Abstract
The suitability of Pseudomonas putida GPo1 for large-scale cultivation and production of poly(3-hydroxyoctanoate) (PHO) was investigated in this study. Three fed-batch cultivations of P. putida GPo1 at the 350- or 400-liter scale in a bioreactor with a capacity of 650 liters were done in mineral salts medium containing initially 20 mM sodium octanoate as the carbon source. The feeding solution included ammonium octanoate, which was fed at a relatively low concentration to promote PHO accumulation under nitrogen-limited conditions. During cultivation, the pH was regulated by addition of NaOH, NH4OH, or octanoic acid, which was used as an additional carbon source. Partial O2 pressure (pO2) was adjusted to 20 to 40% by controlling the airflow and stirrer speed. Under the optimized conditions, P. putida GPo1 was able to grow to cell densities as high as 18, 37, and 53 g cells (dry mass) (CDM) per liter containing 49, 55, and 60% (wt/wt) of PHO, respectively. The resulting 40 kg CDM from these three cultivations was used directly for extraction of PHO. Three different methods of extraction of PHO were applied. From these, only acetone extraction showed better performance and resulted in 94% recovery of the PHO contents of cells. A novel mixture of precipitation solvents composed of 70% (vol/vol) methanol and 70% (vol/vol) ethanol was identified in this study. The ratio of PHO concentrate to the mixture was 0.2:1 (vol/vol) and allowed complete precipitation of PHO as white flakes. However, at a ratio of 1:1 (vol/vol) of the solvent mixture to PHO concentrate, a highly purified PHO was obtained. Precipitation yielded a dough-like polymeric material which was cast into thin layers and then shredded into small strips to allow evaporation of the remaining solvents. Gas chromatographic analysis revealed a purity of about 99% ± 0.2% (wt/wt) of the polymer, which consisted mainly of 3-hydroxyoctanoic acid (96 mol%).
A wide variety of gram-positive and gram-negative bacteria is able to accumulate polyhydroxyalkanoates (PHAs) as carbon and energy reserves. Pseudomonas putida GPo1 (ATCC 29347), commonly known as Pseudomonas oleovorans GPo1 (68), was first described in 1963 (3). After strain improvement for the production of epoxides (56), it was submitted to the ATCC as P. oleovorans TF4-1L and is available under the accession number ATCC 29347. Both strains are able to accumulate PHAs, like poly(3-hydroxyoctanoate) (PHO), from n-octane, and the 16S sequences of both are identical. P. putida GPo1 is able to utilize n-alkanes through their oxidation to the corresponding fatty acids due to the presence of the catabolic OCT plasmid, which encodes alkane hydrolase (12).
Accumulation of PHAs is an inherent way for bacteria to store carbon and energy when the supply of nutrients is imbalanced. PHAs are accumulated when bacterial growth is limited by deprivation of nitrogen, phosphorous (57), or oxygen and when a surplus amount of a carbon source is still present. While a limitation of nitrogen exists in many bacteria, including the pseudomonads triggering PHA biosynthesis and accumulation, for some others, like Azotobacter spp., the most effective limitation is oxygen (71).
Thermoplastic biopolymers represent an interesting group of environmentally friendly materials for various technical applications. Their application is favorable because they are biodegradable and originate from renewable resources (1). PHAs are cytoplasmatic water-insoluble storage compounds for carbon and energy, occurring exclusively in prokaryotes. PHAs have attracted much interest in the past decades because they can be molded and processed into different devices, such as compostable packaging materials, or as resorbable materials for medical applications (45). The chain lengths of the 3-hydroxy fatty acids range from 3 to 5 carbon atoms in short-chain-length PHA, with PHB [poly(3-hydroxybutyric acid)] as the best studied example, or from 6 to 14 carbon atoms in medium-chain-length PHA (PHAMCL), with PHO [poly(3-hydroxyoctanoic acid)] as a typical polymer for this group. The large variation in the compositions, in particular of PHAMCL, can be obtained from substrates, like carbohydrates (27, 65), aliphatic or aromatic substrates (15, 19, 28), and substituted substrates, in which the structure of the desired constituent is already contained (23, 24, 54). Moreover, the key enzymes of PHA biosynthesis (PHA synthases) are well known for their low substrate specificity, which is responsible for the large number of different PHA constituents found in microbial PHAs (59). As in the case of PHB synthases, different PHA synthases were cloned and expressed in various prokaryotic organisms (46, 47, 55, 58, 67); eukaryotic organisms, such as Saccharomyces cerevisiae (40); insect cells (73); and plants (21, 48, 53). Recently, the range of polymers synthesized by PHA synthases was extended to polythioesters consisting of 3-mercaptoalkanoates by employing PHA-accumulating bacteria, such as Cupriavidus necator (formerly Ralstonia eutropha), which typically accumulate PHB and other short-chain-length PHA, or engineered Escherichia coli strains and various organic thiochemicals as precursor substrates (42, 43). Polythioesters exhibit interesting physical and material properties (37, 44), and their homopolymers are not biodegradable (37).
PHO is an elastomer and exhibits rubber-like material properties, like low crystallinity. Its extension to break value is about 1,000%. The melting point temperature is only about 60°C (1, 18, 29, 69). It has been described in the literature that PHO, in particular PHO containing small fractions of unsaturated medium-chain-length hydroxyalkanoic acids, can be cross-linked by reactive chemicals or by irradiation yielding materials with interesting properties resembling natural rubber or even more vulcanized rubber (25, 38, 69). Therefore, many different applications have been described for PHO and structurally related polyesters (68). PHO is a promising candidate for many applications due to its physical properties that have been reported elsewhere (69). These applications involve pressure-sensitive adhesives, paint binders (69), biodegradable rubbers (2, 11, 16, 17), and cheese coatings (69). The previous applications rely mostly on the elasticity, hydrophobicity, low oxygen permeability, water resistance, and biodegradability of the polymer. The aim of this work was to optimize the large-scale cultivation process of P. putida GPo1 to produce large amounts of cells with high PHO contents and to establish a simple but efficient method for extraction and purification of PHO at the kilogram scale.
MATERIALS AND METHODS
Bacterial strain, media, and culture conditions.
P. putida GPo1 (ATCC 29347) was used in all cultivations (56, 68). The strain was maintained as lyophilized cell material or at 4°C on nutrient agar plates for regular cultivations. In addition to E medium (72), mineral salts medium (MSM) (14) was preferentially used for precultures for large-scale cultivation experiments. In this case, the medium did contain 20 mM sodium octanoate as a maximum, since concentrations higher than 30 mM are toxic to P. putida GPo1. Solutions of 2.8 to 20 mM NH4Cl were added according to each individual cultivation process, as were 75 mM KH2PO4, 4.1 mM MgSO4 · 7H2O (sterilized and added separately), and 1 ml of trace elements solution, which contained 750 mM EDTA (pH 6.8), 220 mM FeSO4 · 7H2O, 5.0 mM CuCl2 · 2H2O, 5.9 mM CoCl2 · 6H2O, 32 mM ZnSO4 · 7H2O, 43 mM MnCl2 · 4H2O, 3.7 mM Na2MoO4 · 2H2O, and 26 mM H3BO4.
