Abstract
We investigated the microbial community in a pilot plant for treatment of acid mine water by biological ferrous iron oxidation using clone library analysis and calculated statistical parameters for further characterization. The microbial community in the plant was conspicuously dominated by a group of Betaproteobacteria affiliated with “Ferribacter polymyxa”.
The operation of opencast pits requires hoisting huge amounts of low-pH groundwater and of iron and sulfate. Due to these contaminants the pumped groundwater must be treated before it is drained into water courses. Pretreatment of mine water by biological oxidation at low pH prior to the conventional chemical treatment involving neutralization with lime can decrease the iron load in the chemical treatment step considerably. By using biological oxidation of ferrous iron at a pH of about 3, iron hydroxysulfates, primarily schwertmannite {Fe16[O16(OH)9-12(SO4)3.5-2]}, can be precipitated, removed from the treatment system, and used for industrial applications (5). Since the rate of oxidation of ferrous iron only at pH values greater than 5 increases 100-fold per pH unit and the process is very slow at pH values below 4 (1, 15), iron-oxidizing bacteria, which can increase the oxidation rate up to 5 orders of magnitude (1), must be involved in the pretreatment technology. For this biological oxidation step to be performed on a large scale, it is helpful to have some insight into the microbial community responsible for the process. Although iron-oxidizing communities in extremely acidic habitats have been analyzed in various studies, including studies of the Rio Tinto (pH 2) (12), Iron Mountain (pH 0.5 to 1) (2), and a stirred tank for bioleaching (pH 1.3 to 1.6) (14), the treatment system investigated in this study is a different habitat that has not been analyzed previously with respect to flow, higher pH, and forced aeration.
In brief (detailed methods are described in the supplemental material), the microbial communities in the pilot plant and in the groundwater pumped into the plant were investigated by using 16S rRNA gene clone library analysis. Altogether, we analyzed six clone libraries from the pilot plant and one clone library from the groundwater, each comprising 144 to 150 clones. Further characterization of the microbial communities was performed by statistical calculation of diversity and similarity indices based on amplified rRNA gene restriction analysis data, and the habitat was characterized by determining chemical parameters (Table 1).
TABLE 1.
Chemical parameters for the inflowing groundwater and the oxidation basin of the planta
| Location | pH | Eh (mV) | Temp (°C) | Fe2+ concn (mg/liter) | Fe3+ concn (mg/liter) | SO42− concn (mg/liter) | Total inorganic carbon concn (mg/liter) | Total organic carbon concn (mg/liter) | NH4 concn (mg/liter) | Cl− concn (mg/liter) |
|---|---|---|---|---|---|---|---|---|---|---|
| Inflow | 4.9 | 270 | 17 | 630 | 3 | 2,700 | 98 | 50 | 0.6 | 21 |
| Oxidation basin | 3 | 480 | 17 | 100 | 300 | 2,400 | NDb | ND | ND | ND |
The oxygen concentration 20 cm below the water surface decreased from approximately 8 mg/liter around the inflow area to about 5 mg/liter in the middle of the oxidation basin.
ND, not determined.
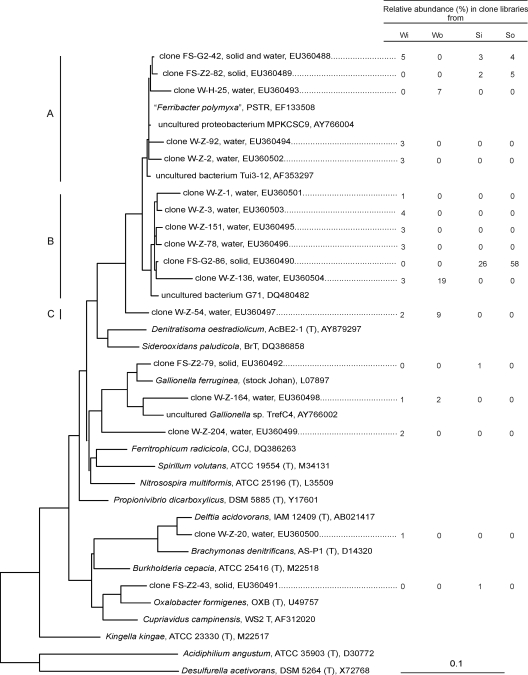
According to previous studies, amplification with archaeal primers does not yield a PCR product (2, 7, 9). Analysis of the bacterial 16S rRNA gene revealed that representatives of the Betaproteobacteria conspicuously dominated the microbial diversity in the solid samples from the oxidation basin, as well as the microbial diversity in both water samples, and were also present in other samples from the plant at significant frequencies (Table 2). The majority of the sequences were affiliated with “Ferribacter polymyxa,” which was recently isolated from an abandoned copper mine (accession number EF133508) (unpublished data). The species designated “F. polymyxa” in sequence databases has not been formally described, and there is considerable evolutionary distance between this species and cultivated relatives, such as the iron-oxidizing organisms Ferritrophicum radicicola and Siderooxidans paludicola, which were recently isolated from different wetland plants at pH 4 and circumneutral pH (16), and the ammonium-oxidizing organism Nitrosospira multiformis (Fig. 1). The average high rate of oxidation of ferrous iron in the plant, which was 35 g m−3 h−1, along with the autotrophic growth of an isolate closely related to “F. polymyxa” on ferrous iron, corroborated the assumption that iron was oxidized by the Betaproteobacteria detected (7). The conspicuous dominance of these species