Abstract
The novel signal peptide SLPmod was used for the secretion of murine interleukin-12 (mIL-12) by Lactococcus lactis. A >4-fold increase in secretion was observed when SLPmod was used instead of the Usp45-derived secretion signal. Oral delivery of this cytokine using the autoinducible host L. lactis FI5876 utilizing SLPmod resulted in a significant increase in mIL-12 plasma levels in mice.
Many heterologous proteins and peptides have been successfully expressed in Lactococcus lactis for different biotechnological applications. Despite its lack of invasiveness, L. lactis is able to deliver heterologous antigens and cytokines to the systemic and mucosal immune system (11). Cytokine mucosal delivery is dependent upon its release into the mucosa when L. lactis cells are in contact with the mucosa-associated lymphoid tissue. The secretion of recombinant cytokines has generally involved fusion of the relevant protein to the signal peptide of the abundant Usp45 protein (1, 13, 14, 15); however, poor secretion efficiency of the large murine cytokine interleukin-12 (mIL-12) was observed (1, 17). Here, we report the development of an improved secretion system for the delivery of large cytokines, specifically mIL-12. We have engineered the self-inducing, nisin-producing L. lactis strain FI5876 to express mIL-12 and have compared this with an exogenously induced system using strain UKLc10.
The SLPmod signal peptide has been developed on the basis of an alignment of lactobacillus S-layer proteins (unpublished data). After retrieving the respective S-layer protein sequences from the UniProt database (http://www.uniprot.org/) and the removal of incomplete sequences, the first 70 amino acids of the remaining proteins were aligned using ClustalW (10). The sequences clustered into three groups (data not shown), the largest of which contained sequences from Lactobacillus acidophilus, Lactobacillus crispatus, and Lactobacillus helveticus. Because a clear majority of available sequences cluster into one group and because there was a strong similarity to the sequence of a secreted lactococcal protein (llmg_0851), the respective consensus sequence was chosen as the basis for the design of SLPmod.
Construction of nisin promoter (PnisA)-controlled mIL-12 expression vectors.
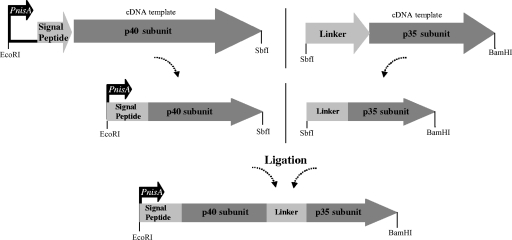
The regions encoding the mIL-12 p40 and p35 subunits were amplified by PCR employing mouse spleen cDNA as a template (AMS Biotechnology, Abingdon, United Kingdom). Primer pairs 5′-ATGTGGGAGCTGGAGAAAGAC-3′ and 5′-CCTGGATCCGACCCTGCAG-3′ for the p40 fragment and 5′-AGGTGGAGGAGGATCTAGGGTCATTCCAGTCTC-3′ and 5′-CTGGATCCTTTCAGGCGGAGC-3′ for the p35 fragment were used to create fragments of 938 bp and 578 bp, respectively. Subsequently, spliced overlap extension PCR was used to create two fragments, representing SignalPeptide_p40 under the control of the nisin A promoter or Linker_p35. These two subfragments were cut using restriction enzymes and cloned as PnisA_SignalPeptide_p40_Linker_p35 into the shuttle vector pTG262 (12) (Fig. 1), resulting in the fragments coding for the two protein subunits being connected by an artificial linker coding for (Gly4-Ser)3 (8). The signal peptides included were either SLPmod (pFI2596) or Usp45 (pFI2602) signal peptides. Subsequently, L. lactis strain FI5876 or UKLc10 was transformed with plasmid pFI2596 or pFI2602 in order to generate the recombinant strains listed in Table 1. Bacterial growth conditions were as described previously (5, 7). Strains were grown to an optical density at 600 nm of ∼1.0 before supernatants and cell pellets were collected. UKLc10-derived strains were induced with 10 ng ml−1 of nisin at an optical density at 600 nm of ∼0.6 (optimized conditions).
FIG. 1.
