Abstract
Research protocols frequently necessitate procedures or design elements that differ from those used in routine clinical care. An example is the inclusion of a placebo arm in many randomized clinical trials. Because there are risks to taking a placebo when one has a chronic disorder such as schizophrenia, ascertaining how well people with severe mental illness understand placebos is an important task for empirical research ethics. We investigated whether schizophrenia patients' understanding of placebo controls could be improved with a brief educational intervention. We randomized 49 middle-aged and older patients with schizophrenia or schizoaffective disorder to receive either (1) a routine explanation of placebos in the context of consent for a hypothetical double-blind placebo-controlled clinical trial, or (2) the consent for the hypothetical trial plus a brief educational module explaining placebos in more depth. Understanding of placebos was assessed with a 12-item questionnaire, and we examined demographic, clinical, neurocognitive, and decision-making correlates of understanding of placebos. Those participants who received the intervention obtained higher scores on the placebo post-test compared to those who received the standard information alone. Performance on the placebo post-test was positively correlated with measures of decisional capacity and neurocognitive abilities and negatively correlated with severity of negative symptoms, but it showed no relationship with positive or general symptoms. Some participants interpreted the common phrase “sugar pill” as relating somehow to diabetes. We conclude that the level of understanding of important research design–related information is not static but may be influenced by how investigators approach the consent process.
Keywords: research ethics, decision making, informed consent, research designs, clinical trials
Introduction
Concerns have been mounting for some time about the adequacy of informed consent in clinical research.1–3 Inadequate comprehension of certain research-specific procedures has been found across various diagnostic groups.4–7 At least some of this problem is likely attributable to overly long and legalistic consent forms,8 as well as to insufficient attention to the consent process itself. Although the consent process should not be construed as transferring the investigator's beliefs to the participant, there is certainly a learning component to the consent process, in that participants must understand the consent information before they can appreciate or reason with that information. Thus, in recent years an important development in informed consent research has consisted of efforts to develop evidence-based methods to improve the consent process by various means, including incorporating educational strategies to enhance the learning/understanding component of the consent process.9–11
The failure of participants to understand the use of randomization, placebo controls, and blinding is ethically problematic—particularly if it means participants do not recognize how these procedures may affect their health or individual care.7,12,13 Placebo-controlled trials, in particular, carry a risk of personal consequences, especially for people who may be forgoing standard or effective care for a given time period, during which symptoms or clinical status could worsen.
This placebo dilemma is notably problematic with chronic conditions like schizophrenia, for which existing treatments are generally more beneficial than no treatment for most individuals, but for which currently available treatments remain less than ideal in both effectiveness and side-effect profiles. A clear need exists for better and safer antipsychotic medications, yet the process of developing and testing such agents may entail clinical trials in which some persons go off their current medication and receive a placebo. The use of placebos in schizophrenia research thus continues to be a focus of discussion and debate.14–17 Although normative questions about the ethical acceptability of placebos in schizophrenia trials are likely to remain unresolved for some time,17 empirical data can serve an important role by helping to frame the issues.13
Three factors implicate the need to examine how placebos are explained and how well they are understood: (1) the pivotal place of informed consent in arguments for continued use of placebo controls in schizophrenia;16,18 (2) concerns about the abilities of people with schizophrenia to provide capable consent19 (which encompasses not just an understanding of key aspects of the proposed protocol, but also an appreciation of the significance of the information provided for one's own situation, reasoning with the information provided, and making a choice);20 and (3) growing evidence that many people may confuse some aspects of clinical care (ie, individualization of treatment) with those of research.7,12,21 Despite these compelling factors, few studies have examined in any depth how well research participants understand placebo controls.4,22 One exception was a recent survey of 190 participants enrolled in 14 different cardiology, rheumatology, and ophthalmology clinical trials, in which understanding of the placebo's purpose was assessed using an open-ended question. The authors reported that 13% of participants demonstrated full understanding and 56% showed partial understanding, while 30% simply did not know.22 These results suggest that for clinical research participants with various physical disorders, some confusion or uncertainty may exist about placebos.
Whether and how well participants in schizophrenia research actually understand the purpose, nature, and potential effects of placebos is essentially unknown.13,23 Furthermore, minimal efforts have been made specifically to identify and remediate gaps in the understanding of placebos or other research design elements.7 Here, we describe a preliminary study of understanding of placebos among middle-aged and older people with schizophrenia and schizoaffective disorder, in which we piloted in a randomized fashion a brief educational intervention designed to provide more information about placebos than is typically conveyed in consent forms. Based on our earlier findings of improved general consent understanding, in which information was presented in a structured educational format,10 as well as on other successful consent educational interventions,9,11,24,25 we hypothesized that the placebo educational intervention would result in better understanding of placebo controls compared to routine information provided in consent for a hypothetical clinical trial. We evaluated the correlates of understanding of placebos; based on the pattern of correlates seen for decision-making capacity in general, we hypothesized that we would find significant correlations with overall cognitive functioning but not with severity of psychopathology.
METHOD
Participants
Participants were 49 middle-aged and older people with schizophrenia or schizoaffective disorder. These participants were recruited as part of a larger sample and study on enhancing informed consent for older people with psychotic disorders. Inclusion criteria were (1) DSM-IV diagnosis of schizophrenia or schizoaffective disorder (as determined by the participant's treating physician), (2) fluency in English, and (3) absence of a diagnosis of dementia. A variety of recruitment sites (eg, board-and-care residences, academic and county psychiatric clinics, and the local Veterans Administration hospital) were used to create a sample representing the broad range of symptoms, cognitive deficits, and decision-making capacities typically seen among community-dwelling people with psychosis.