Large-scale cultivations.
High-cell-density cultivations were done in a Biostat D 650 stainless steel reactor (B. Braun, Biotech International, Melsungen, Germany), which had a total volume of 650 liters (64-cm inner diameter and 198-cm height) and a d/D value (relation of stirrer diameter to vessel diameter) of 0.375. Probes and sensors were connected to the bioreactor to measure dissolved oxygen, temperature, pH, foam, and optical density during fermentation. Carbon dioxide and oxygen concentrations in the spent gas leaving the bioreactor were measured with a URAS 10 P NDIR spectrophotometer and a Magnos 6 G oxygen analyzer (both from Mannesmann, Hartmann and Braun, Frankfurt, Germany), respectively. Foam was controlled by means of a mechanical foam destroyer and also by addition of the antifoam agent Silikon Antischaum emulsion SLE (Wacker, Darwin Vertriebs GmbH, Ottobrunn, Germany), whenever necessary. The dissolved oxygen was maintained at 20 to 30% air saturation during the different cultivations by automatically adjusting the stirrer speed and manually increasing or decreasing the airflow rate between 100 and 600 liters per min. The pH was kept at 6.9 automatically by addition of 25% (wt/vol) NH4OH or pure octanoic acid. Temperature was maintained at 30°C. The cultivation was started by inoculation (1 or 2% [vol/vol]) with a preculture grown for 18 to 36 h at 30°C, with shaking in Erlenmeyer flasks equipped with baffles. All precultures used for large-scale cultivations contained 20 mM NH4Cl.
The aim of the feeding strategy was to maintain a surplus of octanoate as the carbon source and simultaneously maintain a limited concentration of the nitrogen source during the entire cultivation process. Therefore, two separate feeding solutions were prepared for the fed-batch cultivations, one containing 3 M ammonium octanoate (pH 7.5) and the other containing 1 M MgSO4·7H2O. Both were connected to the bioreactor, and the ways of feeding were varied for optimization from one cultivation to the other, as will be indicated later. Cells were allowed to grow for 4 or 5 h, and when the pH started to drop, either 4 M NaOH or 25% (wt/vol) NH4OH was used to adjust the pH. Feeding in the fed-batch phase of cultivation was designed as three phases. The first phase started after 6 or 7 h of inoculation by feeding 0.5 mM ammonium octanoate and 0.05 mM magnesium sulfate per h for 5 successive h. In the second phase, feeding was exponentially increased to 5 mM ammonium octanoate and 0.5 mM magnesium sulfate per hour for 5 further h. In the final phase, 1.8 mM and 0.27 mM of ammonium octanoate and magnesium sulfate, respectively, were fed per each h to the end of cultivation period. The cells were then harvested as mentioned below.
Cells harvested from 400-liter-scale cultivations.
Cells were harvested from 400-liter-scale cultivations by centrifugation in CEPA type Z41 and type Z61 continuous centrifuges (Carl Padberg Zentrifugenbau GmbH, Lahr, Germany). Both types are characterized by a slow flow rate for better separation of cells. For faster separation, a Westfalia separator CSA-8 (Westfalia Separators, Germany) was used (a separation capacity of about 1,000 liters/h). Harvested cells were frozen at −30°C and then lyophilized (Beta 1-16, Christ, Osterode, Germany).
Determination of cell dry matter.
Growth of P. putida GPo1 was followed by measuring the increase of optical density at 600 nm (OD600). Moreover, cell dry weight was determined gravimetrically from an aliquot withdrawn from the culture medium in which cells were sedimented by centrifugation for 20 min at 12,000 × g and washed twice with sterile saline solution. Then cells were freeze-dried until the weight remained constant.
Analyses of ammonium concentrations in the medium.
The concentration of ammonium was determined during the fermentation in cell-free supernatants using a gas-sensitive type 152303000 ammonium electrode (Mettler Toledo GmbH).
Small-scale extraction of PHO.
PHO was isolated from freeze-dried cells at a small scale to select the best method that would be applicable in the large-scale extraction process. Extraction with chloroform as an example of halogenated solvents was examined using 2 liters of chloroform in a Soxhlet apparatus (Schneider and Schüll, Dassel, Germany) containing 50 g cells under reflux. Detergents such as sodium dodecyl sulfate (SDS) were used at a ratio of 1:0.5 (wt/wt) of cells to SDS. The solution was stirred for 2 h at room temperature and was then centrifuged at 12,000 × g for 40 min. The resulting pellets were washed three times with double-distilled water, and the remaining solids were freeze-dried. The resulting fluffy powder was dissolved in 100 ml chloroform and filtered, the volume of chloroform was then reduced to 50 ml, and 10 volumes of ethanol was added to precipitate PHO. Cold extraction using acetone at room temperature was also applied. For this, 50 g of cells was suspended in 500 ml acetone and stirred for 24 h. The mixture was allowed to decant, and the supernatant was filtered; acetone containing PHO was then evaporated to one-tenth of the original volume. The polymer concentrate was precipitated using 20 volumes of ethanol, whereas the supernatant was decanted, and the precipitated material was dissolved in acetone, poured into petri dishes, and allowed to dry before further analysis.
Large-scale extraction of PHO.
To scale up the downstream process, the following system was established in the laboratory. The system is composed of a 20-liter bottle and a stirrer to agitate the cell suspension in acetone at a speed of up to 1,200 rpm. In these bottles, 2 kg of freeze-dried cells was suspended in about 20 liter of acetone. The suspension was stirred for 24 h at room temperature and afterwards left to decant, and the supernatant was then passed through a Sartopore 2 mini cartridge filter (Sartorius, Göttingen, Germany) to remove remaining cells. To concentrate the diluted PHO dissolved in acetone, a 20-liter distillation apparatus was assembled. Diluted PHO solution was heated in a water bath to 70°C, thereby allowing the acetone (boiling point, 56°C) to evaporate. The acetone was recovered and was used again in subsequent extraction processes.
Extraction of PHO at larger scales was carried out in a stainless steel vessel with a total volume of 128 liter in which 10 kg of cells was agitated in 100 liters acetone for 24 h. Cells were allowed to decant, and the supernatant was filtered as mentioned before. After filtration, the filtrate was distilled and the concentrated PHO solution was used for precipitation.
Large-scale precipitation of PHO.
