Abstract
The relationship between compost amendment, plant biomass produced, and bacterial root colonization as measured by fluorescence in situ hybridization was examined following plant growth in mine tailings. Mine tailings can remain devoid of vegetation for decades after deposition due to a combination of factors that include heavy metal toxicity, low pH, poor substrate structure and water-holding capacity, and a severely impacted heterotrophic microbial community. Research has shown that plant establishment, a desired remedial objective to reduce eolian and water erosion of such tailings, is enhanced by organic matter amendment and is correlated with significant increases in rhizosphere populations of neutrophilic heterotrophic bacteria. Results show that for the acidic metalliferous tailings tested in this study, compost amendment was associated with significantly increased bacterial colonization of roots and increased production of plant biomass. In contrast, for a Vinton control soil, increased compost had no effect on root colonization and resulted only in increased plant biomass at high levels of compost amendment. These data suggest that the positive association between compost amendment and root colonization is important in the stressed mine tailings environment where root colonization may enhance both microbial and plant survival and growth.
A long-term goal in the revegetation of mine tailings is to develop a sustainable ecosystem that is able to survive perturbation and minimize wind and water erosion processes (9, 12). Mine tailings have drastically reduced and functionally altered microbial communities that are not suited to support plant establishment in tailings (13). In fact, previous work has documented major transitions in the microbial communities of tailings during successful plant establishment in tailings (8, 11, 14, 16). For example, in a recent field study in a neutral tailings site, a temporal sample series taken in the rooting zone underwent significant bacterial community changes during an 18-month field trial compared to unplanted controls (16). Similarly, a recent greenhouse study showed a 1- to 5-log decrease in iron and sulfur oxidizers accompanied by a 6-log increase in neutrophilic heterotrophs, following plant establishment in acidic tailings (11). One question that has arisen from these studies is whether the transitions in specific microbial populations observed in bulk and rhizosphere soils during plant establishment in mine tailings are reflected in the colonization of roots by bacteria. This question was prompted by recent research indicating that plants which have undergone root colonization by beneficial microorganisms can subsequently become “primed” and respond more effectively to subsequent stress, including abiotic stress (6).
Revegetation of tailings generally requires the addition of large amounts of amendments, which can include compost, biosolids, lime, or topsoil, a factor that helps dictate remediation costs (9, 12, 14). Recent research has explored the minimum compost amendment required for sustained plant growth (7, 11, 16). Significant incremental increases were observed in total plant biomass for plants grown in extremely (pH 2.7) and moderately (pH 5.7) acidic mine tailings amended with 0, 5, and 10% compost. While plant establishment was accompanied by 4- to 6-log increases in rhizosphere populations of neutrophilic heterotrophs over initial bulk soil counts, final rhizosphere counts were the same for all treatments (0 to 10% compost). Thus, no correlation was observed between the increases in plant biomass associated with increasing levels of compost amendment and the final rhizosphere bacterial counts. The specific question addressed in this effort is whether the rhizosphere population dynamics observed in previous studies (11, 16) accurately reflect the actual bacterial colonization patterns of the root surface. Our goal is to better understand how the compost amendment of mine tailings affects the development of root-microbe associations during the revegetation process, as the rhizosphere community is critical for plant health.
Documented effects of compost addition to a soil include the obvious effects, such as increased organic matter and plant nutrients like phosphorus and nitrogen, as well as increased soil respiration and microbial biomass (for an example, see reference 20). Less studied is the impact on root colonization. Root colonization can be measured by using surrogates or by direct microscopic examination. Surrogate measures of muramic acid to indicate bacterial colonization have shown that soils with naturally higher levels of organic matter have higher levels of root colonization (2). Values ranged from 1.7 to 21.6 mg muramic acid g−1 dry root mass in one study of 15 different species, although the effect was plant species specific (1). One disadvantage of such surrogate measurements is that they are not able to discriminate between live and dead cells. Direct measurements have been performed using electron and direct microscopy, with data showing that 4 to 10% of the root surface is normally colonized by bacteria that also depend on the organic matter content of soil (19). A study of hydroponically grown roots showed slightly higher, 12% ± 3.5%, surface colonization (15). This study used confocal laser scanning microscopy and the nucleic acid stain SYTO9.
In the present study, fluorescence in situ hybridization (FISH) was used to determine the relationship between root colonization and compost amendment following plant establishment in an acidic tailings sample. FISH analysis using rRNA probes was selected to target metabolically active populations. Specifically, we report that (i) modified FISH image analysis methodology allows quantitation of root colonization in mine tailings and that (ii) root colonization results as a function of compost amendment and substrate type (tailings versus a control soil), following a 12-week greenhouse revegetation study.
