for all combinations of sample sizes and sample frequencies, which were subsequently analyzed by the Bayesian method described above to obtain posterior means for 1,000 samples of the posterior distribution.
In addition to these 10,000 simulations, where the population distribution of β (used in the simulations) was identical to the prior distribution in the Bayesian analysis, we assessed the accuracy by simulation with other distributions, based on the confidence intervals for μβ (2.19 to 2.58 day−1) and σβ (0.144 to 0.488 day−1). Four new data sets were simulated, including two data sets with different μβ (2.19 and 2.58 day−1, with σβ kept at 0.295 day−1) and two data sets with different σβ (0.144 and 0.488 day−1, with μβ kept at 2.37 day−1). This resulted in four sets of 1,000 simulated data sets for 10 samples every 1, 3, or 7 days. These data were used to estimate t0 as described above.
RESULTS
Data set.
The flock age at the time of first detection of Campylobacter-positive droppings varied from 24 to 54 days. For 14 of 42 flocks the prevalence was more than 95% at the time of first detection. For an additional 10 flocks in which the prevalence was up to 10% at the time of first detection, sampling was continued until the flocks were found to be fully colonized (>95% prevalence), which took 4.4 days on average (range, 3 to 6 days) (see the supplemental material for details).
For 38 of 42 flocks, all Campylobacter isolates were identified as Campylobacter jejuni. Both C. jejuni and Campylobacter coli were found in four flocks. Because insufficient data concerning C. coli outbreaks were available, our analyses were based solely on C. jejuni outbreaks.
Estimation of β and t0 for the study population.
For eight flocks with sufficient data, flock-specific β and t0 were estimated by logistic regression. The point estimates for β ranged from 1.3 to 3.1 day−1 (Fig. 1), and t0 ranged from 21 to 35 days (see the supplemental material for details).
FIG. 1.
(Top) Eight estimates of Campylobacter β (with 95% confidence intervals), obtained by logistic regression. (Bottom) Estimated density of the normal distribution of β in the Australian study population, obtained by maximum likelihood estimation (μβ = 2.37 day−1; σβ = 0.295 day−1).
The estimates for the distribution of β, which were based on 42 outbreaks, were as follows: μβ = 2.37 day−1 (95% confidence interval, 2.19 to 2.58 day−1) and σβ = 0.295 (95% confidence interval, 0.144 to 0.488 day−1) (Fig. 1). Figure 2 shows how fast flocks of 20,000 broilers were colonized for the mean β, a low β, and a high β within the distribution of β.
FIG. 2.
Epidemic curves for three different values of the estimated distribution of β (lower limit, point estimate, and upper limit) and an experimentally derived β estimate (17) for flocks consisting of 20,000 broilers.
For 40 Campylobacter outbreaks in which the population size was known we estimated t0 by Bayesian analysis. The results (Fig. 3) show that colonization did not take place before chickens reached an age of 3 weeks. On average, t0 was 4.8 days (range, 2.2 to 9.3 days) earlier than the first detection of Campylobacter-positive samples (see the supplemental material for details).
FIG. 3.
Histogram showing the t0 for 40 Australian C. jejuni outbreaks.
The estimates obtained by logistic regression and Bayesian analysis did not differ more than 0.6 day except for one flock (3.4-day difference).
Accuracy of t0 estimation.
The accuracy of the Bayesian method was assessed by analyzing simulated outbreaks; the results are shown in Table 1. If the prior distribution used for the analysis reflected the underlying β in the population correctly, the t0 was estimated within 3 days even with weekly sampling of only 10 birds.
TABLE 1.