Absolute ethanol, technical ethanol (96%, vol/vol), absolute methanol, technical methanol (97%, vol/vol), and water were tested alone or in a mixture to precipitate PHO from the solution in acetone and to obtain highly purified polymer. The ratios of PHO concentrate to precipitation solvent were usually between 1:10 and 1:50 (vol/vol) (39, 63). In another case, pretreatment of cells prior to PHAMCL extraction using 20 volumes of cold methanol was reported (30). However, in this study, novel procedures were applied using 70% (vol/vol) methanol and ethanol as precipitants at a ratio of 1:1, and we obtained highly purified PHO. The PHO was collected after precipitation and washing; it exhibited a dough-like consistency and could be easily brought into any form. The purity of the dried polymer was determined by gas chromatography in comparison to analytical grade PHO on a weight basis.
Determination of PHO contents and composition.
The PHO contents of cells were determined by methanolysis of 10 to 15 mg of lyophilized cells in the presence of 15% (vol/vol) sulfuric acid (5). The resulting methyl esters of the constituent hydroxyalkanoic acids were analyzed by gas chromatography (6, 66). For identification of the methylesters, gas chromatography-tandem mass spectrometry (GC-MS-MS) was also performed using an HP 6890 gas chromatograph equipped with a model 5973 mass selective detector (Hewlett Packard, Waldbronn, Germany). Isolated polymer samples were analyzed correspondingly.
RESULTS
Fed-batch cultivation of P. putida GPo1.
Cultivation of P. putida GPo1 at the 400-liter scale was done in MSM initially containing 20 mM sodium octanoate and 2.8 mM NH4Cl. Medium was inoculated with 1% (vol/vol) seed culture grown for 48 h in 250-ml Erlenmeyer flasks at 30°C. Partial O2 pressure (pO2) was regulated to 30 to 40% saturation by varying the airflow and stirrer speed. The fed-batch cultivation mode was started 6 h after inoculation by applying the feeding scheme mentioned in Materials and Methods, but in the form of pulses per hour. The pH was manually controlled by adding octanoic acid (6.2 M); however, the pH value was allowed to rise above 7, as shown in Table 1. During cultivation, the airflow was raised to 600 liters/min when the pO2 dropped drastically. As shown in Table 1, the maximum cell density at the end of the cultivation period was 17.8 g cells (dry mass) (CDM) per liter, which corresponded to an OD600 value of 95. The growth rate during the exponential phase of growth was 0.05 h−1, and the productivities of biomass and PHO over the fermentation period were 0.38 and 0.18 g liter−1 h−1, respectively. At the end of this cultivation, 6.5 kg of CDM was recovered by centrifugation, and only about 0.7 kg cells was lost during the separation process. The PHO content of cells in this cultivation was 48.9% (wt/wt).
TABLE 1.
Growth of P. putida GPo1 during cultivation at the 400-liter scalea
| Cultivation period (h) | OD600 | CDM (g/liter) | PHO (%, wt/wt) | pH |
|---|---|---|---|---|
| 1 | 0.01 ± 1 × 10−4 | 0.02 ± 0.001 | ND | 6.86 |
| 15 | 4.32 ± 0.02 | 1.4 ± 0.2 | 10 ± 0.5 | 6.92 |
| 33 | 54.2 ± 2.2 | 11.2 ± 0.8 | 30 ± 1.3 | 7.32 |
| 48 | 95.4 ± 4.1 | 17.8 ± 0.3 | 48 ± 2.6 | 7.42 |
Analyses were done in triplicate; the averages and standard deviations of values are presented. ND, not determined.
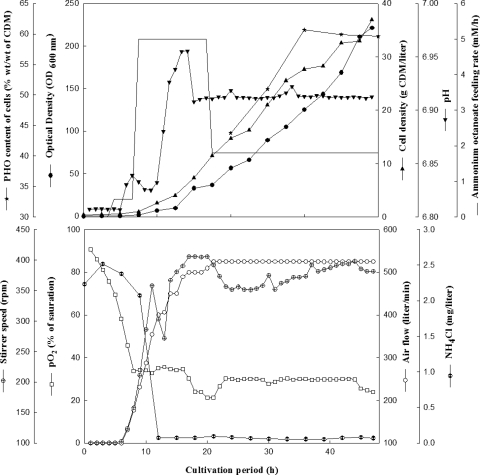
To optimize the cultivation process, a second cultivation at the 400-liter scale was accomplished. Composition of the cultivation medium was the same as that in the previous cultivation, except that a higher initial concentration of NH4Cl (18.7 mM) was used. In addition, the inoculum size was slightly increased to 1.3% (vol/vol). The feeding scheme was changed to the continuous mode to avoid the need for excessive aeration and stirring; as a result, the pO2 remained between 25 and 30% (vol/vol) of saturation. Cells grew to an OD600 of 215 ± 2.7, corresponding to 37 ± 0.23 g CDM/liter, as shown in Fig. 1. The pH of the medium was controlled using 4 M NaOH or 6.2 M octanoic acid. The latter was consumed as the carbon source for growth, whereas growth-limiting amounts of ammonium were provided as ammonium octanoate (Fig. 1). Cells grew at a μ of 0.15 h−1 during the batch phase. Biomass and PHO productivities were calculated to be 0.95 and 0.62 g liter−1 h−1, respectively, during the fed-batch phase of cultivation, and about 15.5 kg of CDM was finally obtained at the end of the cultivation process. The amount of PHO present in the cells was estimated to be 43% ± 0.2% (wt/wt) after 24 h of cultivation and 55.4% ± 0.6% (wt/wt) at cell harvest (48 h) (Fig. 1). For this, the cells had consumed about 18.3 kg of octanoic acid during the cultivation.
FIG. 1.
Fed-batch cultivation of P. putida GPo1 in a Biostat D-650 stirred tank reactor. The bioreactor contained 400 liters of MSM with sodium octanoate as the carbon source, and the pH was controlled by adding 6.2 M octanoic acid or 4 M NaOH. The medium was inoculated with 1.3% (vol/vol) of a preculture grown in the same medium. Aeration and agitation were controlled automatically by adjusting the pO2 to 30% (vol/vol) saturation. Cells were grown for 48 h at 30°C. Cultivation parameters, the concentration of residual ammonium in the medium during cultivation, CDM, and PHO contents were determined as described in Materials and Methods. At the end of cultivation, cells were harvested in a Westfalia separator.