Cloning strategy used for the introduction of the fragment PnisA_SignalPeptide_p40_Linker_p35 into the vector pTG262 (not to scale).
TABLE 1.
Strains used in this study
| Strain no. | Host | Presence of:
|
Plasmid | Signal peptide | mIL-12 concn (pg/ml)c | Reference or source | |
|---|---|---|---|---|---|---|---|
| nisa | nisRKb | ||||||
| FI5876 | + | 0 | 3 | ||||
| UKLc10 | + | 0 | 16 | ||||
| FI10632 | FI5876 | + | pTG262 | 0 | This study | ||
| FI10724 | UKLc10 | + | pTG262 | 0 | This study | ||
| FI10611 | FI5876 | + | pFI2596 | SLPmod | 185 | This study | |
| FI10608 | FI5876 | + | pFI2602 | Usp45 | 40 | This study | |
| FI10610 | FI5876 | + | pFI2595 | None | This study | ||
| FI10615 | UKLc10 | + | pFI2596 | SLPmod | 150 | This study | |
| FI10626 | UKLc10 | + | pFI2602 | Usp45 | 35 | This study | |
| FI10614 | UKLc10 | + | pFI2595 | None | This study | ||
nis, entire nisin gene cluster.
nisRK, genes nisRK integrated into the fivefold-peptidase-deficient mutant IM16, a derivative of L. lactis MG1363.
The concentration of mIL-12 (pg ml−1) in culture supernatants of lactococcal strains, as determined by sandwich enzyme-linked immunosorbent assay. The values represent the mean results from quadruplicate samples (standard deviation, ≤8%).
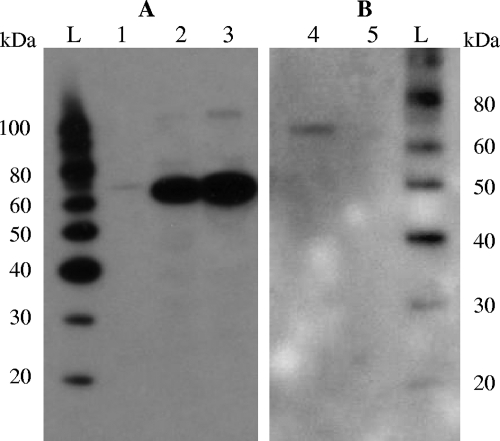
Western blot analysis was carried out on both the intracellular content and culture supernatant (Fig. 2), as previously described (5). Culture supernatants from FI5876 and UKLc10 derivatives carrying the plasmid pTG262, pFI2596, or pFI2602 revealed that a clear band of the expected 70 kDa was detected in the strains with mIL-12-encoding plasmids (Fig. 2B; data shown for pFI2596). Cytokine concentration was measured by enzyme-linked immunosorbent assay. L. lactis FI10611 utilizing the SLPmod signal peptide for secretion produced the highest level of secreted mIL-12 (185 pg ml−1). In contrast, a much lower level (40 pg ml−1) was obtained with strain FI10608 that utilized the Usp45 signal peptide. The levels of mIL-12 secretion were comparable in both FI5876 and UKLc10 mIL-12-producing derivatives (Table 1).
FIG. 2.
Western blot detection of mIL-12 in the intracellular content (A) and culture supernatants (B). Lane 1, mIL-12; lane 2, L. lactis FI10614; lane 3, L. lactis FI10610; lane 4, L. lactis FI10611; lane 5, L. lactis FI10632; lanes L, MagicMark Western protein standard.
Interestingly, the buffering of media with 2% NaHCO2 led to a further threefold increase in mIL-12 production. Furthermore, an additional plasmid (pFI2595) encoding mature mIL-12 (no signal peptide) was also included. The resulting Western blot of the intracellular contents of FI10610 and FI10614 revealed a strong single band in each (Fig. 2A), indicating the presence of an intact cytokine, unlike the result published by Bermúdez-Humarán et al. (1), which showed a smeared range of bands, suggesting that degradation had occurred.
Using the bioactivity assay described previously (1), the addition of culture supernatants of an L. lactis strain secreting mIL-12 to mouse splenocytes resulted in the induction of IFN-γ production. These induction levels were comparable to those obtained using standard mIL-12, indicating that bacterial produced cytokine was biologically active.