Accuracy of Bayesian estimation (with prior distribution μβ = 2.37 day−1 and σβ = 0.295 day−1) of the posterior mean time of Campylobacter introduction into a flock of 20,000 broilers, with different sample sizes and sampling intervals
| μβ (day−1) | σβ (day−1) | No. of data sets | Sample size | 90% Interval of estimation error
|
||
|---|---|---|---|---|---|---|
| Each day | Each 3rd day | Each 7th day | ||||
| 2.37 | 0.295 | 10,000 | 10 | −0.9 to 1.7 | −1.0 to 1.8 | −2.1 to 2.7 |
| 10,000 | 20 | −0.8 to 1.6 | −0.9 to 1.7 | −1.8 to 2.4 | ||
| 10,000 | 100 | −0.6 to 1.4 | −0.8 to 1.6 | −1.3 to 2.1 | ||
| 2.19 | 0.295 | 1,000 | 10 | −0.7 to 2.2 | −0.8 to 2.4 | −1.7 to 3.1 |
| 2.58 | 0.295 | 1,000 | 10 | −1.1 to 1.3 | −1.4 to 1.3 | −2.5 to 2.3 |
| 2.37 | 0.144 | 1,000 | 10 | −0.8 to 1.4 | −0.8 to 1.4 | −1.9 to 2.5 |
| 2.37 | 0.488 | 1,000 | 10 | −1.0 to 2.3 | −1.3 to 2.7 | −2.4 to 3.3 |
The Bayesian estimation method was not very sensitive to the correctness of the prior distribution (within the margins of the 95% confidence intervals), and the sensitivity was greatest if μβ was lower or σβ was higher, resulting in a margin of 3.3 days with weekly sampling of 10 birds.
DISCUSSION
The first aim of this study was to determine the β of Campylobacter in commercial broiler flocks. β, based on the field data, was estimated to be 2.37 ± 0.295 day−1, which means that one colonized bird could, on average, infect 2.37 birds per day. This implies that in a flock of 20,000 broilers the within-flock prevalence of Campylobacter would increase from a prevalence of one colonized bird to a prevalence of 95% within 1 week.
Previous studies showed that flocks determined to be Campylobacter negative at one moment could appear to be fully colonized within 1 week, suggesting that Campylobacter was introduced in the intervening week (2, 9, 11). This seems consistent with our findings, but the sample size used in these studies did not allow detection of positive flocks at the start of an epidemic. This implies that bacteria could have been introduced at some previous time.
The second aim of this study was to estimate the moment of colonization of the first bird in a flock (t0) by using the estimated β. The estimates of t0 were all greater than a flock age of 21 days. The estimate of t0 was accurate within 3 days in 90% of the cases, even if only 10 birds were sampled weekly. The sensitivity to incorrectness of the prior distribution was low. The apparent lack of Campylobacter transmission in the first 3 weeks of life, which seemed to have occurred in our study, has been described previously (11). It is difficult or probably impossible to determine whether in the first 3 weeks no introduction occurred or whether introduction of bacteria did occur but did not result in colonization of the birds. The risk of introduction of Campylobacter can be high throughout the rearing period, but it could also be hypothesized that this risk may increase over time. Another possibility is that young chicks are less susceptible, as indicated by Ringoir et al. (13) and Sahin et al. (14), who demonstrated that age and maternally derived immunity had an effect on susceptibility. More insight into the underlying mechanism of this phenomenon may provide clues for prevention of Campylobacter colonization.
Conclusions.
A unique data set describing the change in prevalence of Campylobacter-colonized birds in commercial broiler flocks was used to quantify C. jejuni transmission under field conditions. The estimated β implies that in a flock of 20,000 broilers the within-flock prevalence of Campylobacter increases to 95% within 1 week after colonization of the first bird. Since such rapid spread has not been described previously on the basis of experimental transmission experiments, this study provides important new quantitative information on the epidemiology of Campylobacter in broilers.
This study also showed how the β can be used to estimate when the first bird is colonized. We showed that this method for estimation was accurate, and therefore this method is a promising method for further studies of mechanisms of Campylobacter introduction, because it allows focusing on the chronology of events.
Additionally, t0 estimation can result in an accurate description of the period in which no transmission of Campylobacter occurs, like the first 3 weeks for our study population. Consequently, interventions aimed at prevention of introduction of and subsequent colonization by Campylobacter might better be targeted at the second half of the rearing period, which in our study population could be considered a high-risk period.
Supplementary Material
Acknowledgments
This work was funded in part by the Australian funding body RIRDC.
