This paper describes several experiments that were performed to evaluate the accuracy of a technique developed to measure skeletal kinematics using dual fluoroscopic imaging. Thorough validation should be a prerequisite for the clinical application of any new instrumentation, and the authors are to be commended for investing considerable time and effort to characterize the performance of their system. This is also a timely topic, because dynamic radiographic imaging has become increasingly popular for assessing bone and joint motion over the last several years. The widespread availability of mobile digital fluoroscopic systems with dynamic imaging capabilities (“C-arms”) places this type of motion analysis within reach for many research groups.
These systems, however, have inherent limitations for dynamic studies, which are not clearly presented in the Li, et. al. paper. Clarification of these issues is essential for interpreting the findings of the paper in the proper context, and could be important for others attempting to develop and use similar systems. In particular, this manuscript creates confusion about the frame rate and exposure time capabilities of the imaging system, both of which are critical for determining its suitability for dynamic musculoskeletal studies. The lack of clarity concerning imaging system performance is particularly evident in these two verbatim passages from the manuscript:
First paragraph, Section 2.1: The DFIS consisted of two fluoroscopes (BV Pulsera, Philips) set to generate 8 ms width X-ray pulses every 33 ms with a dose rate of 13 mGy/s. Therefore, the fluoroscopic system samples the object’s motion at a frequency of 125 Hz (1000 ms/8 ms), but only takes 30 snap shots. In the actual setup, the snap shot can be generated every 67 ms, so that 15 images can be obtained at the actual frequency of 125 Hz.
First paragraph, Section 2.2.1: The knee motion was imaged with 30 frames per second with a frame rate of 125 Hz. Each frame was a snap shot with a pulse of 8 ms.
These passages imply a 125 Hz frame rate based on the use of 8 ms x-ray pulses, regardless of the actual sampling frequency. However, it is incorrect to infer frame rate or frequency based on the inverse of the pulse width. It is important to specify pulse width, frame rate and radiographic protocols precisely when describing radiographic measurements of moving objects, since these all affect image quality, measurement accuracy and radiation exposure to subjects. Thus, the aims of this Letter to the Editor are to promote proper use of imaging terminology, to discuss implications of radiographic system design on dynamic imaging performance and to encourage design of validation studies that replicate anticipated testing conditions.
In all imaging applications (video or x-ray), frame rate refers to the actual number of images acquired per second, and is analogous to sample rate for other types of data acquisition. It should be selected based on the expected frequency content of the motion under study. Exposure time is the duration for each frame during which an image is acquired. This parameter should be based on the object speed in the image plane, since any motion that occurs during the exposure period results in blur. For systems with continuous illumination and no shutter, exposure time is equal to the frame interval (the inverse of the frame rate). However, exposure time can be reduced relative to the frame interval either by employing a shutter in the camera or by using pulsed illumination (e.g. a strobe light to freeze motion). Modern clinical fluoroscopy systems typically use pulsed x-rays for reducing exposure time, since this avoids exposing the patient to radiation that is not used to make an image, consistent with the widely recognized ALARA (As Low As Reasonably Achievable) principle for minimizing radiation exposure. For pulsed systems, the exposure time per frame is equal to the width of the x-ray pulse, and frame rate and pulse width can be adjusted independently (with the restriction that the pulse width cannot exceed the frame interval).
In the specific case of the Philips BV Pulsera system used by Li, et. al., the maximum frame rate is 30 Hz (for a frame interval of 33 ms), and the minimum pulse width is 8 ms (manufacturer’s specifications, Philips. Inc.). Contrary to what is implied in the passages above (frequency or frame rate of 125 Hz), actual sample rate can never exceed 30 Hz for this system, regardless of pulse width. In fact, it appears that the sphere tests were actually performed at a frame rate of 15 Hz (“In the actual setup, the snap shot can be generated every 67 ms”; 1/0.067 s = 15 Hz), and the cadaver experiments were performed at 30 Hz (“The knee motion was imaged with 30 frames per second…”).
Why is this important? The Li, et al. paper uses gait as an example application, but 30 Hz is generally considered to be inadequate for kinematic gait studies. According to Gill, et al., significant events in gait (such as the sudden changes in limb direction that occur at foot strike) require a sample rate of 100 Hz or greater for accurate analysis (Gill, et al., 1997). Nearly all commercial gait analysis systems are capable of at least 60 Hz acquisitions, and most modern systems sample at 100 Hz or higher. Of note is the treadmill speed used to generate the data in Appendix A of the Li, et al. paper (1.2 miles/hour, or 0.54 m/s), which is only about 36% of average walking speed for healthy young men (Murray, et al., 1964). A sample rate of 30 Hz may be adequate for this abnormally slow gait but would likely miss important details of normal walking, and would certainly be inappropriate for faster movements with higher-frequency components (running, jumping, etc.).