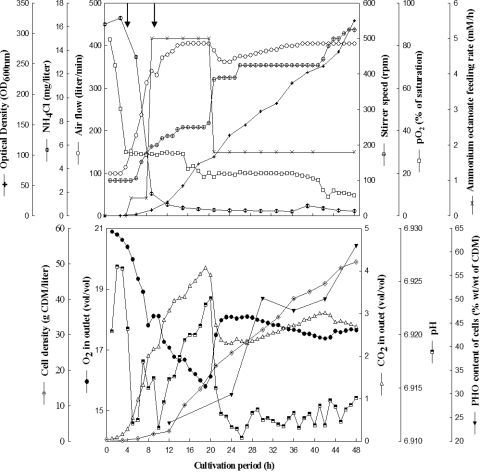
To further increase cell densities and PHO contents, the cultivation was repeated at the 350-liter scale with minor modifications in controlling the pH. The volume of the preculture (24 h) was further increased to 2% (vol/vol), and the initial concentration of NH4Cl was increased to 20 mM. The batch phase of this cultivation continued for only 4 h with a μ of 0.2 h−1, while the first fed-batch phase (7 h) (Fig. 2) was started by feeding ammonium octanoate (0.5 mM/h) and magnesium sulfate (0.05 mM/h). Unlike in the two previous cultivations, the pH was controlled by adding 25% (wt/vol) NH4OH instead of 4 M NaOH (Fig. 2, top panel). This strategy resulted in an OD600 value as high as 321, and the productivities of biomass and PHO reached 1.2 and 0.76 g liter−1 h−1, respectively. At the end of the cultivation period, about 22.5 kg of octanoic acid was consumed and 18.7 kg CDM was harvested. The amount of cells recovered from the 350-liter medium indicated a cell density of at least 53 g CDM per liter. The final PHO content of the cells was 60% ± 2% (wt/wt) of CDM, as revealed by gas chromatography (Fig. 2).
FIG. 2.
Fed-batch cultivation of P. putida GPo1 in a Biostat D-650 stirred tank reactor. The bioreactor contained 350 liters of medium with sodium octanoate and was fed with ammonium octanoate and magnesium sulfate as described in Materials and Methods. The pH was controlled by adding 6.2 M octanoic acid or 25% (wt/vol) NH4OH. Medium was inoculated with 2% (vol/vol) of a preculture grown in the same medium. Aeration and agitation were controlled automatically by adjusting the pO2 to 20 to 25% (vol/vol) saturation. Cells were grown for 48 h at 30°C. The cultivation parameters, concentration of residual ammonium during cultivation, CDM, PHO contents of cells, feeding rate, and O2 and CO2 contents in the outlet gas were determined as described in Materials and Methods. Arrows (top panel) indicate the addition of NH4OH to control pH from the 3rd to the 10th h of cultivation. At the end of cultivation, cells were harvested in a Westfalia separator.
The specific yield coefficients (YPHO/S) (in grams per gram), which indicate the conversion of octanoic acid into PHO, were calculated as 0.39 ± 0.007 and 0.41 ± 0.004 g/g for the second and third cultivations, respectively. These are relatively high values.
Cells examined by phase-contrast microscopy during the three fed-batch fermentations showed large refractile PHO granules, while in the presence of the hydrophobic dye Nile red, fluorescent PHO granules were observed under the fluorescence microscope. Cells were filled with granules, but in specific locations some protuberances were observed during three-dimensional examination of cells. Such protuberances indicate the protrusion and extension of the cell caused by the intracellular accumulation of PHO granules (data not shown).
Small- and large-scale extraction of PHO.
To reveal the best method suitable for simple and efficient large-scale extraction of PHO from cells, small-scale extraction experiments were done first. Chloroform extraction of PHO with reflux for 15 h and subsequent precipitation with ethanol yielded only about 61% ± 1.5% of the total PHO content of the cells. On the other hand, using SDS for lysis of cell walls and omitting any solvent in a laborious procedure yielded only 41.5% ± 2.5% of the PHO content. The third method, applying acetone at room temperature, retrieved 93.5% ± 0.8% of the PHO content. This clearly provided a good alternative to the use of detergents and halogenated solvents, such as chloroform, which are known for their negative environmental impacts.
Large-scale recovery of PHO.
Several precipitation solvents were applied at a small scale to determine the purity of the obtained PHO. As shown in Table 2, the most highly purified PHO was obtained if a mixture of 70% (vol/vol) methanol and 70% (vol/vol) ethanol was used. When each solvent was used alone or when only water was used, a much lower purity was obtained, as indicated by the appearance of peaks for different fatty acids in the GC chromatogram (data not shown).
TABLE 2.
Purity of PHO according to the solvent used for precipitation
| Solvent used for precipitation | % Purity (wt/wt)a |
|---|---|
| Absolute methanol | 91 ± 0.4 |
| Methanol (90%, vol/vol) | 86 ± 1.1 |
| Methanol (70%, vol/vol) | 96 ± 0.7 |
| Absolute ethanol | 98 ± 0.6 |
| Ethanol (70%, vol/vol) | 97 ± 0.5 |
| Ethanol-methanol (70%, vol/vol) mix | 99 ± 0.2 |
| Water | 91 ± 0.7 |
| Standardb | 84 ± 1.5 |
The purity of the obtained PHO samples was determined by GC analysis of dried polymer material. Analyses were done in triplicate, and mean values and standard deviations are shown.
Standard PHO available at our laboratory was prepared by at least three repeated cycles of dissolution in chloroform and precipitation with ethanol; afterwards the polymer was dried to a constant weight and analyzed by GC.
To extract PHO from cells, it was previously recommended that 10 to 20 volumes of solvents such as acetone or chloroform be used. In this study the ratio of dried cells to acetone was 1:10 (wt/vol). After extraction of PHO from 25 kg of freeze-dried cells of P. putida GPo1 employing 250 liters of acetone, the acetone solution obtained was first concentrated to 75 liters. Acetone was recovered by distillation and was used several times again to minimize the extraction costs. Likewise, for precipitation of PHO from the solution in acetone, in other studies 10 volumes of ethanol or methanol was used. Besides the problems from handling such large amounts of solvents, enormous costs occurred. In our case, large amounts of ethanol, methanol, or a mixture of the two would be required to precipitate the PHO, even from the concentrate. This is practically not convenient for applications in industry. Therefore, the newly applied mixture consisting of a 1:1 (vol/vol) mixture of 70% methanol and 70% ethanol was applied. This aspect is one of the most interesting findings of this study, because 75 liters of this mixture was sufficient to precipitate PHO from the entire concentrate obtained from 25 kg CDM very efficiently and with high yield. White flocs of PHO, whose size increased rapidly when the ratio of mixture to PHO came close to 0.2:1 (vol/vol), occurred. However, a 1:1 (vol/vol) ratio of precipitation solvent to PHO concentrate was used to wash the polymer and to remove all lipids from the precipitated material. Removal of the liquid over the precipitated PHO was the most critical phase of the entire downstream process, since prolonged contact of the liquid with the precipitated PHO resulted in increasing amounts of impurities in the polymer. Adding the methanol-plus-ethanol mixture at an excess increased the purity of the polymer only if it was vigorously agitated.
The dough-like material obtained by the procedure described above was cast into thin layers of 2- or 3-cm thickness and was allowed to dry. It was then shredded with a knife into small strips to obtain maximum dryness (Fig. 3). The application of this method led to the extraction of about 94% ± 0.8% (wt/wt) of the PHO previously present in the cells. This is significantly higher than the yield of all other small-scale methods applied during this study.
FIG. 3.
Final form of extracted PHO as plates (thickness of about 2 or 3 cm) or as long and thin strips shredded to allow maximal evaporation of the used solvents.