Participant Safeguards
Informed consent to participate in the consent enhancement study was obtained in writing from each participant after the purpose of the study was carefully explained. As this was a procedurally simple and minimal-risk protocol, we employed a “sliding scale” concept of capacity, accepting as adequate understanding the participant's ability to understand that participation was voluntary, that they were not enrolling in a real medication study, and that the primary risks were related to boredom or fatigue from the procedures. Standard measures for the protection of confidentiality were taken. All appeared to have adequate understanding of the basic purpose and procedures of this protocol, and no potential participants were excluded on the basis of impaired decisional capacity. The University of California, San Diego, Institutional Review Board for the protection of human subjects reviewed and approved the protocol.
Measures and Procedures
Clinical trial vignette
The hypothetical clinical trial that was presented to all participants involved randomization to 1 of 3 study arms: approved medication, experimental medication, or placebo. The risks described were similar to those of common clinical trials for antipsychotic medications, and the consent form was as or nearly as detailed as a typical consent form. The placebo module study was a substudy of a larger, overall parent study of consent. Within the overall study of consent, each participant was first randomized to a routine paper-based or computer-based consent procedure, each containing the same information, with the computer version additionally providing graphics, summary slides, and several video clips.
Placebo educational intervention
Each participant was randomly assigned to learn about placebos via 1 of 2 methods. Of the 49 participants, 25 received standard information about placebos within the overall consent procedure, and 24 received the standard information plus the educational placebo module (described below). The standard information (whether delivered in the overall consent procedure via paper or computer, as described above) defined placebos as follows: “A placebo pill is a sugar pill with no active medication inside it.” The overall consent form that all participants received also discussed randomization and double-blind procedures.
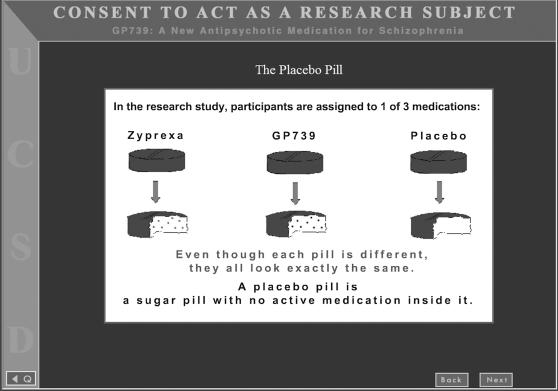
After completing the overall consent procedure, those participants who were randomized to the educational placebo module also viewed four slides on a laptop computer. These were designed to convey more information about the purpose, nature, and risks of placebos in research than what is typically provided in consent forms. The first slide (Figure 1) described the placebo both graphically and verbally. The module then described risks of placebos, as well as contextual information that symptoms could get better or worse regardless of which agent people received.
Fig. 1.
First Screen of Placebo Educational Module.
We gave considerable thought to how we would describe placebos in the materials. We ultimately included the phrase “sugar pill,” a phrase commonly used by investigators to describe placebos in lay terms. We considered using a more technically precise definition; however, we were concerned that an overly technical description could confuse participants further. The explanation we ultimately chose was modeled in part after suggestions made by Lidz and Appelbaum.26
Decisional abilities
Integrated into the overall consent assessment for the hypothetical clinical trial, each participant's decisional capacity was evaluated with the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR).27 This instrument consists of a semistructured interview, administered by a trained research assistant, with questions that assess one's understanding of the factual information about the study, appreciation of the significance of the information for one's own situation, reasoning with the information (comparing options and generating consequences), and expression of a choice. Per standard MacCAT-CR procedures, our analyses focused on the 4 subscale scores (understanding, appreciation, reasoning, and expression of a choice) rather than a MacCAT-CR total score.27 Interrater reliability for the MacCAT-CR, based on a randomly selected subsample of 15 interviews, was high, with the following intraclass correlation coefficients: Understanding 0.98, Appreciation 0.84, and Reasoning 0.78. (Because of very little variability in the Choice subscale, reliability scores are not meaningful for this portion.)
Placebo questionnaire
After completing the MacCAT-CR, each participant was also interviewed with a 12-item questionnaire designed to evaluate more comprehensively his or her understanding of key aspects of the placebo-controlled design. This questionnaire included 6 closed-ended and 6 open-ended questions (for the questions, see Table 2). The closed-ended items were true/false statements (eg, “It will be decided at random whether I will get a placebo” and “There are no risks associated with taking a placebo”). The open-ended items asked participants for their understanding in more depth (eg, “Why do the researchers use a placebo pill?” and “Do you think that some people who get the placebo will feel their symptoms are better/worse? What makes you think this?”). The research assistants who administered this questionnaire were trained to probe for clarification of any unclear responses. Individuals were not able to refer to the consent form to access the information to answer these questions as they were being questioned. The first author scored the open-ended items while unaware of all other variables (ie, assignment to educational module versus standard placebo information, overall randomization within parent study to paper- or computer-based consent, demographic information, clinical and neurocognitive status, and decision-making abilities). Items were scored as 0 or 1 (0 for an incorrect/marginal response, and 1 for a correct response). A total score was created by summing the true/false and open-ended items, for a possible total score range of 0 to 12.