Tailings were collected from a State Superfund site, the Klondyke Mill located in Graham County, Arizona. The tailings subsample used, called T2, has a sandy loam texture (pH 5.4), a total organic carbon content of 0.41 ± 0.03 g kg−1, a total nitrogen content of 0.07 ± 0.01 g kg−1 (variation represents one standard deviation; n = 3), and heavy metals, including the following (mg kg−1): As (229), Cd (18.3), Cu (1,610), Fe (36,700), Mn (12,600), Pb (13,800), and Zn (5,610). Vinton sand (sandy, mixed, thermic Typic Torrifluvents, pH 7.7; total organic carbon, 1 g kg−1) was used as a control soil (21). The Vinton soil was used as the control in previous phytoremediation studies (7) to simulate southwestern ecosystems typical of locations where many of these tailings were deposited. Many abandoned tailings piles in the southwest are found along river banks where entisols predominate, the majority of which are alkaline. Thus, native plants used in mine tailing revegetation studies in the southwest are drought and salt tolerant and are adapted to pH levels of 7 to 8. In addition, sandy soils, like mine tailings, are low in organic matter and water-holding capacity. The compost was obtained from the University of Arizona Campus Agricultural Center where landscape waste, cow manure, and river sand are used in commercial field scale composting operations.
Tailings and Vinton soil were mixed with compost at 5 and 10% (wt/wt) in bulk until completely homogenized and then distributed into replicate pots. Immediately following mixing, triplicate samples were taken for heterotrophic bacterial counts as indicators of viable bacterial activity at the time of planting (11). Heterotrophic plate counts were used as a measure of soil health because previous phytostabilization research has demonstrated a positive relationship between these counts and final plant biomass (11). Both neutrophilic (pH 7) and moderately acidic (pH 5) heterotrophs were enumerated (7). Results showed that unamended T2 had low neutrophilic counts, (2.34 ± 0.10) × 103 (standard deviation; n = 3), which increased by 1.5 and 2 log units, respectively, with the addition of 5% and 10% compost (Table 1). In contrast, compost amendment did not impact neutrophilic heterotrophic counts in Vinton soil, which in all cases exceeded 107 CFU g dry soil−1, well within the expected range for normal soil (18). The pH 5 and 7 heterotrophic counts were similar in the unamended T2 tailings, suggesting that the heterotrophs present are predominantly acid tolerant. In contrast, as compost was added, the difference between the pH 5 and pH 7 counts increased to 1 and 2 logs, respectively. In the Vinton soil, counts at pH 5 were 2 to 3 logs lower than counts at pH 7, indicating that the heterotrophs present were generally not acid tolerant.
TABLE 1.
Neutrophilic and moderately acidic R2A heterotrophic bacterial counts from unamended and compost-amended T2 tailings and Vinton soil prior to planting
| Sample type (% compost) | CFU g dry soil−1 (±SD) ata:
|
|
|---|---|---|
| pH 5 | pH 7 | |
| T2 (0) | (9.85 ± 2.56) × 102 | (2.34 ± 0.10) × 103 |
| T2 (5) | (2.59 ± 0.41) × 103 | (6.06 ± 3.64) × 104 |
| T2 (10) | (2.60 ± 0.46) × 103 | (2.98 ± 0.79) × 105 |
| Vinton soil (0) | (1.19 ± 0.14) × 105 | (2.42 ± 2.42) × 107 |
| Vinton soil (5) | (4.29 ± 0.32) × 104 | (5.07 ± 4.06) × 107 |
| Vinton soil (10) | (4.39 ± 0.20) × 104 | (3.88 ± 4.39) × 107 |
SD, one standard determination; a total of three plates were studied.