GC and GC-MS-MS analysis of the isolated polymer samples revealed that 3-hydroxyoctanoate constituted up to 96% (wt/wt) of the polymer, whereas the other, only minor constituents were 3-hydroxyhexanoate (2.8%, wt/wt) and 3-hydroxydecanoate (0.9%, wt/wt). Other constituents could not be identified in the isolated polymer by GC-MS-MS (data not shown).
DISCUSSION
This study aimed at cultivating the well-known PHAs accumulating the bacterium P. putida GPo1 at a large scale while developing a simple and efficient process for PHO recovery. High-cell-density cultivation is a crucial factor for efficient production of intracellular bacterial polymers, such as PHO. P. putida GPo1 and other pseudomonads are frequently used for the production of PHAs at small- and pilot-scale cultivations (13, 60, 61). The environmental conditions that influence the expression driven by the PcI promoter region in P. putida GPo1 (upstream of phaC1 and phaC2, which encode PHA synthase) include, besides nitrogen limitation, the nature of the carbon source present in the medium. This strain is an exception among the pseudomonads in that it is able to accumulate PHA from alkanes and alkanoates but not from glucose or other carbohydrates (45, 50). When citric acid or glucose is used as the carbon source, PcI is less active than in the presence of octanoic acid. Therefore, octanoic acid and ammonium octanoate were selected as carbon and nitrogen sources, respectively. The feeding solutions that contained magnesium sulfate and ammonium octanoate were fed continuously and at a low concentration to maintain nitrogen limitation, which promotes PHO accumulation and inhibits its degradation.
The maximum cell density ever obtained with P. putida KT2442 was 141 g CDM/liter containing 51% (wt/wt) of PHA; however, this was achieved when oleic acid was used as the carbon source (41). When octanoic acid was used as the carbon source, cells could be grown only to densities of about 55 g CDM/liter, with a maximum PHA content of 75% (wt/wt) (35); it has to be mentioned that these experiments were done at the 2.5-liter scale only (36). Other carbon sources can also be used for PHA production but appear less efficient (45). The third large-scale cultivation presented in this study resulted in 53 g/liter of CDM and a PHO content of 60% ± 2% (wt/wt) of CDM, which represent, at the 350-liter scale, increases of 13% and 9% in biomass production and PHO contents, respectively, compared to those previously reported (14) using a similar strategy but only at the 6-liter cultivation scale.
To obtain a polymer with high 3-hydroxyoctanoate content, the use of octanoic acid as the carbon source is preferred (22, 26, 31, 35, 49). Using fatty acids with a greater carbon chain length resulted in PHAs consisting of a larger variety of constituents. For example, the use of oleic acid yielded PHAs consisting mainly of constituents of C6, C8, C10, C12, C12:1, C14, and C14:1 carbon chain lengths (27, 33, 75), and the use of nonanoic acid preferentially yielded constituents with C9 and C7 carbon chain lengths (61), due to the ability of PHA synthase to add a wide range of monomer fatty acid derivatives to the growing PHA chain.
Oxygen transfer is one of the most important factors for PHA production. With a low temperature (18°C) during cultivation, a cell density of 35 g/liter CDM was obtained, and the cells contained 33% (wt/wt) PHA (49). On the other hand, when cells of P. putida Gpo1 were grown with a better oxygen transfer and in optimized medium, a cell density of 112 g CDM /liter was obtained; however, the cells contained only 25% PHA (32, 34). One has also to consider that all previous cultivations were conducted at a small scale, not exceeding 30 liters (33). Automated continuous feeding in combination with pH control, as shown for the second and third cultivations of this study, demonstrated the importance of this strategy in PHO production, where foam formation resulted from extensive stirring and aeration when the pulse type of feeding was applied. Much less foam was formed when the continuous feeding strategy was applied. The production of about 40 kg of CDM of P. putida GPo1 over three cultivations in the present study provides, according to our knowledge, the first published large-scale cultivation using octanoic acid as the carbon source.
Separation processes can easily account for up to 30% of the overall process costs in PHA production (62). Different separation processes have been described before, starting from filtration (10), froth flotation (70), and continuous centrifugation (20). Continuous separation, also with continuous cell release from the separator, as used in this study, provided a fast and efficient method for separation of cultivation broth up to 1,000 liter/h, as it is possible with a CSA-8 Westfalia separator. In contrast, continuous centrifuges have a limited rotor capacity and require time-consuming operations. Recovery procedures for PHO principally resemble those developed for PHB, which usually involve the application of halogenated solvents, such as chloroform (39, 52). PHB recovery using hypochlorite (4, 14, 51), SDS in an in situ extraction process (64), or an enzyme cocktail (9, 34) were also reported. The disadvantages related to applications of hypochlorite and detergents are the severe reduction in polymer molecular weight and the requirement of extended washing steps to get rid of detergent residuals, respectively. Furthermore, the use of acetone or hexane extraction of PHAs in combination with subsequent precipitation of the extracted polymer using nonsolvents of PHAs, such as methanol, has been reported (74). Acetone is inexpensive, relatively safe, and even available from renewable sources as a by-product of microbial fermentations (7). Such PHA solvents are also considered far less harmful to the environment than halogenated solvents. Quite different ratios of the solvent containing the PHA to the solvent used for the precipitation of PHA have been reported, with values ranging from 2.5:1 (52) to 10:1 (6, 39) or even to 50:1 (63).
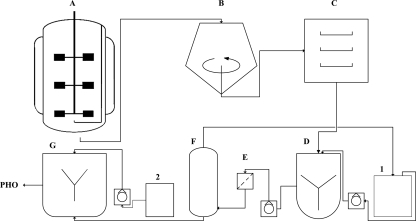
In an industrial-scale extraction of PHB (8), 200 to 500 kg of freeze-dried cells were extracted with 5,000 liters chloroform and precipitated with 15,000 liters of hexane or heptane, which indicates the application of 3 volumes of PHA nonsolvent in comparison to the PHA solvent, whereas in this study only 1 volume of the nonsolvent was successfully used, yielding a polymer with a high degree of purity. Considering that the PHA solution, in which PHA from 25 kg cells was extracted, was concentrated to about 75 liters prior to precipitation, only about 750 to 1,500 liters of the nonsolvent, instead of 15,000 liters, would have to be used in the example cited above. The precipitation of PHO as “dough” did still allow a fast and efficient evaporation of the remaining solvents. Our method (Fig. 4) for production, extraction, and precipitation of PHO is therefore simple and efficient, yields highly purified PHO, and is also economically much more feasible than previously reported methods.
FIG. 4.
Schematic diagram comprising the entire process for PHO production. (A) Fermentative production of PHO by P. putida GPo1; (B) separation of bacterial cells from the spent medium; (C) freeze-drying; (D) extraction of polymer by acetone (1); (E) filtration process; (F) distillation to concentrate the polymer solution and to recycle acetone for extraction of the polymer (1); (G) precipitation of the polymer by the methanol-plus-ethanol mixture (2). Polymer was then dried and prepared as shown in Fig. 3.