in microbial communities was recently also reported in studies of other mine waters with pHs ranging from 2.4 to 3 (7, 9). Other Betaproteobacteria in the plant were related to Gallionella ferruginea, a neutrophilic autotrophic iron oxidizer. Relatives of G. ferruginea have often been detected in microbial mine water communities, and in some acid mine drainage investigation areas these bacteria seemed to be a dominant group (3, 4, 7). Moreover, sequences related to Delftia acidovorans and Oxalobacter formigenes were detected at very low relative abundance in all clone libraries. A further significant phylogenetic group was the Alphaproteobacteria, which clearly dominated the solid sample from the inflow area (see Fig. S2 in the supplemental material) and was also frequently found in the other clone libraries. The majority of the Alphaproteobacteria sequences were related to Acidocella species, and several other clones showed 16S rRNA gene similarity to Acidiphilium, Sphingomonas, Caulobacter, and Caedibacter species. The function of the heterotrophic Alphaproteobacteria in the iron-oxidizing community has not been completely clarified, but the presence of Acidocella and Acidiphilium species in acidic mine waters is quite common and has been reported in various studies (8, 11, 13). Gammaproteobacteria, including the well-known iron oxidizer Acidithiobacillus ferrooxidans, as well as other representatives, such as Acinetobacter, Stenotrophomonas, and Legionella, constituted another major sequence group. Sequences related to A. ferrooxidans were detected in all of the clone libraries obtained from the plant except the library from the water sample from the inflow area. For other clones that could be assigned to this phylogenetic group there were no close cultivated relatives. Representatives of the Deltaproteobacteria formed a considerable group only in clone libraries from water samples. Except for one clone, which was related to the sulfur-reducing Desulfuromonas species, all these clones were distantly related to the sulfate-reducing bacterium Desulfobacca acetooxidans. The Actinobacteria were represented in the microbial community by species related to “Ferrimicrobium acidiphilum,” a heterotrophic iron oxidizer which has been observed in several mine waters (4, 6), by relatives of Rhodococcus, and by other uncultured species. Sequences affiliated with the Firmicutes were detected mainly in water samples (see Fig. S3 in the supplemental material). Besides a few sequences related to Bacillus subtilis, the majority of the clones were related to isolate SLC66, which has been described as a gram-positive iron-oxidizing acidophile (10). Within the Nitrospira class, relatives of Leptospirillum ferrooxidans, a well-studied iron oxidizer which has often been detected in extremely acidic environments (2, 6), were detected only in two clone libraries from the inflow area. It is remarkable that the Leptospirillum-related sequences were detected close to the point where there was continuous inflow of higher-pH groundwater and not in other areas of the plant. Representatives of Acidobacteria detected in clone libraries from the water samples were distantly related to the heterotrophic organism Acidobacterium capsulatum.
TABLE 2.
Distribution of phylogenetic groups in seven clone libraries
| Putative phylum or class | % of clones in clone librariesa
|
||||||
|---|---|---|---|---|---|---|---|
| Solid samples
|
Water samples
|
Groundwater sample (n = 145) | |||||
| Inflow
|
Oxidation basin
|
Inflow (n = 150) | Oxidation basin (n = 150) | ||||
| Preparation step 1 (n = 160)b | Preparation step 2 (n = 144) | Preparation step 1 (n = 147) | Preparation step 2 (n = 150) | ||||
| Betaproteobacteria | 34 | 29 | 60 | 76 | 31 | 37 | 1 |
| Alphaproteobacteria | 51 | 54 | 32 | 5 | 17 | 12 | 1 |
| Gammaproteobacteria | 14 | 14 | 6 | 5 | 4 | 8 | 16 |
| Deltaproteobacteria | 0 | 1 | 0 | 0 | 13 | 13 | 11 |
| Actinobacteria | 0.5 | 1 | 1 | 10 | 7 | 7 | 23 |
| Nitrospira | 0.5 | 0 | 0 | 0 | 3 | 0 | 0 |
| Verrucomicrobia | 0 | 0 | 1 | 2 | 3 | 2 | 6 |
| Chloroflexi | 0 | 0 | 0 | 1 | 1 | 0 | 6 |
| Firmicutes | 0 | 0 | 0 | 1 | 14 | 13 | 3 |
| Acidobacteria | 0 | 0 | 0 | 0 | 7 | 8 | 6 |
| Chlorobi | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| Candidate division OP11 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Gemmatimonadetes | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Planctomycetes | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Unclassified | 0 | 1 | 0 | 0 | 0 | 0 | 9 |
Assignment of clones resulted from digestion with RsaI (and in some cases also with TaqI) and sequencing of at least one representative for each digestion pattern.