Animal study and plasma levels of mIL-12.
Five groups of C3H/HeJ mice were intragastrically treated by gavage with 50 μl of 2% NaHCO2 containing 109 CFU, as previously described (6), of freshly prepared L. lactis cells of either the parental strains UKLc10 and FI5876 carrying the control vector (FI10724 and FI10632), their mIL-12 engineered derivatives (FI10615 and FI10611), or a buffer solution (2% NaHCO2). Plasma samples were collected 6 h after gavage treatment, and mIL-12 levels were measured. Wilcoxon rank tests were used for statistical analysis of the data. A significant (P < 0.05) increase in mIL-12 levels was obtained only when the mIL-12-secreting FI5876 strain was used (FI10611, an autoinduced strain). Independent studies were carried out, and a mean value of 40 ± 11 pg ml−1 was obtained when animals had been treated by gavage with FI10611. In contrast, a mean value of 30 ± 10 pg ml−1 was found when mice had been treated by gavage either with the mIL-12-secreting UKLc10 strain (FI10615, requiring exogenous induction) or the controls.
In this study, the use of a more effective signal peptide has enhanced the secretion of biologically active mIL-12 by L. lactis. In comparison to the secretion achieved when the signal peptide of Usp45 was used, the presence of the alternative SLPmod signal peptide lead to a >4-fold increase in mIL-12 secretion, which in turn resulted in an eightfold increase in IFN-γ production in the biological activity assays using mouse splenocytes (data not shown). The new SLPmod signal peptide will be useful for the heterologous production of proteins not only in L. lactis but potentially also in other gram-positive bacteria, where an enhancement of protein secretion is required.
Here, two L. lactis strains, FI5876 and UKLc10, were engineered to secrete mIL-12 by using a nisin-controlled gene expression system (3, 4, 9). The strong inducible nisin promoter requires the presence of nisin in the media in order to be activated. We compared the abilities of the mIL-12-secreting derivative strains of FI5876 (autoinducible) and UKLc10 (inducible) to influence the plasma levels of mIL-12 after their oral administration in mice. Similar mIL-12 levels of secretion were detected in the culture supernatants of the respective FI5876 and UKLc10 derivative strains. However, as predicted, elevated mIL-12 plasma levels in mice were obtained only when the nisin-producing strain FI10611 was employed because nisin is likely to be present when the metabolically active strain is in contact with the intestinal mucosa. This local supply of nisin (2) is able to autoinduce PnisA, leading to the subsequent secretion of mIL-12 and its delivery to the mucosal tissue. In contrast, L. lactis UKLc10 derivatives are non-nisin-producing strains that depend upon the exogenous addition of nisin in order to produce mIL-12. We conclude that a nisin-producing strain is the ideal host for oral delivery.
Intragastric administration of a recombinant noncolonizing L. lactis strain that secretes biologically active mIL-12 could be of great benefit in reducing toxic side effects associated with systemic delivery. In addition, mucosal delivery of mIL-12 has been shown to act as an adjuvant in vaccine delivery, which might be enhanced by the application of the FI10611 strain developed here.
Acknowledgments
This work was partially supported by an Intra-European Marie Curie Fellowship.
We are grateful to Jeffrey Temblay (IFR) and Simon Deakin (DMU, University East of Anglia, United Kingdom) for advice and technical assistance. We also thank Carmen Pin (IFR) for the statistical analysis performed. We thank Bernhard Henrich (University of Kaiserslautern, Germany) for providing plasmid pUK200 and the lactococcal strain UKLc10.
Footnotes
Published ahead of print on 5 December 2008.