Table 2.
Performance on Placebo Questionnaire: Individual Items
|
n (%) Answering Item Correctly |
χ2 | p | ||
| True/False or Open-Ended Item (Correct answer) | No placebo module (n = 25) | Placebo module (n = 24) | ||
| It will be decided at random whether I will get a placebo. (True) | 22 (88.0%) | 23 (95.8%) | 1.002 | .317 |
| The research physician will not know whether I'm getting a real medication or the placebo pill. (True) | 19 (76.0%) | 17 (70.8%) | .168 | .682 |
| Most people can tell if they are taking a placebo pill. (False) | 14 (56.0%) | 22 (91.7%) | 7.991 | .005 |
| There are no risks associated with taking a placebo pill. (False) | 14 (56.0%) | 17 (70.8%) | 1.159 | .282 |
| Some people who get the placebo pill might experience improvement in their symptoms. (True) | 14 (56.0%) | 24 (100%) | 13.617 | <.001 |
| People who get the placebo pill won't experience any side effects; that would only happen if they were taking a real medication. (False) | 10 (40.0%) | 17 (70.8%) | 4.705 | .030 |
| Why do the researchers use a placebo pill? (To tell difference between groups in effects of medications; to have a baseline to compare) | 7 (28.0%) | 9 (37.5%) | .503 | .478 |
| Can the research participants tell the difference between a placebo pill and a pill with medication inside of it? (No) | 11 (44.0%) | 19 (79.2%) | 6.379 | .012 |
| Do you think that some people who get the placebo will feel their symptoms are better? What makes you think this? (Answer showing awareness of possible placebo effect) | 7 (28.0%) | 18 (75.0%) | 10.824 | .001 |
| Do you think that some people who get the placebo will feel that their symptoms are worse? What makes you think this? (Answer showing awareness of possibility of symptoms worsening) | 13 (52.0%) | 13 (54.2%) | .023 | .879 |
| Can placebos cause side effects? (Answer showing awareness that people on placebos can sometimes feel they are having side effects) | 6 (24.0%) | 12 (50.0%) | 3.562 | .059 |
| Do you think there are any risks to taking a placebo pill? What kind of risks? (Awareness of possibility of symptoms reemerging or worsening) | 9 (36.0%) | 7 (29.2%) | .260 | .610 |
Note: df for χ2 = 1.
Additional measures
Severity of psychopathology was evaluated with the Positive and Negative Syndrome Scale (PANSS),28 severity of depressive symptoms with the 17-item version of the Hamilton Depression Rating Scale (HAM-D),29 and awareness of having a mental illness and of the need for treatment with the Birchwood Insight Questionnaire.30 Severity of cognitive deficits was evaluated with the Mattis Dementia Rating Scale (DRS).31 The DRS is a 20–30 minute battery that provides a total score reflecting overall level of cognitive impairment (potential range 0 to 144; lower scores represent more severe cognitive impairment), as well as five subscale scores (Attention, Initiation/Perseveration, Construction, Conceptualization, and Memory). The DRS has been shown to be sensitive to functional impairment in schizophrenia.32 In addition, we collected demographic information through patient interview and/or review of available records, as well as other data to address hypotheses for the larger parent study (not presented here).
Each of the above measures was administered by trained research assistants. The MacCAT-CR and 12-item placebo questionnaire were given by one staff member, although this person did not score the open-ended items (as described above, this was done blindly by the first author). A second research assistant administered the psychiatric rating scales and DRS; this person was kept unaware of the scores on the MacCAT-CR and placebo questionnaire and was blind as well to randomization to overall consent arm and to placebo information version.
Data Analysis
We compared the two groups (no placebo module versus placebo module) on all relevant variables, using t-tests for continuous variables and chi-square for categorical variables. Due to the commonly observed significant skew in several of the key variables, we used nonparametric correlations (Spearman's rho) to evaluate the bivariate associations among the variables. Because scoring some of the responses to open-ended items has a level of subjectivity, we also examined the effects of the intervention by comparing total scores on the 6 true/false items using a t-test; we evaluated the bivariate associations between total score on these 6 items with cognitive deficits, decision-making abilities, and psychopathology using Spearman's rho. We also examined the bivariate associations between individual open-ended items and the DRS. For all analyses, significance was defined as p < 0.05 (two-tailed).
Results
Participant Characteristics
Demographic, clinical, neurocognitive, and decision-making characteristics of the 49 participants are shown in Table 1. Although there were more men in the group who received the educational intervention than in the standard consent condition, there were no sex effects on the MacCAT-CR or 12-item placebo questionnaire. The two consent groups did not differ significantly on any of the other demographic or clinical characteristics or in their general levels of decisional capacity (MacCAT-CR Understanding, Appreciation, Reasoning, or Expression of a Choice).
Table 1.