Acknowledgments
Financial support by Honda R & D Europe (Offenbach, Germany) is gratefully acknowledged.
We are indebted to Herbert Ahlers and Ahmed Sallam for technical assistance.
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Anderson, A. J., and E. A. Dawes. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54:450-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashby, R. D., A. M. Cromwick, and T. A. Foglia. 1998. Radiation cross linking of a bacterial medium-chain-length poly(hydroxyalkanoate) elastomer from tallow. Int. J. Biol. Macromol. 23:61-72. [DOI] [PubMed] [Google Scholar]
- 3.Baptist, J. N., R. K. Gholson, and M. J. Coon. 1963. Hydrocarbon oxidation by a bacterial enzyme system: I. Products of octane oxidation. Biochim. Biophys. Acta 69:40-47. [DOI] [PubMed] [Google Scholar]
- 4.Berger, E., B. A. Ramsay, J. A. Ramsay, C. Chaverie, and G. Braunegg. 1989. PHB recovery by hypochlorite digestion of non-PHB biomass. Biotechnol. Tech. 3:227-232. [Google Scholar]
- 5.Brandl, H., E. J. Knee, and R. C. Fuller. 1989. Ability of the phototrophic bacterium Rhodospirillum rubrum to produce various poly(β-hydroxyalkanoates): potential sources for biodegradable polyesters. Int. J. Biol. Macromol. 11:49-55. [DOI] [PubMed] [Google Scholar]
- 6.Brandl, H., R. A. Gross, R. W. Lenz, and R. C. Fuller. 1988. Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 54:1977-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, S. W., X. Ma, L. S. Wang, and X. H. Zhao. 1998. Acetone-butanol fermentation of rice straw enzymatic hydrolysate. Ind. Microbiol. 28:30-34. [Google Scholar]
- 8.Chen, G. Q., G. Zhang, S. J. Park, and S. Y. Lee. 2001. Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl. Microbiol. Biotechnol. 57:50-55. [DOI] [PubMed] [Google Scholar]
- 9.de Koning, G. J. M., and B. Witholt. 1997. A process for the recovery of poly(hydroxyalkanoates) from Pseudomonads. Part 1: solubilization. Bioprocess Biosyst. Eng. 17:7-13. [Google Scholar]
- 10.de Koning, G. J. M., M. Kellerhals, C. van Meurs, and B. Witholt. 1997. A process for the recovery of poly(hydroxyalkanoates) from Pseudomonads. Part 2: process development and economic evaluation. Bioprocess Biosyst. Eng. 17:15-21. [Google Scholar]
- 11.de Koning, G. J. M., H. M. M. van Bilsen, P. J. Lemstra, W. Hazenberg, B. Witholt, H. Preusting, J. G. van der Galien, A. Schirmer, and D. Jendrossek. 1994. A biodegradable rubber by crosslinking poly(hydroxyalkanoate) from Pseudomonas oleovorans. Polymer 35:2090-2097. [Google Scholar]
- 12.de Smet, M. J., G. Eggink, B. Witholt, B. Kingma, and H. Wynberg. 1983. Characterization of intracellular inclusions formed by Pseudomonas oleovorans during growth on octane. J. Bacteriol. 154:870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diard, S., J. P. Carlier, E. Ageron, P. A. Grimont, V. Langlois, P. Guerin, and O. M. Bouvet. 2002. Accumulation of poly(3-hydroxybutyrate) from octanoate in different pseudomonads belonging to the rRNA homology group I. Syst. Appl. Microbiol. 25:183-188. [DOI] [PubMed] [Google Scholar]
- 14.Dufresne, A., and E. Samain. 1998. Preparation and characterization of a poly(β-hydroxyalkanoate) latex produced by Pseudomonas oleovorans. Macromolecules 31:6426-6433. [Google Scholar]
- 15.Eggink, G., P. de Waard, and G. N. M. Huijberts. 1992. The role of fatty acid biosynthesis and degradation in the supply of substrates for poly(3-hydroxyalkanoate) formation in Pseudomonas putida. FEMS Microbiol. Rev. 103:159-164. [Google Scholar]
- 16.Gagnon, K. D., R. W. Lenz, R. J. Farris, and R. C. Fuller. 1994. Chemical modification of bacterial elastomers. 1. Peroxide crosslinking. Polymer 35:4358-4367. [Google Scholar]
- 17.Gagnon, K. D., R. W. Lenz, R. J. Farris, and R. C. Fuller. 1994. Chemical modification of bacterial elastomers. 2. Sulfur vulcanization. Polymer 35:4368-4375. [Google Scholar]
- 18.Gagnon, K. D., R. W. Lenz, R. J. Farris, and R. C. Fuller. 1992. Crystallization behaviour and its influence on the mechanical properties of a thermoplastic elastomer produced by Pseudomonas oleovorans. Macromolecules 25:3723-3728. [Google Scholar]
- 19.García, B. R., E. Olivera, B. Miñambres, M. F. Valverde, L. M. Cañedo, M. A. Prieto, J. L. García, M. Martínez, and J. M. Luengo. 1999. Novel biodegradable aromatic plastics from a bacterial source. J. Biol. Chem. 274:29228-29241. [DOI] [PubMed] [Google Scholar]
- 20.Gorenflo, V., G. Schmack, R. Vogel, and A. Steinbüchel. 2001. Development of a process fort the biotechnological large-scale production of 4-hydroxyvalerate-containing polyesters and characterization of their physical and mechanical properties. Biomacromolecules 2:45-57. [DOI] [PubMed] [Google Scholar]
- 21.Hahn, J. J., A. C. Eschenlauer, M. H. Narrol, D. A. Somers, and F. Srienc. 1997. Growth kinetics, nutrient uptake, and expression of the Alcaligenes eutrophus poly(β-hydroxybutyrate) synthesis pathway in transgenic maize cell suspension cultures. Biotechnol. Prog. 13:347-354. [DOI] [PubMed] [Google Scholar]
- 22.Hazenberg, W., and B. Witholt. 1997. Efficient production of medium-chain-length poly-(3-hydroxyalkanoates) from octane by Pseudomonas oleovorans: economic considerations. Appl. Microbiol. Biotechnol. 48:588-596. [Google Scholar]
- 23.Hazer, B., R. W. Lenz, and R. C. Fuller. 1994. Biosynthesis of methyl branched poly(β-hydroxyalkanoate)s by Pseudomonas oleovorans. Macromolecules 27:45-49. [Google Scholar]
- 24.Hazer, B., R. W. Lenz, and R. C. Fuller. 1996. Bacterial production of poly-3-hydroxyalkanoates containing arylalkyl substituent groups. Polymer 37:5951-5957. [Google Scholar]
- 25.Hazer, B., S. I. Demirel, M. Borcakli, M. S. Eroglu, M. Cakmak, and B. Erman. 2001. Free radical crosslinking of unsaturated bacterial polyesters obtained from soybean oily acids. Polym. Bull. 46:389-394. [Google Scholar]