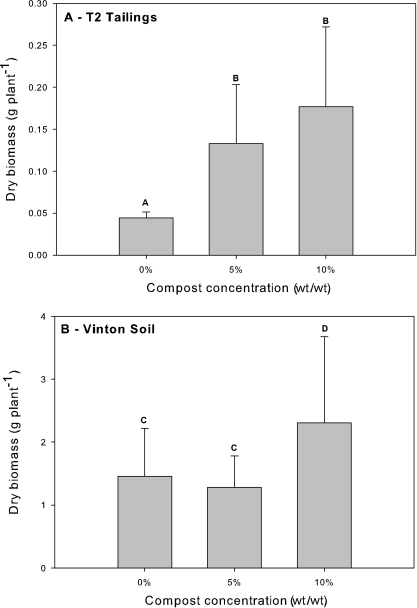
Each treatment consisted of five replicate pots sown with 10 seeds of the native plant Buchloe dactyloides (Nutt.) Engelm. (buffalo grass) per pot as described previously (7). Plants were harvested for dry biomass measurements at the end of 12 weeks. Results show that the average total biomass for the B. dactyloides grown in T2 tailings with either 5% (0.13 ± 0.07 g pot−1) or 10% (0.18 ± 0.10 g pot−1) compost was up to fourfold greater than that for the tailings treatment without compost (0.045 ± 0.007 g pot−1) (Fig. 1A). However, T2-grown plants had much less overall biomass than those grown in the Vinton control soil, with a 10-fold biomass difference between Vinton and T2 plants in compost treatments and a 30-fold difference in no compost treatments. Stress in T2-grown plants was indicated in a number of ways other than the large reduction in plant biomass. Shoot/root ratios were higher in Vinton controls (2.3) than in T2 (1.7). Stolons, vegetative extensions of B. dactyloides that have the potential to root in nearby soil and produce viable clones, were observed in Vinton soil at 5% and 10% compost but were rare in T2-grown plants. In Vinton, below-ground biomass appeared as a profusion of fine roots, creating a web of plant matter throughout the coarse sand matrix. This phenomenon was not observed in the T2 tailings, which had large, less-complex roots. It is unclear whether the observed difference in root structure was a stress response of the T2 plants to the toxicity and acidity of the tailings or if it resulted from physical differences between the two substrates. As with total biomass, a 10-fold or greater reduction in root biomass was observed for the T2 plants than for the Vinton plants, both in treatments with and without compost (data not shown). This difference could be associated with inhibition to either plant growth or microbial activity (13, 17), both of which influence root structure. In addition, the tailings become compacted in the pots following irrigation due to their fine texture and lack of aggregation, thus potentially impeding root elongation.
FIG. 1.
Average dry biomass of uninoculated B. dactyloides grown in T2 tailings (A) and Vinton sand (B) following 12 weeks of growth. A one-way ANOVA determined that there were significant differences between treatments for both substrates (T2 tailings, P = 0.00003; Vinton soil, P = 0.03); means with significant differences were identified using Duncan's multiple-range test (α = 0.05; a total of 10 to 15 plants) and are labeled with different letters. Error bars represent one standard deviation.
At the 12-week plant harvest, triplicate root samples from each treatment were taken for FISH analysis to examine root colonization. Multiple studies have demonstrated a high degree of variability in bacterial colonization along the length of the root, with the greatest densities occurring at the root tips due to the high concentration of root exudates (3, 10, 22). Thus, root tips (also representing the youngest and fastest-growing part of the root system), <0.1-mm thick, were chosen to physically and physiologically standardize samples. The FISH analysis was adapted from that described by Daims et al. (5). The probe mix EUB338 (an equimolar mixture of EUB338-I, EUB338-II, and EUB338-III) (4), labeled with the fluorophore Cy5 to target the domain Bacteria, was obtained from Integrated DNA Technologies, Inc. (Coralville, IA). The fluorophore Cy5 was used because it emits in the far-red range and has been shown to minimize root/tailings autofluorescence (22). The nonsense probe NON338 was used to check root samples for nonspecific binding. A slide cell was designed to immobilize root fragments for hybridization. Briefly, Mμlti hybridization slide frames (VWR International) were applied to gelatin-coated slides with 2-mm vents cut into a corner of the frame to allow diffusion of the washing buffer. Roots were placed within the frames and then covered with dilute, low-melt agarose (0.02%) to protect the root during the hybridization procedure. Positive controls using pure bacterial cultures were run to confirm that the low-melt agarose allowed diffusion of hybridization solutions and probes. Slides were suspended in an ethanol series for dehydration (3 min each at 50, 80, and 96% ethanol) and then dried at 37°C. For hybridization, 30 μl of fresh 25% stringency buffer (25% formamide, 0.2% 1 M Tris-HCl, 1.8% 5 M NaCl, 1 μl 10% sodium dodecyl sulfate, and double-distilled water [ddH2O]) was mixed with 3 μl of each FISH probe (30 ng μl−1), applied to the hybridization frame, and incubated for 2.5 h at 46°C in a hybridization oven (VWR International). Following hybridization, the slides were immersed in prewarmed washing buffer (1 M Tris-HCl, 1 ml; 5 M NaCl, 1.49 ml; 0.5 M EDTA, 0.5 ml; ddH2O to 50 ml; pH 7.0 to 7.2) for 15 min at 48°C. Finally, slides were rinsed with ice-cold ddH2O, dried with compressed air, and stored in a dark box containing desiccant at −20°C.