Demographic, Clinical, Neuropsychological, and Decision-Making Characteristics
| Characteristic | No placebo module (n = 25) | Placebo module (n = 24) | t or χ2[df] | p |
| Age, years | 55.8 (6.0) | 55.3 (3.9) | t[47] = 0.352 | 0.727 |
| Education, years | 13.4 (1.7) | 12.7 (2.1) | t[47] = 1.408 | 0.166 |
| Sex | χ 2[1] = 4.751 | 0.029 | ||
| Male (%) | 60.0% | 87.5% | ||
| Female (%) | 40.0% | 12.5% | ||
| Diagnosis | χ2[1] = 0.234 | 0.628 | ||
| Schizophrenia (%) | 60.0% | 66.7% | ||
| Schizoaffective disorder (%) | 40.0% | 33.3% | ||
| Ethnicity | χ2[2] = 3.381 | 0.184 | ||
| Caucasian (%) | 72.0% | 91.7% | ||
| African American (%) | 8.0% | 4.2% | ||
| Hispanic/Latino (%) | 20.0% | 4.2% | ||
| MacCAT-CR Subscales (possible range) | ||||
| Understanding (0–26) | 18.9 (5.8) | 17.5 (6.0) | t[47] = 0.847 | 0.401 |
| Appreciation (0–6) | 4.5 (1.6) | 4.1 (1.6) | t[47] = 0.877 | 0.385 |
| Reasoning (0–8) | 5.4 (1.9) | 4.9 (2.5) | t[47] = 0.844 | 0.403 |
| Choice (0–2) | 1.9 (0.3) | 2.0 (0.2) | t[40.2] = −1.000 | 0.323 |
| PANSS (possible range) | ||||
| Positive subscale (7–49) | 16.4 (5.2) | 13.9 (4.5) | t[47] = 1.833 | 0.073 |
| Negative subscale (7–49) | 14.2 (5.6) | 14.5 (6.4) | t[47] = −0.190 | 0.850 |
| General subscale (16–112) | 28.6 (7.1) | 28.4 (7.9) | t[47] = 0.086 | 0.932 |
| HAM-D (17-item version, possible range 0–50) | 11.0 (6.6) | 9.8 (6.6) | t[47] = 0.641 | 0.525 |
| Birchwood Insight Questionnaire (possible range 0–16, N = 48) | 11.8 (2.2) | 10.7 (4.8) | t[46] = 1.037 | 0.307 |
| DRS raw scores (N = 46) (range) | ||||
| Total (poss. 0–144; obs. 83–143) | 128.0 (15.3) | 128.4 (10.6) | t[44] = −0.120 | 0.905 |
| Attention (poss. 0–37; obs. 21–37) | 33.8 (3.7) | 34.8 (1.8) | t[44] = −1.151 | 0.256 |
| Initiation/Perseveration (poss. 0–37; obs. 14–37) | 33.2 (5.6) | 31.6 (4.4) | t[44] = 1.082 | 0.285 |
| Construction (poss. 0–6; obs. 2–6) | 5.2 (1.2) | 5.5 (1.0) | t[44] = −0.835 | 0.408 |
| Conceptualization (poss. 0–39; obs. 25–39) | 34.4 (4.3) | 34.8 (4.0) | t[44] = −0.307 | 0.760 |
| Memory (poss. 0–25; obs. 11–25) | 21.4 (4.0) | 21.7 (2.8) | t[44] = −0.250 | 0.803 |
Note: Unless otherwise indicated, the values represent means (and SDs). MacCAT-CR = MacArthur Competence Assessment Tool for Clinical Research; PANSS = Positive and Negative Syndrome Scale; HAM-D = Hamilton Depression Rating Scale; DRS = Mattis Dementia Rating Scale; Poss. = Possible range; Obs. = Observed range.
Performance on the Placebo Questionnaire
Overall, the participants had a mean of 7.0 (SD = 2.9) of a possible 12 points on the placebo questionnaire (range 2 to 12). Those participants who were given the extra educational module regarding placebos obtained significantly higher scores than the standard information group on the placebo questionnaire (mean = 8.2 [SD = 2.7] versus mean = 5.8 [SD = 2.5], respectively; t = −3.203, df = 47, p = 0.002).
We also examined the effects of consent condition on specific items from the placebo questionnaire (Table 2). We found that items related to 3 specific themes were most responsive to the educational intervention: the abilities of participants to tell the difference between placebos and the active medications; the idea that some people who receive the placebo may feel their symptoms are better; and the possibility that people who receive the placebo could experience side effects. (This information was contained explicitly in the placebo educational module condition, whereas the regular consent form simply explained that symptoms could get worse if one received the placebo.) On the other hand, we did not find any differences between the 2 groups in participants' abilities to describe why a placebo is used or to identify risks associated with taking a placebo. We also did not find any significant difference in scores between those who received the overall, routine paper-based consent (ie, presenting the entire hypothetical trial) and those who received the overall computer-based consent.
Correlates of performance on placebo questionnaire
Across the two groups (with or without placebo module), worse cognitive deficits (lower scores on DRS Total and on Construction and Conceptualization subscales) were related to worse scores on the placebo measure. Worse negative symptoms were associated with worse performance on the placebo post-test questionnaire, whereas no association was found between the placebo questionnaire and positive symptoms, general psychopathology, depressive symptoms, or level of insight. We also found that better performance on the MacCAT-CR Understanding and Reasoning, but not Appreciation, subscales was associated with better total scores on the placebo questionnaire (Table 3).
Table 3.