- 26.Huijberts, G. N. M., and G. Eggink. 1996. Production of poly(3-hydroxyalkanoates) by Pseudomonas putida KT2442 in continuous cultures. Appl. Microbiol. Biotechnol. 46:233-239. [Google Scholar]
- 27.Huijberts, G. N. M., G. Eggink, P. de Waard, G. W. Huisman, and B. Witholt. 1992. Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl. Environ. Microbiol. 58:536-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huisman, G. W., O. de Leeuw, G. Eggink, and B. Witholt. 1989. Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl. Environ. Microbiol. 55:1949-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyun, D. S., J. N. Lee, and Y. H. Lee. 2002. In vivo blending of medium chain length polyhydroxyalkanoates and polyhydroxybutyrate using recombinant Pseudomonas putida harboring phbCAB operon of Ralstonia eutropha. Biotechnol. Lett. 24:1729-1735. [Google Scholar]
- 30.Jiang, X., J. A. Ramsay, and B. A. Ramsay. 2006. Acetone extraction of mcl-PHA from Pseudomonas putida KT2440. J. Microbiol. Methods 67:212-219. [DOI] [PubMed] [Google Scholar]
- 31.Jung, K., W. Hazenberg, M. Prieto, and B. Witholt. 2001. Two-stage continuous process development for the production of medium-chain-length poly(3-hydroxyalkanoates). Biotechnol. Bioeng. 72:19-24. [DOI] [PubMed] [Google Scholar]
- 32.Kellerhals, M. B., B. Kessler, and B. Witholt. 1999. Closed-loop control of bacterial high-cell-density fed-batch cultures: production of mcl-PHAs by Pseudomonas putida KT2442 under single-substrate and cofeeding conditions. Biotechnol. Bioeng. 56:306-315. [PubMed] [Google Scholar]
- 33.Kellerhals, M. B., B. Kessler, B. Witholt, A. Tchouboukov, and H. Brandl. 2000. Renewable long-chain fatty acids for production of biodegradable medium-chain-length polyhydroxyalkanoates (mcl-PHAs) at laboratory and pilot plant scale. Macromolecules 33:4690-4698. [Google Scholar]
- 34.Kellerhals, M. B., W. Hazenberg, and B. Witholt. 1999. High cell density fermentations of Pseudomonas oleovorans for the production of mcl-PHAs in two liquid phase media. Enzyme Microb. Technol. 24:111-116. [Google Scholar]
- 35.Kim, B. S. 2002. Production of medium chain length polyhydroxyalkanoates by fed-batch culture of Pseudomonas oleovorans. Biotechnol. Lett. 24:125-130. [Google Scholar]
- 36.Kim, D. Y., Y. B. Kim, and Y. H. Rhee. 2000. Evaluation of various carbon substrates for the biosynthesis of polyhydroxyalkanoates bearing functional groups by Pseudomonas putida. Int. J. Biol. Macromol. 28:23-29. [DOI] [PubMed] [Google Scholar]
- 37.Kim, D. Y., K. Elbanna, N. Thakor, T. Lütke-Eversloh, and A. Steinbüchel. 2005. Poly(3-mercaptopropionate): a non-biodegradable biopolymer? Biomacromolecules 6:897-901. [DOI] [PubMed] [Google Scholar]
- 38.Kim, Y. B., R. W. Lenz, and R. C. Fuller. 1995. Poly-3-hydroxyalkanoates containing unsaturated repeating units produced by Pseudomonas oleovorans. J. Polym. Sci. A 33:1367-1374. [Google Scholar]
- 39.Lageveen, R. G., G. W. Huisman, H. Preusting, P. Ketelaar, G. Eggink, and B. Witholt. 1988. Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly(R)-3-hydroxyalkanoates and poly(R)-3-hydroxyalkenoates. Appl. Environ. Microbiol. 54:2924-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leaf, T. A., M. S. Peterson, S. K. Stoup, D. Somers, and F. Srienc. 1996. Saccharomyces cerevisiae expressing bacterial polyhydroxybutyrate synthase produces poly-3-hydroxybutyrate. Microbiology 142:1169-1180. [DOI] [PubMed] [Google Scholar]
- 41.Lee, S. Y., H. H. Wong, J. Choi, S. H. Lee, S. C. Lee, and C. S. Han. 2000. Production of medium-chain-length polyhydroxyalkanoates by high-cell-density cultivation of Pseudomonas putida under phosphorus limitation. Biotechnol. Bioeng. 68:466-470. [PubMed] [Google Scholar]
- 42.Lütke-Eversloh, T., A. Fischer, U. Remminghorst, J. Kawada, R. H. Marchessault, A. Bögershausen, M. Kalwei, H. Eckert, R. Reichelt, S. J. Liu, and A. Steinbüchel. 2002. Biosynthesis of novel thermoplastic polythioesters by engineered Escherichia coli. Nat. Mater. 1:236-240. [DOI] [PubMed] [Google Scholar]
- 43.Lütke-Eversloh, T., K. Bergander, H. Luftmann, and A. Steinbüchel. 2001. Identification of a new class of biopolymer: bacterial synthesis of a sulfur-containing polymer with thioester linkages. Microbiology 147:11-19. [DOI] [PubMed] [Google Scholar]
- 44.Lütke-Eversloh, T., and A. Steinbüchel. 2004. Microbial polythioesters. Macromol. Biosci. 4:165-174. [DOI] [PubMed] [Google Scholar]
- 45.Madison, L. L., and G. W. Huisman. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park, J. S., H. C. Park, T. L. Huh, and Y. H. Lee. 1995. Production of poly-β-hydroxybutyrate by Alcaligenes eutrophus transformants harbouring cloned phbCAB genes. Biotechnol. Lett. 17:735-740. [Google Scholar]
- 47.Peoples, O. P., and A. J. Sinskey. 1989. Poly-β-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC). J. Biol. Chem. 264:15298-15303. [PubMed] [Google Scholar]
- 48.Poirier, Y., C. Somerville, L. A. Schechtman, M. M. Satkowski, and I. Noda. 1995. Synthesis of high-molecular-weight poly(-(2)-3-hydroxybutyrate) in transgenic Arabidopsis thaliana plant cells. Int. J. Biol. Macromol. 17:7-12. [DOI] [PubMed] [Google Scholar]
- 49.Preusting, H., R. van Houten, A. Hoefs, E. K. van Langenberghe, O. Favre-Bulle, and B. Witholt. 1993. High-cell-density cultivation of Pseudomonas oleovorans: growth and production of poly(3-hydroxyalkanoates) in 2-liquid phase batch and fed-batch systems. Biotechnol. Bioeng. 41:550-556. [DOI] [PubMed] [Google Scholar]
- 50.Prieto, M. A., B. Bühler, K. Jung, B. Witholt, and B. Kessler. 1999. PhaF, a polyhydroxyalkanoate-granule-associated protein of Pseudomonas oleovorans GPo1 involved in the regulatory expression system for pha genes. J. Bacteriol. 181:858-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramsay, J. A., E. Berger, A. Ramsay, and C. Chaverie. 1990. Recovery of poly-3-hydroxyalkanoic acid granules by surfactant-hypochlorite treatment. Biotechnol. Tech. 4:221-226. [Google Scholar]