Footnotes
Published ahead of print on 1 December 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Achen, M., T. Y. Morishita, and E. C. Ley. 1998. Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter age. Avian Dis. 42:732-737. [PubMed] [Google Scholar]
- 2.Allen, V. M., H. Weaver, A. M. Ridley, J. A. Harris, M. Sharma, J. Emery, N. Sparks, M. Lewis, and S. Edge. 2008. Sources and spread of thermophilic Campylobacter spp. during partial depopulation of broiler chicken flocks. J. Food Prot. 71:264-270. [DOI] [PubMed] [Google Scholar]
- 3.Dohoo, I., W. Martin, and H. Stryhn. 2003. Veterinary epidemiologic research. AVC Inc., Charlottetown, Canada.
- 4.EFSA 27 January 2005, posting date. Opinion of the Scientific Panel on Biological Hazards (BIOHAZ) related to Campylobacter in animals and foodstuffs. http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1178620776955.htm.
- 5.Friedman, C. R., R. M. Hoekstra, M. Samuel, R. Marcus, J. Bender, B. Shiferaw, S. Reddy, S. D. Ahuja, D. L. Helfrick, F. Hardnett, M. Carter, B. Anderson, and R. V. Tauxe. 2004. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin. Infect. Dis. 38:S285-S296. [DOI] [PubMed] [Google Scholar]
- 6.Gellynck, X., W. Messens, D. Halet, K. Grijspeerdt, E. Hartnett, and J. Viaene. 2008. Economics of reducing Campylobacter at different levels within the Belgian poultry meat chain. J. Food Prot. 71:479-485. [DOI] [PubMed] [Google Scholar]
- 7.Gelman, A., J. B. Carlin, H. S. Stern, and D. B. Rubin. 2003. Bayesian data analysis, 2nd ed. CRC Press, Boca Raton, FL.
- 8.Gilks, W. R., S. Richardson, and D. J. Spiegelharter. 1996. Markov chain Monte Carlo in practice. CRC Press, Boca Raton, FL.
- 9.Hald, B., E. Rattenborg, and M. Madsen. 2001. Role of batch depletion of broiler houses on the occurrence of Campylobacter spp. in chicken flocks. Lett. Appl. Microbiol. 32:253-256. [DOI] [PubMed] [Google Scholar]
- 10.Hansson, I., L. P. Forshell, P. Gustafsson, S. Boqvist, J. Lindblad, E. O. Engvall, Y. Andersson, and I. Vagsholm. 2007. Summary of the Swedish Campylobacter program in broilers, 2001 through 2005. J. Food Prot. 70:2008-2014. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs-Reitsma, W. F., A. W. van de Giessen, N. M. Bolder, and R. W. Mulder. 1995. Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol. Infect. 114:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keeling, M. J., and P. Rohani. 2008. Modeling infectious diseases in humans and animals. Princeton University Press, Princeton, NJ.
- 13.Ringoir, D. D., D. Szylo, and V. Korolik. 2007. Comparison of 2-day-old and 14-day-old chicken colonization models for Campylobacter jejuni. FEMS Immunol. Med. Microbiol. 49:155-158. [DOI] [PubMed] [Google Scholar]
- 14.Sahin, O., N. Luo, S. Huang, and Q. Zhang. 2003. Effect of Campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl. Environ. Microbiol. 69:5372-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern, N. J., N. A. Cox, and M. T. Musgrove. 2001. Incidence and levels of Campylobacter in broilers after exposure to an inoculated seeder bird. J. Appl. Poult. Res. 10:315-318. [Google Scholar]
- 16.van den Giessen, A. W., J. J. H. C. Tilburg, W. S. Ritmeester, and J. van der Plas. 1998. Reduction of Campylobacter infections in broiler flocks by application of hygiene measures. Epidemiol. Infect. 121:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Gerwe, T. J. W. M., A. Bouma, W. F. Jacobs-Reitsma, J. van den Broek, D. Klinkenberg, J. A. Stegeman, and J. A. P. Heesterbeek. 2005. Quantifying transmission of Campylobacter spp. among broilers. Appl. Environ. Microbiol. 71:5765-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