Bivariate Correlations (Spearman's rho) Between Placebo Questionnaire Scores and Demographic, Clinical, and Neuropsychological Variables
| Characteristic | Spearman's rho | p |
| Age (years) | −.265 | .066 |
| Education (years) | .083 | .570 |
| PANSS | ||
| Positive subscale | −.123 | .399 |
| Negative subscale | −.359 | .011 |
| General subscale | −.146 | .315 |
| HAM-D (17-item version) | .023 | .873 |
| Birchwood Insight Questionnaire | .218 | .137 |
| DRS Raw Scores (N = 46) | ||
| Total | .405 | .005 |
| Attention | .219 | .144 |
| Initiation/Perseveration | .270 | .070 |
| Construction | .359 | .014 |
| Conceptualization | .371 | .011 |
| Memory | .199 | .185 |
| MacCAT-CR Subscales | ||
| Understanding | .623 | < .001 |
| Appreciation | .229 | .114 |
| Reasoning | .443 | .001 |
Note: N = 49, except where indicated. PANSS = Positive and Negative Syndrome Scale; HAM-D = Hamilton Depression Rating Scale; DRS = Mattis Dementia Rating Scale; MacCAT-CR = MacArthur Competence Assessment Tool for Clinical Research.
We also explored whether there were any associations particular to each group (with or without placebo module) in order to understand more fully whether the intervention was compensating for some specific deficit. Negative symptoms were inversely associated with performance on the placebo questionnaire in the group who received the placebo module (n = 24; Spearman's rho = −0.534, p = 0.007) but not in the group that did not receive it (n = 25; Spearman's rho = −0.047, p = 0.824). The difference in the magnitude of these correlations, although large in effect size, did not reach statistical significance (Fischer's r-to-Z transformation: Z = 1.798, p ≤ 0.0721). DRS Total scores were significantly correlated with placebo questionnaire scores among those who received the placebo module (n = 24; Spearman's rho = 0.549, p = 0.006) but not among those not receiving it (n = 25; Spearman's rho = 0.358, p = 0.102); but the difference in the magnitude of these two correlations had a small effect size and was not statistically significant (Fischer's r-to-Z transformation: Z = .794, p ≤ 0.427).
Responses to open-ended items
For some participants the open-ended questions elicited concrete and/or superficial responses. For this reason, the interviewer probed to try to illuminate underlying reasoning. A common misconception seemed to be that the placebo, which we described as a “sugar pill” (a common characterization), is connected to diabetes, suggesting that some interpreted the phrase “sugar pill” quite literally. When asked whether placebos have risks, one participant initially responded that one “might have increased blood sugar, if you were diabetic it might do something to you”; upon further questioning, the participant stated that there were “no other risks.” In contrast, some participants were able to describe the risks of not taking an active medication, eg, “Generally, I think to be taken off medication and given a placebo, even in a controlled environment, the study participant's side effects would worsen. It is likely the illness would manifest more because of lack of medicine.” (This highly rational and well-articulated response serves as a clear reminder that some people with schizophrenia retain very well intact cognitive skills, and it would be a disservice to assume that people lack the capacity to make their own decisions solely because they have a diagnosis of schizophrenia.)
Additional Analyses
When the placebo questionnaire total score was limited to just the true/false items, the benefits of the additional placebo module remained (mean [SD] total = 5.0 [1.1] versus 3.7 [1.5]; t = −3.47, df = 44.6, p = 0.001). The mean score for the latter group was only slightly higher than the level that would be expected by chance (3.0). Correlations with cognitive functioning (DRS Total, Construction, and Conceptualization) were not significant; in part, this may have been due to the constricted range on a 6-item true/false test, although correlations with the MacCAT-CR Understanding (Spearman's rho = 0.401, p = 0.004) and Reasoning (Spearman's rho = 0.334, p = 0.019) subscales remained significant. Of the open-ended items, 2 showed strong correlations with DRS Total scores: “Why do the researchers use a placebo pill?” (Spearman's rho = 0.514, p < 0.001) and “Do you think that some people who get the placebo will feel that their symptoms are worse?” (Spearman's rho = 0.326, p = 0.027).
Discussion
In this preliminary study we found that people with schizophrenia showed a wide range of understanding of placebos as measured by a questionnaire incorporating both true/false and open-ended questions. In addition, a brief educational intervention appeared to improve understanding of placebos. To our knowledge, this is the first study specifically focusing on levels of understanding of placebos among people with serious neuropsychiatric disorders.
In the present study, indeed as with many informed consent studies, the majority of participants did not obtain 100% correct on the questionnaire used. This does not necessarily mean, however, that they lacked decision-making capacity regarding the overall hypothetical clinical trial. This precise dilemma—determining what would constitute an adequate level of performance—still remains unresolved. Our findings may thus represent a “double-edged sword,” because although many patients manifested very good knowledge of placebos, others did not appear to grasp important points, including that there could be a personal downside to being assigned to the placebo condition. This could in turn call into question participants' appreciation of the significance of the information provided for their own situation, underscoring the point that although we refer here mainly to participants' understanding of placebos, we may also have been tapping into their appreciation as well (despite the lack of correlation with scores on the MacCAT-CR Appreciation subscale).
Our finding of heterogeneity of level of understanding of placebos is consistent with the overall literature, which has consistently documented that people with schizophrenia show a wide range of performance on measures of understanding of research in general, as well as on other decision-making abilities,11,19,33 manifesting heterogeneity on virtually every other dimension on which people with schizophrenia may be measured.34–36
The finding that the Conceptualization subscale of the DRS was a good predictor of performance on the 12-item placebo questionnaire fits standard cognitive models of decisional capacity,37 as this subscale taps into executive functions such as abstract thinking. It is also notable that the DRS Memory subscale was not a significant correlate of understanding of placebos; this lack of a significant association may indicate that the placebo questionnaire was in fact measuring more than the ability to repeat back information that the participant had been told. On the other hand, the observed significant correlation with the DRS Construction subscale with the 12-item questionnaire is somewhat puzzling. Constructional ability has no straightforward conceptual link to understanding of placebos (except perhaps for the fact that the placebo module intervention utilized one visuospatial stimulus). As constructional ability is not typically severely impaired in schizophrenia, it may simply have been that impairment on this particular subscale served as a proxy for more general severity of cognitive impairment in our sample.