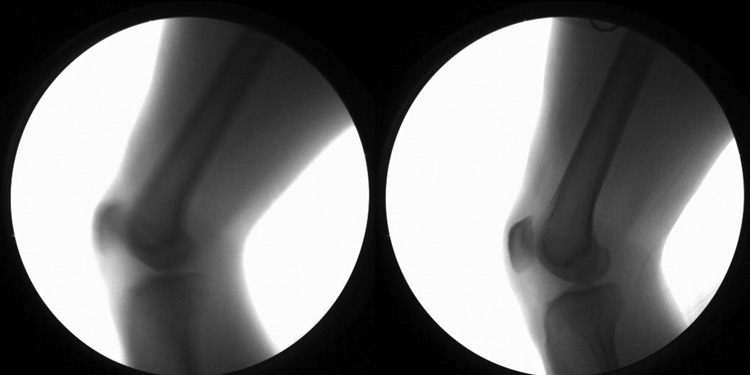
The 8 ms exposure time of the system used by Li, et al. is also a concern for dynamic measurements. Video-based gait analysis systems often use exposures of 1 ms or less (e.g. strobe pulse width for Vicon MX, Vicon Corp., is 0.125 to 1.0 ms) to minimize blur effects. In our studies of patients with knee injuries walking on treadmills at 1 m/s (still less than typical healthy gait speed), we have measured average movement velocity of the distal femur/proximal tibia in the order of 0.25–0.4 m/s, with peak velocities approaching 1 m/s. During an 8 ms exposure, the knee would move from 2 to 8 mm, causing considerable blur. Figure 1 shows two images taken during early stance phase of gait of the same individual at the same treadmill speed (1.3 m/s). The left image employed an 8 ms exposure; the right image used a 1 ms exposure. There is sufficient blur with 8 ms exposure to make quantitative analysis difficult, especially for edge-based matching methods.
Figure 1.
Effects of exposure time on image quality. Both images were acquired at approximately 0.13 s after heelstrike during treadmill gait (1.3 m/s); knee motion speed was approximately 1 m/s. The left image was acquired from a 30 Hz sequence using an 8 ms, 25 mA pulsed exposure (similar to Li, et al.). The right image was acquired from a 60 Hz sequence using a 1 ms, 200 mA pulsed exposure. Radiation exposure was identical at 0.2 mA-s per image.
The authors touch upon these issues, but the validations performed cannot reliably assess the effects of the frame rate and exposure time limitations. The spheres tested in section 2.1 reach a peak speed of about 0.6 m/s, but traversed a very simple movement path. Since the signature of a sphere does not change shape with rotation, the tracking simplifies to a 3 degree-of-freedom problem. A rolling sphere has a very low movement frequency, so sampling rate is not an issue. Since the motion path is linear and easily predictable and the object is a simple shape, an operator performing the manual matching process (as described in section 2.1) might naturally fit the sphere projection to either the center or the leading edge of a blurred marker signature, minimizing effects of blur on the error.
The cadaveric experiments (section 2.2) are far better suited to evaluate effects of sample rate and exposure time on tracking accuracy, as they recreate the complex shapes and soft tissue obstruction that characterize in vivo images. However, neither of the cadaver studies appears to have reproduced the movement speed or complexity one would encounter during in vivo, dynamic studies of functional tasks. Movement speed for the MTS experiments (section 2.2.1) was only 0.017 m/s (less than 2% of anticipated peak movement speed during gait). No movement speeds were provided for the cadaver knee flexion/extension experiments (Section 2.2.2), so it is impossible for the reader to determine if this test was a reasonable simulation of gait in terms of movement speed and its effects on blur and tracking accuracy. However, if the tibia or femur were held fixed during the motion, then bone motion speed would again have been only a small fraction of what would occur during gait.
Similar frame rate and sample limitations to the fluoroscopy system employed by Li, et al. are common to nearly all commercially available C-arm systems (e.g. OEC 9800/9900 series, General Electric Corp.: 30 frames/s max, 10 ms minimum pulse width). This has lead many labs (e.g. Henry Ford Health System, Brown University, University of Pittsburgh) to develop custom systems capable of much shorter exposures and higher frame rates. In our own work validating measurements from dynamic radiography, we have found that actual errors during in vivo studies of functional activities can exceed those measured in static cadaver studies by a factor of 5 or more (Anderst and Tashman, 2008; You, et al., 2001). It is important for anyone considering the use of dynamic x-ray imaging to account for movement frequency and speed during the activities they wish to study, to insure that the imaging hardware they select is capable of meeting their needs. Furthermore, validations studies should be performed and their results interpreted with careful consideration of the planned application.
It is also important that manuscripts describing x-ray based measurements clearly report the imaging parameters employed using a common terminology. It is suggested that this include frame rate, pulse width/exposure time, x-ray protocols (beam current in mA or mA-s and beam energy in kVp) and x-ray system geometry (source-to-detector distance, inter-beam angle for biplane systems). This will enhance comparison of data collected using diverse systems and assist with those developing new applications for this valuable measurement modality.
Acknowledgments
Conflict of Interest Statement:
The Author has received funding from the National Institutes of Health (NIAMS), the Orthopaedic Research and Education Foundation and the University of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderst W, Tashman S. Validation of Three-Dimensional Model-Based Tibio-Femoral Tracking During Running. Medical Engineering & Physics. 2008 doi: 10.1016/j.medengphy.2008.03.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS, Morris J, Biden E, O'Conner J. Optometric methods in biomechanical gait analysis. In: Orr JF, Shelton JC, editors. Optical Measurement Methods in Biomechanics. Springer; 1997. [Google Scholar]
- Murray MP, Drought AB, Kory RC. Walking Patterns of Normal Men. J Bone Joint Surg AM. 1964;46:335–360. [PubMed] [Google Scholar]
- You B-M, Siy P, Anderst W, Tashman S. In-vivo measurement of 3D skeletal kinematics from sequences of biplane radiographs: application to knee kinematics. IEEE Trans. Medical Imaging. 2001;20:514–525. doi: 10.1109/42.929617. [DOI] [PubMed] [Google Scholar]