- 52.Ramsay, J. A., E. Berger, R. Voyer, C. Chavarie, and B. A. Ramsay. 1994. Extraction of poly-3-hydroxybutyrate using chlorinated solvents. Biotechnol. Tech. 8:589-594. [Google Scholar]
- 53.Romano, A., L. H. van der Plas, B. Witholt, G. Eggink, and H. Mooibroek. 2005. Expression of poly-3-(R)-hydroxyalkanoate (PHA) polymerase and acyl-CoA-transacylase in plastids of transgenic potato leads to the synthesis of a hydrophobic polymer, presumably medium-chain-length PHAs. Planta 220:455-464. [DOI] [PubMed] [Google Scholar]
- 54.Scholz, C., R. C. Fuller, and R. W. Lenz. 1994. Growth and polymer incorporation of Pseudomonas oleovorans on alkyl esters of heptanoic acid. Macromolecules 27:2886-2889. [Google Scholar]
- 55.Schubert, P., A. Steinbüchel, and H. G. Schlegel. 1988. Cloning of the Alcaligenes eutrophus genes for the synthesis of poly-β-hydroxybutyrate (PHB) and synthesis of PHB in Escherichia coli. J. Bacteriol. 170:5837-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz, R. D., and C. J. McCoy. 1976. Enzymatic epoxidation: synthesis of 7,8-epoxy-1-octene, 1,2-7,8-diepoxyoctane, and 1,2-epoxyoctane by Pseudomonas oleovorans. Appl. Environ. Microbiol. 31:78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shang, L., M. Jiang, and H. N. Chang. 2003. Poly(3-hydroxybutyrate) synthesis in fed-batch culture of Ralstonia eutropha with phosphate limitation under different glucose concentrations. Biotechnol. Lett. 25:1415-1419. [DOI] [PubMed] [Google Scholar]
- 58.Slater, S. C., W. H. Voige, and D. E. Dennis. 1988. Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-β-hydroxybutyrate biosynthetic pathway. J. Bacteriol. 170:4431-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steinbüchel, A., and H. E. Valentin. 1995. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 128:219-228. [Google Scholar]
- 60.Sun, Z., J. A. Ramsay, M. Guay, and B. A. Ramsay. 2006. Automated feeding strategies for high-cell-density fed-batch cultivation of Pseudomonas putida KT2440. Appl. Microbiol. Biotechnol. 71:423-431. [DOI] [PubMed] [Google Scholar]
- 61.Sun, Z., J. A. Ramsay, M. Guay, and B. A. Ramsay. 2007. Carbon-limited fed-batch production of medium-chain-length polyhydroxyalkanoates from nonanoic acid by Pseudomonas putida KT2440. Appl. Microbiol. Biotechnol. 74:69-77. [DOI] [PubMed] [Google Scholar]
- 62.Sun, Z., J. A. Ramsay, M. Guay, and B. A. Ramsay. 2007. Fermentation process development for the production of medium-chain-length poly-3-hydroxyalkanoates. Appl. Microbiol. Biotechnol. 75:475-485. [DOI] [PubMed] [Google Scholar]
- 63.Taniguchi, I., K. Kagotani, and Y. Kimura. 2003. Microbial production of poly(hydroxyalkanoate)s from waste edible oils. Green Chem. 5:545-548. [Google Scholar]
- 64.Thakor, N., T. Lütke-Eversloh, and A. Steinbüchel. 2005. Application of the BPEC pathway for large-scale biotechnological production of poly(3-mercaptopropionate) by recombinant Escherichia coli, including a novel in situ isolation method. Appl. Environ. Microbiol. 71:835-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Timm, A., and A. Steinbüchel. 1990. Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl. Environ. Microbiol. 56:3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Timm, A., D. Byrom, and A. Steinbüchel. 1990. Formation of blends of various poly(3-hydroxyalkanoic acids) by a recombinant strain of Pseudomonas oleovorans. Appl. Microbiol. Biotechnol. 33:296-301. [Google Scholar]
- 67.Tombolini, R., S. Povolo, A. Buson, A. Squartini, and M. P. Nuti. 1995. Poly-β-hydroxybutyrate (PHB) biosynthetic genes in Rhizobium meliloti 41. Microbiology 141:2553-2559. [DOI] [PubMed] [Google Scholar]
- 68.van Beilen, J. B., S. Panke, S. Lucchini, A. G. Franchini, M. Rothlisberger, and B. Witholt. 2001. Analysis of Pseudomonas putida alkane-degradation gene clusters and flanking insertion sequences: evolution and regulation of the alk genes. Microbiology 147:1621-1630. [DOI] [PubMed] [Google Scholar]
- 69.van der Walle, G. A. M., G. J. M. de Koning, R. A. Weusthuis, and G. Eggink. 2001. Properties, modifications and applications of biopolyesters, p. 263-292. In A. Steinbüchel and W. Babel (ed.), Advances in biochemical engineering/biotechnology: biopolymers, vol. 71. Springer-Verlag, New York, NY. [DOI] [PubMed] [Google Scholar]
- 70.van Hee, P., A. C. M. R. Elumbaring, R. G. J. M. van der Lans, and L. A. M. van der Wielen. 2006. Selective recovery of polydydroxyalkanoates inclusion bodies from fermentation broth by dissolved air flotation. J. Colloid Interface Sci. 297:595-606. [DOI] [PubMed] [Google Scholar]
- 71.Verlinden, R. A. J., D. J. Hill, M. A. Kenward, C. D. Williams, and I. Radecka. 2007. Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 102:1437-1449. [DOI] [PubMed] [Google Scholar]
- 72.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97-106. [PubMed] [Google Scholar]
- 73.Williams, M. D., A. M. Fieno, R. A. Grant, and D. H. Sherman. 1996. Expression and analysis of a bacterial poly(hydroxyalkanoate) synthase in insect cells using baculovirus system. Protein Expr. Purif. 7:203-211. [DOI] [PubMed] [Google Scholar]
- 74.Williams, S. F., D. P. Martin, D. M. Horowitz, and O. P. Peoples. 1999. PHA applications: addressing the price performance issue: I. Tissue engineering. Int. J. Biol. Macromol. 25:111-121. [DOI] [PubMed] [Google Scholar]
- 75.Wuesthuis, R. A., G. N. M. Huijberts, and G. Eggink. 1997. Production of mcl-poly(hydroxyalkanoates) (review). In G. Eggink, A. Steinbüchel, Y. Poirer, and B. Witholt (ed.), 1996 International Symposium on Bacterial Polyhydroxyalkanoates. NRC Research Press, Ottawa, Canada.