We also found that understanding of placebos was correlated with scores on the Understanding and Reasoning subscales of a more general and widely used measure of decision-making abilities (the MacCAT-CR). These findings suggest that we were measuring areas relevant to the decision-making abilities assessed with this instrument. It is notable that none of the MacCAT-CR Understanding or Reasoning subscale items specifically asked about placebos (although note that the MacCAT-CR can be tailored to reflect the specific procedural elements of individual clinical trials). In contrast, we found no significant association with the Appreciation subscale, even though that subscale had one item that indirectly related to placebo methods (ie, evaluating the person's belief about the possibility he or she could receive a medication that would not help.) Further work therefore seems warranted examining how, in the real world of clinical trials, knowledge about research-related procedures maps onto the decisional abilities traditionally studied by empirical ethics investigators.
Our findings suggest the possibility of improving understanding of placebos using a brief educational intervention. Although the group who received the educational module had higher total scores on the placebo questionnaire, our results were somewhat mixed, in that some aspects of understanding of placebos (such as an awareness of risks of placebos) seemed more resistant to remediation. This may have been because the educational materials were focused on several aspects of placebos, including the potential for clinical deterioration, but risks were not the major focus of the intervention.
The absence of differences in scores between those who received the overall consent for the hypothetical clinical trial in the routine paper format versus those who received the overall consent on the computer may suggest that the active ingredient in this substudy of placebo education was not the computer per se, but rather the extra information provided (beyond what was already contained in the consent).
The absence of a nonpsychiatric comparison group is a limitation in the present study that must be kept in mind to avoid misinterpretation of our present findings. While some schizophrenia patients have difficulty understanding some aspects of the placebo-controlled design, such difficulties do not appear to be limited to those with serious mental illness.22 In fact, numerous studies of consent in medically ill people and healthy controls have shown that many people—not just those with psychiatric disorders—show less than optimal understanding of consent for research; cognitive impairment appears to be the most salient risk factor for inadequate understanding.6,38 As evidenced by the highly articulate and well-reasoned responses given by some participants during open-ended questioning about placebos, some patients with schizophrenia have an excellent grasp of the nature and risks of participation in a placebo-controlled trial. We also were not able, with the data collected, to examine whether past research experience, and of what sort, was associated with better understanding of placebos.
Another possible limitation was the particular description of placebos that was used in the overall consent study and in the placebo educational intervention. Although we selected phrasing (including the descriptor “sugar pill”) that is frequently used in consent forms for clinical trials, we may have inadvertently contributed to confusion of some participants about diabetic risk from placebos by use of this terminology. We considered using a more technical methodological description of a placebo, but we ultimately opted for language that would reflect the kinds of explanations used in actual consent forms.
As preliminary data, these findings should be seen within the broader context of empirical ethics studies involving people with serious mental illnesses, particularly schizophrenia. For example, Roberts and colleagues examined differences and patterns among 59 patients with schizophrenia and 70 psychiatrists in ratings of harms of four hypothetical studies: blood draw, blood draw with medication washout, medication trial, and medication trial with random assignment to new medication, traditional medication, or placebo. They found that both patients and psychiatrists rated the harms of the medication washout and placebo-controlled studies as higher than those of the blood draw and medication study without placebo.39 The authors did not assess how well patients understood placebo controls, and their description of placebo differed from ours, in that they described the possibility of receiving a placebo as follows: “Or [the medicine] could be a fake pill that doesn't help with schizophrenia, making it so that the patient could get sicker, not better.”
Roberts and colleagues' data are important because they suggest that patients with schizophrenia can distinguish meaningfully among protocols of varying levels of potential harm. What is less clear, however, is whether there is some subset of potential research participants who simply fail to grasp the significance of important design elements for the specific study to which they are being asked to consent, perhaps due to cognitive impairments. If this is the case—and the present findings suggest it may be—then finding ways to enhance understanding of research designs is a critical endeavor for upholding the ethical principle of respect for persons as embodied in informed consent. Promising findings related to enhancing consent procedures for biomedical research have been described among psychiatric and medical research populations,6,10,11,24,40 although more rigorous studies are needed, particularly comparing different methods.40 As a follow-up to the work described here, head-to-head testing of several different methods of explaining research-specific elements (eg, placebos, randomization, algorithmic treatment, limitations on individualization of care within specific trials) would be informative. Moreover, such trials should examine a range of psychiatric, medical, and general population groups to determine which methods are best and for whom. Such studies would provide long-needed empirical assessments of suggestions made nearly two decades ago,7 as well as more recently,26 regarding specific methods to improve explanations of research design features.
The importance of dialogue with participants during the consent process—a straightforward method for enhancing consent—is highlighted by the finding that even seemingly basic phrases such as “sugar pill” may be misinterpreted. It is also important to note that participants' understanding of risks of placebos did not appear to be affected by the intervention, emphasizing the need for evidence-based ethics research to focus on some of the specific areas where more work may be required to inform participants. These findings also highlight one of the present limitations of informed consent research in general, namely, the lack of broad consensus about how much information to provide to participants and how to provide it. Greater agreement on these issues will ultimately enable the field to move forward, bringing suggestions for assessing capacity and enhancing consent procedures that would be more widely adopted by the research community.