Prior to being viewed, all slides were warmed to room temperature in the dark. A drop of Citifluor AF-1 antifadent (Electron Microscopy Sciences) was placed on the sample field, and a glass coverslip was applied. Root slides were viewed on a Zeiss LSM 510 Meta NLO laser-scanning confocal microscope. The universal EUB338 probe mix was viewed with a 633-nm HeNe laser for excitation of Cy5. The detector collected emissions between 650 and 710 nm. The pinhole was set to 1 Airy unit, and the optical slice was 1.0 μm. Z-series images were acquired for the upper 12.6 μm of each root sample with an interval of 0.6 μm, similar to the method described by Watt et al. (22). The pixel time was 1.60 μs for all images. Measurements were taken on every other interval for a total of 11 samples per root tip to yield composite images from triplicate root tips for each treatment. Confocal images were analyzed with the freeware program ImageJ (NIH) by setting the maximum feature area to 300 as needed and the minimum fluorescence of bacterial features to 45. These parameters helped to minimize root-based fluorescence. The total area of fluorescence meeting these criteria was recorded and divided by the area of the root visible in the slice to give a ratio of bacterial area to root area. Colonization data are reported here based on the fraction of fluorescence area over root area, which helps minimize the influence of tailings-conferred autofluorescence. A single fluorescence value was generated for each root by dividing the sum of fluorescence for all 11 z slices by the total root area for all slices. Significant differences between means were determined by a one-way analysis of variance (ANOVA) using Duncan's multiple-range test (α = 0.05, n = 3) in the statistics package SAS v9.1 (SAS Institute, Inc.).
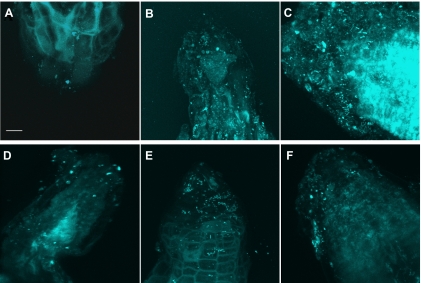
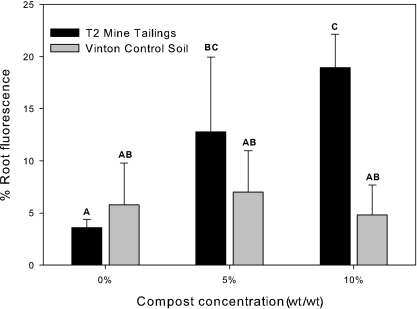
Composite FISH Z-series images from representative roots qualitatively show that T2 tailings with compost were much more heavily colonized than unamended tailings (Fig. 2). Quantitative FISH analysis shows a similar level of root colonization in unamended T2 (3.6% ± 0.8%) and Vinton soil (5.8% ± 4.0%) (Fig. 3). Given the small amount of organic matter in T2 and Vinton soil, these values compare closely to the previously discussed electron and direct microscopy data which show that 4 to 10% of the root surface is normally colonized by bacteria, depending on soil organic matter content (19).
FIG. 2.
Confocal composite Z-stack images of B. dactyloides roots grown in T2 with no compost (A), T2 with 5% compost (B), T2 with 10% compost (C), Vinton soil with no compost (D), Vinton soil with 5% compost (E), and Vinton soil with 10% compost (F). Magnification, ×100; bar, 10 μm. FISH analysis was performed with the universal EUB338 probe mix labeled with Cy5.
FIG. 3.
Shown is the average percent fluorescence, based on the fluorescence fraction (fluorescence area/root area) for B. dactyloides roots grown in T2 tailings (black bars) or Vinton sand (gray bars) and harvested at 12 weeks. FISH analysis was performed with the universal EUB338 probe mix (4) labeled with Cy5 on triplicate root tips for each treatment. A one-way ANOVA determined that there were significant differences between treatments (P = 0.005); means with significant differences were identified using Duncan's multiple-range test and are labeled with different letters (α = 0.05; n = 3). Error bars represent one standard deviation.