n is the number of clones analyzed.
FIG. 1.
Phylogenetic tree based on 16S rRNA gene sequences of Betaproteobacteria from the pilot plant. The relative abundances of the operational taxonomic units were based on the frequencies of the characteristic restriction patterns of the sequences belonging to one operational taxonomic unit divided by the total number of clones in the samples as shown in Table 2. 16S rRNA gene sequences of Acidiphilium angustum and Desulfurella acetivorans were used as outgroups. Lines A, B, and C indicate single clusters in the class Betaproteobacteria. Wi, water sample from the inflow area; Wo, water sample from the oxidation basin; Si, solid sample from the inflow area; So, solid sample from the oxidation basin. The relative abundances from the first and second DNA preparation steps (see the supplemental material) were not divided.
The clones obtained from the groundwater sample were partially sequenced, which yielded 600 to 900 bases. The majority of the clones did not exhibit high levels of similarity to cultivated species, and Alphaproteobacteria and Betaproteobacteria, which dominated the microbial community in the pilot plant, were detected at only very low frequencies. However, individual phylotypes discovered in the groundwater that were relatives of “F. acidiphilum” and Legionella, as well as of uncultured Actinobacteria, were similar to phylotypes in the clone libraries from the pilot plant. Characterization of the microbial community by statistical evaluation revealed that the Shannon indices of the clone libraries decreased from the inflowing groundwater to the oxidation basin, whereas the Shannon indices of the clone libraries from the water samples were higher than the corresponding indices of the clone libraries from the solid samples (see Table S1 in the supplemental material). Calculation of similarity indices revealed that despite the different characteristics of the groundwater and the water in the inflow area, the clone libraries from these sampling points exhibited significantly higher levels of similarity than the clone libraries from solid and water samples from the same sampling point in the pilot plant, which were very similar habitats based on the pH (see Table S2 in the supplemental material). The high level of similarity between the groundwater sample and the water sample from the inflow showed the impact of the groundwater bacteria on the composition of the unattached population in the pilot plant. This impact was also shown by the similar frequencies of the Deltaproteobacteria in the clone libraries from the groundwater and from the water samples from the pilot plant (11 to 13%), whereas several sequences were affiliated with sulfate-reducing species. The aerobic, acidic conditions in the plant presumably did not provide a favorable environment for these bacteria, which were expected to just pass through the oxidation basin. However, since the frequencies of the phylogenetic groups that dominated the pilot plant were very low in the groundwater sample, the low similarity indices for solid and water samples from one sampling point did not result only from the impact of inflowing groundwater bacteria on the unattached population. In fact, the low level of similarity resulted from the conspicuously higher relative number of Alphaproteobacteria in the clone libraries from the solid samples (Table 2). We concluded that the microbial community in the pilot plant, which was conspicuously dominated by relatives of “F. polymyxa,” could be divided into an attached population and an unattached population.
Further investigations of the pilot plant may show how different operating conditions, particularly the relative flow rate and retention time, as well as the pH, affect the microbial diversity in the plant and may provide evidence of the stability of this microbial community.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences have been deposited in the GenBank database under accession numbers EU360471 to EU360508.
Supplementary Material
Acknowledgments
We are grateful to the BMBF for funding this study (project 01RI05014), to the Max Buchner Research Foundation for sponsorship (grant 2721), and to Vattenfall Europe Mining & Generation for support of the project.
We thank Ulrike Bretschneider and Beate Erler for providing technical assistance in the lab, Daniel Terno, Mario Kohl, Günter Rätsel, and Klaus-Dieter Herbach for providing technical service at the pilot plant, and Melissa Wos for reviewing the manuscript.
Footnotes
Published ahead of print on 1 December 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Appelo, C. A. J., and D. Postma. 1993. Geochemistry, groundwater and pollution. A.A. Balkema, Rotterdam, The Netherlands.