Despite these caveats, this and other studies4,7,41,42 illustrate the need not just to attend to overall understanding and appreciation, but also to consider participants' comprehension of quite specific key elements of research design. Something as basic to research as “placebo control,” a common and seemingly simple (to investigators) research method, may be difficult to accurately convey to participants (whether they are patients or healthy comparison subjects). To the degree that a research participant does not understand that a placebo arm means there is a chance he or she will receive a known ineffective treatment, then that person's decision to enter the clinical trial is not fully and appropriately informed. At this juncture, we should not assume such difficulties represent a deficit of the research participants themselves, unless and until sufficient research is done to test other feasible means of effectively communicating such concepts to laypersons. There appears to be considerable room to improve the way research participants are informed about key research-specific concepts such as randomization and placebo control.
Acknowledgments
Dr. Dunn gratefully acknowledges the support of the National Institute of Mental Health (K23 MH66062), the National Alliance for Research on Schizophrenia and Depression, and the Greenwall Foundation. Dr. Palmer gratefully acknowledges the support of the National Institute of Mental Health (R01 MH64722). The authors also wish to thank Rebecca Rodriguez and Deannah Neal for their assistance.
References
- 1.US Department of Health and Human Services, Office of Inspector General. Recruiting Human Subjects: Pressures in Industry-Sponsored Clinical Research. June 2000. OEI-01-97-00195
- 2.Simon C, Zyzanski SJ, Eder M, Raiz P, Kodish ED, Siminoff LA. Groups potentially at risk for making poorly informed decisions about entry into clinical trials for childhood cancer. J Clin Oncol. 2003;21:2173–2178. doi: 10.1200/JCO.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Wendler D. Can we ensure that all research subjects give valid consent? Arch Intern Med. 2004;164:2201–2204. doi: 10.1001/archinte.164.20.2201. [DOI] [PubMed] [Google Scholar]
- 4.Criscione LG, Sugarman J, Sanders L, Pisetsky DS, St Clair EW. Informed consent in a clinical trial of a novel treatment for rheumatoid arthritis. Arthritis Rheum. 2003;49:361–367. doi: 10.1002/art.11057. [DOI] [PubMed] [Google Scholar]
- 5.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet. 2001;358:1772–1777. doi: 10.1016/S0140-6736(01)06805-2. [DOI] [PubMed] [Google Scholar]
- 6.Dunn LB, Jeste DV. Enhancing informed consent for research and treatment. Neuropsychopharmacology. 2001;24:595–607. doi: 10.1016/S0893-133X(00)00218-9. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum PS, Roth LH, Lidz CW, Benson P, Winslade W. False hopes and best data: consent to research and the therapeutic misconception. Hastings Cent Rep. 1987;17:20–24. [PubMed] [Google Scholar]
- 8.Paasche-Orlow MK, Taylor HA, Brancati FL. Readability standards for informed-consent forms as compared with actual readability. N Engl J Med. 2003;348:721–726. doi: 10.1056/NEJMsa021212. [DOI] [PubMed] [Google Scholar]
- 9.Wirshing DA, Sergi MJ, Mintz J. A videotape intervention to enhance the informed consent process for medical and psychiatric treatment research. Am J Psychiatry. 2005;162:186–188. doi: 10.1176/appi.ajp.162.1.186. [DOI] [PubMed] [Google Scholar]
- 10.Dunn LB, Lindamer LA, Palmer BW, Golshan S, Schneiderman LJ, Jeste DV. Improving understanding of research consent in middle-aged and elderly patients with psychotic disorders. Am J Geriatr Psychiatry. 2002;10:142–150. [PubMed] [Google Scholar]
- 11.Carpenter WT, Gold JM, Lahti AC, et al. Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry. 2000;57:533–538. doi: 10.1001/archpsyc.57.6.533. [DOI] [PubMed] [Google Scholar]
- 12.Appelbaum PS, Lidz CW, Grisso T. Therapeutic misconception in clinical research: frequency and risk factors. IRB. 2004;26:1–8. [PubMed] [Google Scholar]
- 13.Roberts LW, Lauriello J, Geppert C, Keith SJ. Placebos and paradoxes in psychiatric research: an ethics perspective. Biol Psychiatry. 2001;49:887–893. doi: 10.1016/s0006-3223(01)01111-8. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter WT, Appelbaum PS, Levine RJ. The declaration of Helsinki and clinical trials: a focus on placebo-controlled trials in schizophrenia. Am J Psychiatry. 2003;160:356–362. doi: 10.1176/appi.ajp.160.2.356. [DOI] [PubMed] [Google Scholar]
- 15.Weijer C. Placebo-controlled trials in schizophrenia: are they ethical? are they necessary? Schizophr Res. 1999;35:211–218. doi: 10.1016/s0920-9964(98)00127-3. [DOI] [PubMed] [Google Scholar]
- 16.Miller FG. Placebo-controlled trials in psychiatric research: an ethical perspective. Biol Psychiatry. 2000;47:707–716. doi: 10.1016/s0006-3223(00)00833-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY. Benefits and burdens of placebos in psychiatric research. Psychopharmacology. 2003;171:13–18. doi: 10.1007/s00213-003-1458-2. [DOI] [PubMed] [Google Scholar]