For the Vinton control soil, the organic matter addition did not influence the percent root colonization, which ranged from 5 to 7% (Fig. 3). In contrast, the compost addition to T2 increased the percent root colonization from 3.6% ± 0.8% to 12.8% ± 7.2% to 18.9% ± 3.2% as the compost amendment was increased from 0 to 5 to 10% (Fig. 3). Duncan's analysis of triplicate samples for each treatment showed a significant increase in root colonization following compost amendment at either 5 or 10% (Fig. 3). These data suggest that the root surface is a preferred habitat for bacteria in T2 tailings. Specifically, though initial heterotrophic counts on R2A were 2 to 3 logs lower in the 5% and 10% composted T2 tailings than in the Vinton control soil with comparable compost amendment, root colonization was two- to fourfold higher. Possible explanations for the observed enhanced colonization of T2 plant roots include the possibility that the bulk tailings are a stressful environment that is less preferable than the root surface or that the stressed T2 plants produce more root exudates than those growing in the Vinton soil, thus stimulating microbial growth.
Taken together, these data show a positive association between increased plant biomass, percent root colonization, and compost amendment in T2 tailings (Fig. 1A and 3). In addition, compost amendment resulted in a 1.5- to 2-log increase in initial neutrophilic heterotrophic counts in the tailings. This supports the previous observation by Mendez et al. (11) that significant differences in initial neutrophilic heterotrophic counts are indicators of plant growth potential in tailings and compost-amended tailings. This study, also conducted in Klondyke tailings, showed that increases in initial culturable, neutrophilic heterotrophs with compost addition paralleled significant increases in plant biomass of the native shrub Atriplex lentiformis in two tailings subsamples amended with five different compost concentrations. In contrast to the percent root colonization results of the present study, no significant differences were observed between culturable rhizosphere counts for all established plants in the Mendez et al. study. Thus, the FISH analysis provides a more accurate measurement of plant-microbe interaction at the root surface. Acquiring a more specific understanding of such interactions as well as the minimum microbial diversity required to sustain these interactions may be instrumental to efforts to enhance plant establishment in mine tailings.
In summary, this study demonstrates that FISH can be used in conjunction with ImageJ software to quantify the bacterial colonization of roots. Although FISH was performed here with a universal bacterial probe, specific probes could be used to identify targeted root-colonizing bacterial populations. In this study, FISH analysis helped determine key differences in the relationship between compost amendment, biomass production, and root colonization in the two substrates studied, tailings and Vinton soil. Specifically, while compost amendment resulted in parallel increases in initial counts, root colonization, and plant biomass for the T2 tailings studied, this was not true for the Vinton control soil, suggesting that this relationship is particularly important in stressed systems. The level of stress in the T2 tailings is evidenced by a 10- to 30-fold reduction in plant biomass production compared to that in the Vinton control. Finally, FISH analysis showed that the level of bacterial colonization of compost-amended, T2-grown roots was almost double that previously reported in the literature (up to 19%), suggesting that this type of stressed environment results in enhanced root colonization which may be necessary for both microbial and plant survival and growth in the tailings.
Acknowledgments
Special thanks to David Bentley and Barbara Carolus from the Biotechnology Imaging Facilities at the University of Arizona for microscopy assistance.
Funding for this research was provided by the University of Arizona Superfund Basic Research Program grant no. 2 P42 ESO4940-11 from the National Institute of Environmental Health Sciences Superfund Basic Research Program, the National Institutes of Health.
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Appuhn, A., and R. G. Joergensen. 2006. Microbial colonisation of roots as a function of plant species. Soil Biol. Biochem. 38:1040-1051. [Google Scholar]
- 2.Appuhn, A., E. Scheller, and R. G. Joergensen. 2006. Relationships between microbial indices in roots and silt loam soils forming a gradient in soil organic matter. Soil Biol. Biochem. 38:2557-2564. [Google Scholar]
- 3.Assmus, B., P. Hutzler, G. Kirchhof, R. Amann, J. R. Lawrence, and A. Hartmann. 1995. In situ localization of Azospirillum brasilense in the rhizosphere of wheat with fluorescently labeled rRNA-targeted oligonucleotide probes and scanning confocal laser microscopy. Appl. Environ. Microbiol. 61:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daims, H., A. Bruhl, R. Amann, K. H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 5.Daims, H., K. Stoecker, and M. Wagner. 2005. Fluorescence in situ hybridization for the detection of prokaryotes, p. 213-239. In A. M. Osborn and C. J. Smith (ed.), Molecular microbial ecology. Taylor & Francis, New York, NY.