- 2.Bond, P. L., S. P. Smriga, and J. F. Banfield. 2000. Phylogeny of microorganisms populating a thick, subaerial, predominantly lithotrophic biofilm at an extreme acid mine drainage site. Appl. Environ. Microbiol. 66:3842-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruneel, O., R. Duran, C. Casiot, F. Elbaz-Poulichet, and J. C. Personne. 2006. Diversity of microorganisms in Fe-As-rich acid mine drainage waters of Carnoules, France. Appl. Environ. Microbiol. 72:551-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coupland, K., and D. B. Johnson. 2004. Geochemistry and microbiology of an impounded subterranean acidic water body at Mynydd Parys, Anglesey, Wales. Geobiology 2:77-86. [Google Scholar]
- 5.Glombitza, F., E. Janneck, I. Arnold, W. Rolland, and W. Uhlmann. 2007. Eisenhydroxysulfate aus der Bergbauwasserbehandlung als Rohstoff, p. 31-40. In M. GDMB Gesellschaft für Bergbau, Rohstoff- und Umwelttechnik e.V. (ed.), Consulting-Erfahrungen und Kontakte für Neuanfänge-Untertägiger Bergbau auf Industrieminerale in Deutschland. vol. 110. GDMB Medienverlag, Clausthal-Zellerfeld, Germany. [Google Scholar]
- 6.Gonzalez-Toril, E., E. Llobet-Brossa, E. O. Casamayor, R. Amann, and R. Amils. 2003. Microbial ecology of an extreme acidic environment, the Tinto River. Appl. Environ. Microbiol. 69:4853-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallberg, K. B., K. Coupland, S. Kimura, and D. B. Johnson. 2006. Macroscopic streamer growths in acidic, metal-rich mine waters in North Wales consist of novel and remarkably simple bacterial communities. Appl. Environ. Microbiol. 72:2022-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamamura, N., S. H. Olson, D. M. Ward, and W. P. Inskeep. 2005. Diversity and functional analysis of bacterial communities associated with natural hydrocarbon seeps in acidic soils at Rainbow Springs, Yellowstone National Park. Appl. Environ. Microbiol. 71:5943-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He, Z., S. Xiao, X. Xie, H. Zhong, Y. Hu, Q. Li, F. Gao, G. Li, J. Liu, and G. Qiu. 2007. Molecular diversity of microbial community in acid mine drainages of Yunfu sulfide mine. Extremophiles 11:305-314. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, D. B., P. Bacelar-Nicolau, N. Okibe, A. Yahya, and K. B. Hallberg. 2001. Role of pure and mixed cultures of gram-positive eubacteria in mineral leaching, p. 461-470. In V. T. Ciminelli and O. J. Garcia (ed.), Biohydrometallurgy: fundamentals, technology and sustainable development, vol. 11A. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 11.Johnson, D. B., S. Rolfe, K. B. Hallberg, and E. Iversen. 2001. Isolation and phylogenetic characterization of acidophilic microorganisms indigenous to acidic drainage waters at an abandoned Norwegian copper mine. Environ. Microbiol. 3:630-637. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Archilla, A. I., E. Gerard, D. Moreira, and P. Lopez-Garcia. 2004. Macrofilamentous microbial communities in the metal-rich and acidic River Tinto, Spain. FEMS Microbiol. Lett. 235:221-228. [DOI] [PubMed] [Google Scholar]
- 13.Okabayashi, A., S. Wakai, T. Kanao, T. Sugio, and K. Kamimura. 2005. Diversity of 16S ribosomal DNA-defined bacterial population in acid rock drainage from Japanese pyrite mine. J. Biosci. Bioeng. 100:644-652. [DOI] [PubMed] [Google Scholar]
- 14.Okibe, N., M. Gericke, K. B. Hallberg, and B. D. Johnson. 2003. Enumeration and characterization of acidophilic microorganism isolated from a pilot plant stirred-tank bioleaching operation. Appl. Environ. Microbiol. 69:1936-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stumm, W., and J. J. Morgan. 1996. Aquatic chemistry, chemical equilibria and rates in natural waters, 3rd ed. John Wiley & Sons, New York, NY.
- 16.Weiss, J. V., J. A. Rentz, T. Plaia, S. C. Neubauer, M. Merrill-Floyd, T. Lilburn, C. Bradburne, J. P. Megonigal, and D. Emerson. 2007. Characterization of neutrophilic Fe(II)-oxidizing bacteria isolated from rhizosphere of wetland plants and description of Ferritrophicim radicicola gen. nov. sp. nov., and Sideroxydans paludicola sp. nov. Geomicrobiol. J. 24:559-570. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.