- 18.Levine RJ. The need to revise the Declaration of Helsinki. N Engl J Med. 1999;341:531–534. doi: 10.1056/NEJM199908123410713. [DOI] [PubMed] [Google Scholar]
- 19.Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: Abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19:149–174. doi: 10.1007/BF01499323. [DOI] [PubMed] [Google Scholar]
- 20.Grisso T, Appelbaum PS. Assessing Competence to Consent to Treatment: A Guide for Physicians and Other Health Professionals. New York: Oxford University Press; 1998. [Google Scholar]
- 21.Henderson GE, Easter MM, Zimmer C, et al. Therapeutic misconception in early phase gene transfer trials. Soc Sci Med. 2005; Published online July 4. [DOI] [PubMed]
- 22.Pope JE, Tingey DP, Arnold JM, Hong P, Ouimet JM, Krizova A. Are subjects satisfied with the informed consent process? a survey of research participants. J Rheumatol. 2003;30:815–824. [PubMed] [Google Scholar]
- 23.Frank E, Novick DM, Kupfer DJ. Beyond the question of placebo controls: ethical issues in psychopharmacological drug studies. Psychopharmacology. 2003;171:19–26. doi: 10.1007/s00213-003-1477-z. [DOI] [PubMed] [Google Scholar]
- 24.Wirshing DA, Wirshing WC, Marder SR, Liberman RP, Mintz J. Informed consent: assessment of comprehension. Am J Psychiatry. 1998;155:1508–1511. doi: 10.1176/ajp.155.11.1508. [DOI] [PubMed] [Google Scholar]
- 25.Stiles PG, Poythress NG, Hall A, Falkenbach D, Williams R. Improving understanding of research consent disclosures among persons with mental illness. Psychiatr Serv. 2001;52:780–785. doi: 10.1176/appi.ps.52.6.780. [DOI] [PubMed] [Google Scholar]
- 26.Lidz CW, Appelbaum PS. The therapeutic misconception: problems and solutions. Med Care. 2002;40:V55–63. doi: 10.1097/01.MLR.0000023956.25813.18. [DOI] [PubMed] [Google Scholar]
- 27.Appelbaum PS, Grisso T. MacCAT-CR: MacArthur Competence Assessment Tool for Clinical Research. Sarasota, Fla: Professional Resource Press; 2001. [Google Scholar]
- 28.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M. A self-report Insight Scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand. 1994;89:62–67. doi: 10.1111/j.1600-0447.1994.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 31.Mattis S. Dementia Rating Scale. Odessa, Fla: Psychological Assessment Resources, Inc.; 1973. [Google Scholar]
- 32.Twamley EW, Doshi RR, Nayak GV, et al. Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry. 2002;159:2013–2020. doi: 10.1176/appi.ajp.159.12.2013. [DOI] [PubMed] [Google Scholar]
- 33.Palmer BW, Dunn LB, Appelbaum PS, Jeste DV. Correlates of treatment-related decision-making capacity among middle-aged and older patients with schizophrenia. Arch Gen Psychiatry. 2004;61:230–236. doi: 10.1001/archpsyc.61.3.230. [DOI] [PubMed] [Google Scholar]
- 34.Palmer BW, Heaton RK, Paulsen JS, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 35.Palmer BW, Heaton RK, Gladsjo JA, Evans JD, Patterson TL, Golshan S, Jeste DV. Heterogeneity in functional status among older outpatients with schizophrenia: employment history, living situation, and driving. Schizophr Res. 2002;55:205–215. doi: 10.1016/s0920-9964(01)00218-3. [DOI] [PubMed] [Google Scholar]
- 36.Tsuang MT, Lyons MJ, Faraone SV. Heterogeneity of schizophrenia: conceptual models and analytic strategies. Br J Psychiatry. 1990;156:17–26. doi: 10.1192/bjp.156.1.17. [DOI] [PubMed] [Google Scholar]
- 37.Marson D, Harrell L. Executive dysfunction and loss of capacity to consent to medical treatment in patients with Alzheimer's disease. Semin Clin Neuropsychiatry. 1999;4:41–49. doi: 10.1053/SCNP00400041. [DOI] [PubMed] [Google Scholar]
- 38.Sugarman J, McCrory DC, Hubal RC. Getting meaningful informed consent from older adults: a structured literature review of empirical research. J Am Geriatr Soc. 1998;46:517–524. doi: 10.1111/j.1532-5415.1998.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 39.Roberts LW, Warner TD, Brody JL, Roberts B, Lauriello J, Lyketsos C. Patient and psychiatrist ratings of hypothetical schizophrenia research protocols: assessment of harm potential and factors influencing participation decisions. Am J Psychiatry. 2002;159:573–584. doi: 10.1176/appi.ajp.159.4.573. [DOI] [PubMed] [Google Scholar]
- 40.Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 41.Dunn LB, Jeste DV. Problem areas in the understanding of informed consent for research: study of middle-aged and older patients with psychotic disorders. Psychopharmacology (Berl) 2003;171:81–85. doi: 10.1007/s00213-003-1501-3. [DOI] [PubMed] [Google Scholar]
- 42.Vitiello B, Aman MG, Scahill L, et al. Research knowledge among parents of children participating in a randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2005;44:145–149. doi: 10.1097/00004583-200502000-00006. [DOI] [PubMed] [Google Scholar]