- 6.Goellner, K., and U. Conrath. 2008. Priming: it's all the world to induced disease resistance. Eur. J. Plant Pathol. 121:233-242. [Google Scholar]
- 7.Grandlic, C. J., M. O. Mendez, J. Chorover, B. Machado, and R. M. Maier. 2008. Identification of plant growth-promoting bacteria suitable for phytostabilization of mine tailings. Environ. Sci. Technol. 42:2079-2084. [DOI] [PubMed] [Google Scholar]
- 8.Kelly, J. J., M. M. Häggblom, and R. L. Tate. 2003. Effects of heavy metal contamination and remediation on soil microbial communities in the vicinity of a zinc smelter as indicated by analysis of microbial community phospholipid fatty acid profiles. Biol. Fertil. Soil 38:65-71. [Google Scholar]
- 9.Li, M. S. 2006. Ecological restoration of mineland with particular reference to the metalliferous mine wasteland in China: a review of research and practice. Sci. Total Environ. 357:38-53. [DOI] [PubMed] [Google Scholar]
- 10.Macnaughton, S. J., T. Booth, T. M. Embley, and A. G. O'Donnell. 1996. Physical stabilization and confocal microscopy of bacteria on roots using 16S rRNA targeted, fluorescent-labeled oligonucleotide probes. J. Microbiol. Methods 26:279-285. [Google Scholar]
- 11.Mendez, M. O., E. P. Glenn, and R. M. Maier. 2007. Phytostabilization potential of quailbush for mine tailings: growth, metal accumulation, and microbial community changes. J. Environ. Qual. 36:245-253. [DOI] [PubMed] [Google Scholar]
- 12.Mendez, M. O., and R. M. Maier. 2008. Phytostabilization of mine tailings in arid and semiarid environments—an emerging remediation technology. Environ. Health Perspect. 116:278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendez, M. O., J. W. Neilson, and R. M. Maier. 2008. Bacterial community characterization of a historic semiarid lead-zinc mine tailings site. Appl. Environ. Microbiol. 74:3899-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moynahan, O. S., C. A. Zabinski, and J. E. Gannon. 2002. Microbial community structure and carbon-utilization diversity in a mine tailings revegetation study. Restor. Ecol. 10:77-87. [Google Scholar]
- 15.Münch, C., T. Neu, P. Kuschk, and I. Röske. 2007. The root surface as the definitive detail for microbial transformation processes in constructed wetlands—a biofilm characteristic. Water Sci. Technol. 56(3):271-276. [DOI] [PubMed] [Google Scholar]
- 16.Rosario, K., S. L. Iverson, D. A. Henderson, S. Chartrand, C. McKeon, E. P. Glenn, and R. M. Maier. 2007. Bacterial community changes during plant establishment at the San Pedro River mine tailings site. J. Environ. Qual. 36:1249-1259. [DOI] [PubMed] [Google Scholar]
- 17.Solís-Dominguez, F. A., M. C. González-Chávez, R. Carrillo-González, and R. Rodríguez-Vázquez. 2007. Accumulation and localization of cadmium in Echinochloa polystachya grown within a hydroponic system. J. Hazard. Mater. 141:630-636. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson, F. J., and M. A. Cole. 1999. Cycles of soil: carbon, nitrogen, phosphorus, sulfur, micronutrients. John Wiley & Sons, Inc., New York, NY.
- 19.Subba Rao, N. S. 1999. The rhizosphere and the phyllosphere, p. 85. In Soil microbiology, 4th ed. Science Publishers, Inc., Enfield, NH.
- 20.Tiquia, S. M., J. Lloyd, D. A. Herms, H. A. J. Hoitink, and F. C. Michel, Jr. 2002. Effects of mulching and fertilization on soil nutrients, microbial activity and rhizosphere bacterial community structure determined by analysis of TRFLPs of PCR-amplified 16S rRNA genes. Appl. Soil Ecol. 21:31-48. [Google Scholar]
- 21.Torrens, J. L., D. C. Herman, and R. M. Miller-Maier. 1998. Biosurfactant (rhamnolipid) sorption and the impact on rhamnolipid-facilitated removal of cadmium from various soils. Environ. Sci. Technol. 32:776-781. [Google Scholar]
- 22.Watt, M., P. Hugenholtz, R. White, and K. Vinall. 2006. Numbers and locations of native bacteria on field-grown wheat roots quantified by fluorescence in situ hybridization (FISH). Environ. Microbiol. 8:871-884. [DOI] [PubMed] [Google Scholar]