REFERENCES
- 1.Bermúdez-Humarán, L. G., P. Langella, N. Cortez-Perez, A. Gruss, R. S. Tamez-Guerra, S. C. Oliveira, O. Saucedo-Cardenas, R. Montes de Oca-Luna, and Y. Le Loir. 2003. Intranasal administration of recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect. Immun. 71:1887-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernbom, N., T. R. Licht, C. H. Brogen, B. Jelle, A. H. Johansen, I. Badiola, F. V. Vogensen, and B. Norrung. 2006. Effects of Lactococcus lactis on composition of intestinal microbiota: role of nisin. Appl. Environ. Microbiol. 72:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dodd, H. M., N. Horn, Z. Hao, and M. J. Gasson. 1992. A lactococcal expression system for engineered nisins. Appl. Environ. Microbiol. 58:3683-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodd, H. M., N. Horn, W. C. Chan, C. J. Giffard, B. W. Bycroft, G. C. Roberts, and M. J. Gasson. 1996. Molecular analysis of the regulation of nisin immunity. Appl. Environ. Microbiol. 142:2385-2392. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez, A., J. M. Rodriguez, R. J. Bongaerts, M. J. Gasson, and N. Horn. 2007. Nisin-controlled extracellular production of interleukin-2 in Lactococcus lactis strains, without the requirement for a signal peptide sequence. Appl. Environ. Microbiol. 73:7781-7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frossard, C. P., L. Steidler, and P. A. Eigenmann. 2007. Oral administration of an IL-10-secreting Lactococcus lactis strain prevents food-induced IgE sensitisation. J. Allergy Clin. Immunol. 119:952-959. [DOI] [PubMed] [Google Scholar]
- 7.Horn, N., A. Fernandez, H. M. Dodd, M. J. Gasson, and J. M. Rodríguez. 2004. Nisin-controlled production of pediocin PA-1 and colicin V in nisin- and non-nisin-producing Lactococcus lactis strains. Appl. Environ. Microbiol. 70:5030-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huston, J. S., D. Levinson, M. Mudgett-Hunter, M. Tai, J. Novotny, M. N. Margolies, E. J. Ridge, R. E. Bruccoleri, E. Haber, R. Crea, and H. Oppermann. 1988. Protein engineering of antibody binding sites: recovery of specific activity in an antidigoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl. Acad. Sci. USA 85:5879-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuipers, O. P., M. M. Beerthuyzen, P. G. de Ruyter, E. J. Luesink, and W. M. de Vos. 1995. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J. Biol. Chem. 270:27299-27304. [DOI] [PubMed] [Google Scholar]
- 10.Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. ClustalW2 and ClustalX version 2. Bioinformatics 23:2947-2948. [DOI] [PubMed] [Google Scholar]
- 11.Robinson, K., L. M. Chamberlain, K. M. Schofield, J. M. Wells, and R. W. F. Le Page. 1997. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 15:653-657. [DOI] [PubMed] [Google Scholar]
- 12.Shearman, C. A., K. L. Jury, and M. J. Gasson. 1994. Controlled expression and structural organization of a Lactococcus lactis bacteriophage lysin encoded by two overlapping genes. Appl. Environ. Microbiol. 60:3063-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steidler, L., K. Robinson, L. Chamberlain, K. M. Schofield, E. Remaut, R. W. F. Le Page, and J. M. Wells. 1998. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 66:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steidler, L., W. Hans, L. Schotte, S. Neirynck, F. Obermeier, W. Falk, W. Fiers, and E. Remaut. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 15.van Asseldonk, M., W. M. de Vos, and G. Simons. 1993. Functional analysis of the Lactococcus lactis usp45 secretion signal in the secretion of a homologous proteinase and a heterologous alpha-amylase. Mol. Gen. Genet. 240:428-434. [DOI] [PubMed] [Google Scholar]
- 16.Wegmann, U., J. R. Klein, I. Drumm, O. P. Kuipers, and B. Henrich. 1999. Introduction of peptidase genes from Lactobacillus delbrueckii subsp. lactis into Lactococcus lactis and controlled expression. Appl. Environ. Microbiol. 65:4729-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu, C., G. Yang, L. G. Bermúdez-Humarán, Q. Pang, Y. Zeng, J. Wang, and X. Gao. 2006. Immunomodulatory effects of IL-12 secreted by Lactococcus lactis on Th1/Th2 balance in ovalbumin (OVA)-induced asthma model mice. Int. Immunopharmacol. 6:610-615. [DOI] [PubMed] [Google Scholar]